Abstract
In this study, we systematically explored the clinical manifestations, diagnosis, treatment and prognosis of renal epithelioid angiomyolipoma (EAML) retrospectively by analyzing data of 52 patients diagnosed with EAML at four centers. Our results showed that the onset of EAML was usually inconspicuous and so no obvious symptoms or signs had occurred in most patients at diagnosis. Its diagnoses always depended on postoperative pathological examination. The immunohistochemical (IHC) results [HMB45 ( + ), cytokeratin (-) and S100 (-)] could be used to differentiate EAML from other malignancies such as renal cell cancer (RCC) and sarcomas. For treatment, surgery resulted in satisfactory short-term prognosis. The long-term prognosis of patients with EAML was poor, particularly when a large size, a high percentage of epithelioid component, tumor thrombus formation and necrosis were present. In conclusion, EAML is a tumor with malignant potential. Once diagnosed, integrated approaches, including surgery, chemotherapy and targeted therapy, should be considered; a close follow-up regimen is necessary for cases that met: 1) tumor size >9 cm, 2) tumor thrombus formation in the vein, 3) epithelioid cells >70% or atypia cells >60% and 4) necrosis.
Similar content being viewed by others
Introduction
Renal angiomyolipoma (AML) is one of the most common renal benign tumors and originates from mesenchymal tissue. Epidemiological studies have revealed that it accounts for approximately 1% of all renal tumors. According to the classification of tumors by the WHO in 20041 there are two types of renal AML: classical and epithelioid. The former is a benign tumor that is composed of different proportions of mature abnormal thick-walled blood vessels, fusiform or epithelioid smooth muscle cells and adipose tissue. EAML, or monotypic EAML, especially for those with atypia epithelioid cells2 has malignant potential and is mainly composed of a large number of hyperplastic epithelioid cells arranged in sheets; the proportion of mature fat cells tends to be <5%3,4. EAML can be one part of classical AML, or a single tumor element.
However, most studies assessing malignant EAML are case reports or analyses5,6; therefore, current understanding of its clinical and pathological characteristics is insufficient. Whether EAML is benign or malignant is controversial and WHO has not yet commented on whether malignant AML truly exists. Nevertheless, some recent reports raised the concept of malignant EAML because of the histological variability, atypia and some cases with invasive growth patterns and fatal outcomes.
In the present study, we analyzed the clinical data of 52 patients with atypical EAML who were diagnosed at four centers and performed a literature review to systematically clarify the clinical manifestation, diagnosis, treatment and prognosis.
Material and methods
All candidates came from 4 large hospitals in the west of China. Retrospectively, a search with the key words “EAML” (using Chinese) was made in pathology database of each center. The hospitalization and re-examination records of all eligible patients were then reviewed, including clinical features, imaging, type of treatment and so on. Their paraffin-embedded tissue slides were centralized to the West China Hospital and a central pathological review was conducted by 3 independent pathologists by microscopes (YJ, XH and SHX). All the paraffin-embedded tissues came from post-operative specimens. The percentage of the epithelioid cells was estimated visually occupying the total tumor areas of the slides and the percentage of the atypia epithelioid cells was estimated visually occupying the total number of epithelioid cells7. EAML was defined as polygonal cells with clear to eosinophilic cytoplasm and round to oval nuclei8. Atypical EAML was defined as the epithelioid cells with abundant cytoplasm, vesicular nuclei, prominent nucleoli and nuclear size that exceeds 2 times the size of adjacent nuclei. The degree of nuclear atypia was graded to low, moderate and severe referring to the definition of Fadi et al.7. Any disagreements were solved through majority voting within the pathological review group. The Follow-up data were achieved by making phone calls or in out-patient department of urology.
Finally, 52 atypical EAML patients were retrieved: the West China Hospital(n = 26), the Sichuan Provincial Cancer Hospital(n = 12), the First Hospital of Guangxi Medical University (n = 10) and the First Affiliated Hospital of Chengdu Medical College(n = 4). 30 patients were male and 22 were female, with a mean age of 39.2 (21–61) years. Twenty-eight tumors involved the left kidney and 24 involved the right kidney. The study was approved by the Ethics Committee of West China Hospital and the methods were carried out in accordance with the approved guidelines. Oral informed consent was obtained for reviewing their pathological specimens when made phone calls or re-examination with each patient. Then, we extracted and analyzed data from the patients’ medical records, postoperative pathological results and re-examined radiology results [chest X-ray, abdominal computed tomography (CT) and ultrasound] from the inpatient database. Patient survival status was determined by making phone calls. The “Progressors” EAML were defined as the existence of progression, including local recurrence, distal metastasis and death due to disease.
SPSS 13.0 was used for statistics. Discrete variables and continuous variables were compared with the Chi-Square and t test, respectively.
Results
Of the 52 patients enrolled, we were unable to determine the survival status of 9: five did not provide or provided unavailable phone numbers and four didn't want to cooperate. Finally, we obtained the complete clinical data of 43 patients (83%), with a mean follow-up duration of 44.3 (4–71) months. The clinical data of follow-up patients in details were summarized in Table 1. The compare of pathologic features between progressors and non-progressors EAML was shown at Table 2.
When the surgical treatment of the 43 cases in the present study are summarized, 12 underwent radical nephrectomy, 30 used nephron-sparing surgery and one underwent radical nephrectomy with the removal of the tumor thrombus in postcava. After follow-up of approximate 4 years, one patient underwent radical nephrectomy and one underwent nephron-sparing surgery suffered metastasis or recurrence; all other patients, including one with liver metastasis at baseline were alive. AML specimens from 43 patients were stratified into 3 groups based on the percentage of epithelioid/atypia epithelioid cells. Among the six cases with >90% epithelioid cells, three showed obvious focal necrosis and hemorrhage. The tumor cells showed different degrees of polymorphy, with irregular shape that differed from the epithelioid or classical AML. Mitotic figures were readily identified and karyolobism could be seen.
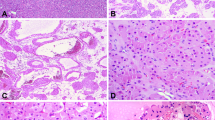
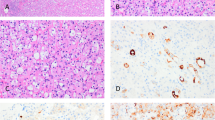
Among these three, two had renal hilum and para-aortic lymph node metastasis at diagnosis. During the follow-up, one of the two patients with lymph node metastasis was diagnosed with hepatic and lung metastasis 12 months postoperatively and died 17 months later (Fig. 1); one patient with renal hilus node metastasis at diagnosis suffered local recurrence 10 months postoperatively and died from serious cancer cachexia 4 months later (Fig. 2); the third case (Fig. 3) and all the others did not suffer any signs of progression and live well.
Discussion
Although some researchers still consider renal AML to be a hamartoma, a tumor-like malformation formed by aberrantly assembled normal renal tissues, many studies have unequivocally demonstrated that it is a tumor. Paradis et al. 9 and Green et al.10 revealed its clonal origination by revealing that chromosome X was inactivated randomly. Kattar et al.11 reported gene deficiencies in chromosome band 16p13 and clonal chromosomal aberrations in other specified locations. Therefore, the latest WHO classification of diseases in 2004 announced that the renal AML was tumor. It also defined EAML as a potentially malignant mesenchymal neoplasm characterized by the proliferation of predominantly epithelioid cells. However, no previous studies have defined clear lower limit of epithelioid cells’ percentage of EAML, which could be called a “real” EAML. Some used the phrase “predominantly” or “mostly”12,13,14,15,16 and others used “pure”17. In the present study, the cases included were stratified into 3 groups based on proportion of epithelioid/atypical epithelioid cells that were present. Herein, we attempted to 1)systematically clarify the clinical manifestation, diagnosis, treatment and prognosis; 2)unfolded the potiential relationship between proportion of epithelioid/atypical epithelioid cells and progression of EAML, as discussed below.
Clinical manifestation
Similar to patients with classical AML, patients with EAML show an insidious onset and tumors are often discovered in imaging results from physical examinations. Dickinson et al.18 discovered that 82%–94% of patients with lesions over 4 cm complained of clinical symptoms and 50%–60% had concomitant bleeding. Of the 43 cases in this study, 8 visited physicians because of significant symptoms or signs: 6 with backache and 2 with hematuria. All of these four tumors were larger than 5 cm. One hematuria case and one backache case were proved to be malignant. These data suggest that the larger the lesion is, the greater the probability of clinical symptoms. Based on our intraoperative findings and postoperative pathological reports, almost all EAMLs had an abundant blood supply. The blood vessels supplying the tumors often communicated with the vessels supplying the adrenal gland, urinary duct, or diaphragm and were usually tortuous with a missing internal elastic membrane. This phenomenon might explain why most cases had different levels of localized hemorrhagic necrosis, which is consistent with the results of Yamakado et al.19.
Diagnosis
Making a preoperative diagnosis for EAML is challenging for several reasons; therefore, it is often misdiagnosed as RCC. This is in part because EAML also has an insidious onset and no specific clinical manifestation. In addition, imaging examination techniques such as CT and MRI are sensitive to adipose tissue and the amount of adipose tissue in EAML is <5%3,4. Therefore, its diagnosis often depends on postoperative pathological examinations when epithelioid cells are detected. Nevertheless, the application of Doppler ultrasound or CT can pick up incidental tumours in asymptomatic patients. Previous studies20,21 demonstrated that EAML was positive for melanoma cell markers (such as HMB45) and smooth muscle cell markers, but negative for epithelial cell markers. Pea et al.18 and L’Hostis et al.22 reported four cases of EAML: three were diagnosed with RCC and one with classical AML preoperatively. However, all cases were diagnosed as EAML based on postoperative HMB45 expression and pathological results.
Under a microscope, epithelioid cells from the 43 cases in the present study were irregular polygon shaped, with enlarged pleomorphic nuclei in the mitotic phase and cytoplasm enriched with eosinophil granules. In addition, IHC results revealed that all 43 cases were HMB45-positive. However, other malignancies such as RCC and sarcomas do not express HMB-45. Ooi et al.23 demonstrated that because Ki-67 is involved in cell proliferation, it could serve as an antigen to indicate the cell proliferation status during the diagnosis of EAML. However, the present study revealed that Ki-67 had a low diagnostic sensitivity because only 10 of 43 cases were positive for Ki-67 and approximately 2–10% cells were stained. Other markers such as cytokeratin and S100 were usually negative. So, the differential expression of HMB-45, cytokeratin and S100 can be used to differentiate EAML from RCC and metastatic melanoma5.
Treatment
To date, no approaches are more effective than surgery for EAML; however, surgery is commonly insufficient. Many previous studies20,24,25,26,27,28,34 have reported that local recurrence or distant metastasis generally occur 1.5–9 years postoperatively. Zomboni et al.29 reported that EAML belonged to a family of perivascular epithelioid cell tumors (PEComa), similar to lymphagioleiomyomas and clear cell “sugar” tumors of the lung and pancreas. Therefore, EAML might be sensitive to chemotherapy, similar to the other two tumor types. Cibas et al.30 and Park et al.12 used doxorubicin, cyclophosphamide and cisplatin to treat EAML and they found that the tumors were responsive. However, long-term follow-up observation was needed to confirm their effects. Heidi et al.31 studied 15 cases of PEComa and found that the mammal rapamycin (mTOR) cascade was always activated, which is related to tumor growth and development. This suggests that patients might benefit from mTOR inhibitors and that further studies should be performed urgently.
Based on the current cohort, we believed that nephron-sparing nephrectomy was safe for patients without lymph node metastasis and tumor thrombus formation; nevertheless, long-term survival requires the support of long-term follow-up data. Multiple therapies, including surgery, chemotherapy and molecular-targeted drugs, might be beneficial for long-term patient survival.
Prognosis
Previous data revealed that patients with EAML had poor prognosis. After Martignoni et al.31 first reported fatal cases of EAML in 1994, many case reports20,24,25,26,27,28,34demonstrated that EAML had malignant potential and might cause patient mortality. Distant metastases were found in different organs 1.5–9 years postoperatively, as confirmed by biopsy or autopsy and most patients died within 1 year of metastasis24,25,26,27,28. Tsai et al.33 reported that at least 30% of EAML patients would progress to typical malignant EAML. Vaema et al.34 performed a systematic review of 10 previously published case studies and, regardless of potential publication bias, they found that most patients died from tumor progression and many included liver and lung metastasis.
Nevertheless, the biological behavior of EAML remains controversial. According to the WHO definition in 2004, EAML are tumors with malignant potential. To predict patient prognosis earlier, some recent studies introduced prediction models for malignant EAML after studying a large number of samples. Fadi et al.7 first defined atypical epithelioid cells (AECs). They then summarized 40 cases of atypical EAML and presented a prediction model for malignant EAML as follows: 1) percentage of AEC ≥70% EC, 2) ≥2 mitotic figures per 10 high power fields (HPF), 3) atypical mitotic figures and 4) necrosis. The model focused on the pathological features of the AEC nuclei and 78% of EAML cases who finally occurred recurrence or metastasis were involved. Therefore, the model had a high reference value. Nese et al.17 summarized 41 cases of “pure” EAML (epithelioid cells accounted for almost 100% of the tumor). They reported that the following five characteristics were related to tumor progression: 1) tuberous sclerosis complications, 2) tumor size >7 cm, 3) involvement of the perirenal and/or renal vein, 4) a carcinoma-like growth pattern and 5) necrosis. After their follow-up, tumor progression was seen all in tumors that fulfilled more than four of these five characteristics. The model by Nese et al., which places emphasis on general features, seems to have more accurate predictive power than the model by Fadi et al. However, it must be considered that Nese’s model was based on “pure” EAML. Therefore, the percentage of epithelioid cells might be associated with tumor progression and a higher probability that all five characteristics would occur. Faraji et al.35 also found that tumors with cytological atypia and tumor necrosis showed an unfavorable outcome.
Our study demonstrated that, after a follow-up of 44.3 months, only 2 patients with node metastases and embolus formation suffered recurrence or metastasis and died finally. The two fatal cases were consistent with the results of previous fatal cases32 in which liver, lung, or bone metastases occurred frequently. The remaining 41 patients, including one patient with liver or bowel metastasis before the surgery, survived. When the 3 progressors were compared with non-progressors (Table 2), it was obvious that the fatal case had a significantly higher percentage of epithelioid cells, percentage of atypia cells and Max diameter. This may enhance the concept that a higher percentage of epithelioid cells facilitates tumor progression. The multi-center study performed by Nese et al.17, which included 41 “pure” EAML cases, revealed recurrence or metastasis in 50% of patients and 30% eventually died from their disease. One explanation for the low percentage of progression in this 43 cohort might be the low percentage of epithelial cells (mean: 43.8 ± 22.2). Another explanation could be the limitation of the present study of a relatively short follow-up period, because a previous case34 did not exhibit distant metastasis until after 9 years of follow-up. In the present study, the three progressors cases met the following characteristics concerning to general and pathological features: 1) tumor size >9 cm, 2) tumor thrombus formation in the vein, 3) epithelioid cells >70% or atypia cells >60% and 4) necrosis. A lesion that met 3 or more of the above features predicted increased risk of malignant behavior and should clinically be closely followed.
Limitations
Given the rare nature of the disease, prospective research design was difficult to conduct. This was the inherent limitation of the study. For this reason, we included patients’ cohort from different hospitals retrospectively. Also, the proper option of surgical method (eg. radical or nephron-sparing nephrectomy) was not available. We couldn’t establish uniform criterion for surgical extirpation; the histopathology of these lesions was mostly determined postoperatively. Therefore, more convincing data from prospective, multicenter, cohort study may reveal the effect of surgical method to prognosis.
Conclusions
EAML is a tumor with potential malignancy. Once diagnosed, particularly when a tumor met the above characteristics like the three malignant cases, active treatment, including radical nephrectomy, chemotherapy and molecular-targeted drugs should be considered and patients should be closely followed.
Additional Information
How to cite this article: Lei, J. H. et al. A Four-Year Follow-up Study of Renal Epithelioid Angiomyolipoma: A Multi-Center Experience and Literature Review. Sci. Rep. 5, 10030; doi: 10.1038/srep10030 (2015).
References
Eble, J. N. et al. World Health Organization classification of tumours. Pathology and genetics of tumours of the urinary system and male genital organs [943–945] IARc Press: Lyon, 2004.
Lopez-Beltran, A. et al. 2004 WHO Classificationof the Renal Tumors. Eur. Urol. 49, 798–805 (2004).
Halpenny, D., Snow, A. & McNeill, G. The radiological diagnosis and treatment of renal angiomyolipomadcurrent status. Clin. Radiol. 65, 99–108 (2010).
Kuo-How, H. et al. Malignant Epithelioid Angiomyolipoma of the Kidney. J. Formos. Med. Assoc. 106, 51–54(2007).
Acikalin, M. F. et al. Epithelioid angiomyolipoma of the kidney. Int. J. Urol. 12, 204–7 (2005).
Chia-Chun, T. et al. Epithelioid angiomyolipoma of the kidney mimicking renal cell carcinoma: a clinicopathologic analysis of cases and literature review. Kaohsiung J. Med. Sci. 25, 133–40 (2009).
Brimo, F. et al. Renal Epithelioid Angiomyolipoma With Atypia: A Series of 40 Cases With Emphasis on Clinicopathologic Prognostic Indicators of Malignancy. Am J Surg Pathol 34, 715–22 (2010).
Aydin, H. et al. Renal Angiomyolipoma Clinicopathologic Study of 194 Cases with Emphasis on the Epithelioid Histology and Tuberous Sclerosis Association. Am J. Surg. Pathol. 33, 289–297 (2009).
Paradis, V. et al. Clonal analysis of renal sporadic angiomyolipomas. Hum. Pathol. 29, 1063–67 (1998).
Green, A. J., Sepp, T. & Yates, J. R. Clonality of tuberous sclerosis hamartomas shown by non-random X-chromosome inactivation. Hum. Genet. 97, 240–243 (1996).
Kattar, M. et al. Chromosomal analysis of renal angiomyolipoma by comparative genomic hybridization: evidence for clonal origin. Hum. Pathol. 30, 295–99 (1999).
Park, H. K. et al. Clinical presentation of epithelioid angiomyolipoma. Int. J. Urol. 14, 21–5 (2007).
Belanger, E. C. et al. Epithelioid angiomyolipoma of the kidney mimickingrenal sarcoma. Histopathology 47, 433–5 (2005).
Takahashi, N. et al. Malignant transformation of renal angiomyolipoma. Int. J. Urol. 10, 271–3 (2003).
Pea, M. et al. Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J. Surg. Pathol. 22, 180–7 (1998).
Martignoni, G. et al. Carcinoma like monotypic epithelioid angiomyolipoma in patients without evidence of tuberous sclerosis: a clinicopathologic and genetic study. Am J. Surg. Pathol. 22, 663–72 (1998).
Nese, N. et al. Pure Epithelioid PEComas (So-Called Epithelioid Angiomyolipoma) of the Kidney: A Clinicopathologic Study of 41 Cases: Detailed Assessment of Morphology and Risk Stratification. Am J. Surg. Pathol. 35, 161–76 (2011).
Dickinson, M. et al. Renal angiomyolipoma: optimal treatment based on size and symptoms. Clin. Nephrol. 49, 281–6 (1998).
Yamakado, K. et al. Renal angiomyolipoma: relationships between tumor size, aneurysm formation and rupture. Radiology 225, 78–82 (2002).
Martignoni, G. et al. Renal epithelioid oxyphilic neoplasm (REON): a pleomorphic monophasic variant of renal angiomyolipoma. Int. J. Surg. Pathol. 2, 539 (1994).
Pea, M. et al. Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identificationof malignant epithelioid angiomyolipoma. Am J. Surg. Pathol. 22, 180–87 (1998).
L’Hostis, M. et al. Renal angiomyolipoma: a clinicopathologic, immunohistochemical and follow up study of 46 cases. Am J. Surg. Pathol. 23, 1011–20 (1999).
Ooi, S. M., Vivian, J. B. & Cohen, R. J. The use of the Ki-67 marker in the pathological diagnosis of the epithelioid variant of renal angiomyolipoma. Int. Urol. Nephrol. 41, 559–65 (2009).
Mai, K. T., Perkins, D. G. & Collins, J. P. Epithelioid cell variant of renal angiomyolipoma. Histopathology 28, 277–80 (1996).
Bjorsson, J. et al. Tuberous sclerosis-associated renal cell carcinoma, clinical, pathological and genetic features. Am J. Pathol. 149, 1201–8 (1996).
Takumi, Y. et al. Rapidly progressive malignant epithelioid angiomyolipoma of the kidney. J. Urol. 168, 190–1 (2002).
Huang, K. H. et al. Malignant epithelioid angiomyolipoma of the kidney. J Formos. Med. Assoc. 106, 51–54 (2007).
Hardman, J. A. et al. Recurrent renalangiomyolipoma associated renal carcinoma in a patient with tuberous sclerosis. Br. J. Urol. 72, 983–4 (1993).
Zamboni, G. et al. Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J. Surg. Pathol. 20, 722–30 (1996).
Cibas, E. S. et al. Malignant epithelioid angiomyolipoma (‘sarcoma ex angiomyolipoma’) of the kidney: a case report and review of the literature. Am J. Surg. Pathol. 25, 121–6 (2001).
Heidi, K. et al. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum. Pathol. 38, 1361–71 (2007).
Martignoni, G. et al. Renal epithelioid oxyphilic neoplasm (REON): a pleomorphic monophasic variant of renal angiomyolipoma. Int. J. Surg. Pathol. 2, 539 (1994).
Tsai, C. C. et al. Epithelioid angiomyolipoma of the kidney mimicking renal cell carcinoma:a clinicopathologic analysis of cases and literature review. Kaohsiung J. Med. Sci. 25, 133–40 (2009).
Varma, S. et al. Renal epithelioid angiomyolipoma: a malignant disease. J. Nephrol. 24, 18–22 (2011).
Faraji, H., Nguyen, B. N. & Mai, K. T. Renal epithelioid angiomyolipoma: a study of six cases and a meta-analytic study. Development of criteria for screening the entity with prognostic significance. Histopathology 55, 525–534 (2009).
Acknowledgements
This work was supported by the Natural Science Foundation of China (project numbers 81370855 and 81270841). We thank Department of Pathology, Sichuan Provincial Cancer Hospital, Department of Pathology, First Affiliated Hospital of Guangxi Medical university and Department of Pathology, First Affiliated Hospital of Chengdu Medical College for providing pathologic data. We wish to express our gratitude to the study participants whose cooperation made the study possible.
Author information
Authors and Affiliations
Contributions
JHL and LRL wrote the first edition of the paper. TRS, LY and HCY obtained patient consent and collected data. YJ, XH and SHX analyzed the pathological results. PH and QW commented in detail on the drafts and approved the final version.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lei, J., Liu, L., Wei, Q. et al. A Four-Year Follow-up Study of Renal Epithelioid Angiomyolipoma: A Multi-Center Experience and Literature Review. Sci Rep 5, 10030 (2015). https://doi.org/10.1038/srep10030
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10030
This article is cited by
-
Hepatic epithelioid angiomyolipoma: magnetic resonance imaging characteristics
Abdominal Radiology (2023)
-
♣Evaluation of clinicopathological profiles and development of a risk model in renal epithelioid angiomyolipoma patients: a large-scale retrospective cohort study
BMC Urology (2022)
-
Renal epithelioid angiomyolipoma: computed tomography manifestation and radiologic–pathologic correlation depending on different epithelioid component percentages
Abdominal Radiology (2022)
-
Differentiating renal epithelioid angiomyolipoma from clear cell carcinoma: using a radiomics model combined with CT imaging characteristics
Abdominal Radiology (2022)
-
Renal epithelioid angiomyolipoma: magnetic resonance imaging characteristics
Abdominal Radiology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.