Abstract
Keratinocyte migration is an early event in the wound healing process. Although we previously found that CD9 downregulation is required for the keratinocyte migration during wound repair, the mechanism of how CD9 expression is regulated remains unclear. Here, we observed the effect of hypoxia (2% O2) on CD9 expression and keratinocyte migration. CD9 expression was downregulated and keratinocyte migration was increased under hypoxic conditions. In addition, CD9 overexpression reversed hypoxia-induced cell migration. We also found that hypoxia activated the p38/MAPK pathway. SB203580, a p38/MAPK inhibitor, increased CD9 expression and inhibited keratinocyte migration under hypoxia, while MKK6 (Glu) overexpression decreased CD9 expression and promoted hypoxic keratinocyte migration. Our results demonstrate that hypoxia regulates CD9 expression and CD9-mediated keratinocyte migration via the p38/MAPK pathway.
Similar content being viewed by others
Introduction
CD9, a member of the tetraspanin superfamily, has been implicated in a variety of cellular and physiological processes, including cell motility1,2, migration and adhesion3,4,5,6. Our previous study revealed that CD9 is involved in wound re-epithelialization and that CD9 expression in the migrating epidermis is downregulated following wounding and then increases, reaching levels comparable to that observed in normal skin epidermis when re-epithelialization is complete7. Our in vitro results also showed that CD9 downregulation promotes keratinocyte migration8. These results indicate that low levels of CD9 are required for re-epithelialization, but little is known about how CD9 expression is regulated during wound healing.
Wound healing is a dynamic and well-ordered biological process, in which re-epithelialization plays an early important role9,10. Re-epithelialization of skin wounds depends upon keratinocytes migration from the wound margins and is enhanced when keratinocytes are covered with occlusive dressings that induce hypoxia11. After acute tissue injury, the wound microenvironment is virtually devoid of oxygen, which results from the vascular disruption caused by the injury, the high oxygen consumption caused by high cell density and cell activity in granulation tissue12,13. Increasing amounts of research have revealed that hypoxia plays a role as an early stimulus for initiation of re-epithelialization and tissue repair/angiogenesis14,15,16. In addition, hypoxia is localized to the leading edge of the healing epidermis area starting from day 2 after wounding and peaking at day 3 after wounding; the oxygen tension in the wound tissue at the day 3 is less than 10 mmHg17,18. The temporal appearance of hypoxia correlates well with the downregulation of CD9 expression during wound repair. Hypoxia also promotes keratinocyte motility over connective tissue components and enhances keratinocyte migration11,19,20. In addition, hypoxia regulates tetraspanin expression in cancer cells, which plays an important role in cell migration21,22,23. Thus, we hypothesize that hypoxia regulates CD9 expression and CD9-mediated keratinocyte migration during wound healing.
In mammalian cells, responses to hypoxia at the molecular transduction level are hallmarks of adaptation and survival under oxygen deprivation conditions. An increasing number of studies have demonstrated that mitogen activated protein kinase (MAPK) systems, especially p38/MAPK, are activated under hypoxia24,25,26. Additionally, wounding activates p38/MAPK in leading keratinocytes27 and the p38/MAPK pathway is required for keratinocyte migration28,29. Inhibition of p38/MAPK impairs the formation of keratinocyte outgrowths in human skin organ cultures, as well as the migration of keratinocytes in an in vitro wound assay30. Accordingly, these data prompted us to investigate the function of p38/MAPK in the regulation of CD9-mediated keratinocyte migration under hypoxia.
In the present study, we used HaCaT cells and primary mouse keratinocytes (MKs) to identify a role for hypoxia in regulation of CD9 expression and keratinocyte migration. We found that CD9 expression is downregulated in hypoxic keratinocytes and CD9 overexpression is sufficient to reverse the hypoxia-induced keratinocyte migration, while CD9 silencing accelerates hypoxic keratinocyte migration. The p38/MAPK pathway plays an important role in regulating CD9 expression and keratinocyte migration under hypoxia. Collectively, our findings suggest that in both HaCaT cells and MKs, the hypoxia-activated p38/MAPK pathway participates in regulation of CD9 expression and cell migration. These results provide novel insights into mechanisms of tetraspanin CD9 regulation and keratinocyte migration during wound healing.
Results
Hypoxia decreases CD9 expression in keratinocytes
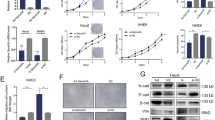
To observe the changes in CD9 expression under hypoxia, HaCaT cells and MKs were subjected to hypoxia for 12 hours, 24 hours and 36 hours. We then analysed CD9 protein levels using western blot. The CD9 protein level in both HaCaT cells and MKs significantly decreased after 12 hours of hypoxia treatment, compared with normoxic keratinocytes (Fig. 1A and Supplementary Fig. S1A). Moreover, real-time PCR analysis also revealed that CD9 mRNA levels were significantly reduced in hypoxic keratinocytes (Fig. 1B). These results indicated that CD9 expression in keratinocytes was downregulated under hypoxic conditions.
Hypoxia decreases CD9 expression in keratinocytes.
(A) Western blot was used to detect CD9 in HaCaT cells and MKs under normoxic and hypoxic conditions (after 12, 24, 36 hours of hypoxia). GAPDH was monitored as a gel-loading control. The graph represents the mean ± SD (n = 3) of the relative integrated signal. (B) CD9 mRNA levels in normoxic and hypoxic HaCaT cells and MKs measured using real-time quantitative PCR. Data are the mean ± SD of three independent experiments performed in triplicate. N, normoxia. *P < 0.05 versus N group.
Effect of CD9 on hypoxia-induced keratinocyte migration
Many reports have revealed that hypoxia promotes keratinocyte migration19,20. Since we previously found that CD9 downregulation also contributes to keratinocyte migration during wound hearing8, we hypothesized that CD9 could account for regulation of keratinocyte migration under hypoxia. Recombinant adenovirus vectors for overexpressing CD9 (Ad-CD9) and silencing CD9 (siCD9) were constructed and used to infect HaCaT cells prior to hypoxia treatment. The effects of adenovirus infection were quantified and verified before further experiments. After infection for 48 hours, more than 90% of the keratinocytes confirmed to be infected by observing GFP expression using a fluorescent microscope (Supplementary Fig. S2A and S2B). The effective silencing of endogenous CD9 or overexpression of CD9-GFP fusion proteins was confirmed using western blot (Supplementary Fig. S2C) and fluorescence-activated cell sorting (FACS) analysis (Supplementary Fig. S2D). We then evaluated the effect of CD9 on hypoxic HaCaT keratinocyte migration using the scratch wound assay. Our results showed that hypoxia induced HaCaT cell migration compared with normoxic cells and CD9-overexpression reversed the hypoxia-induced cell migration, while CD9 silencing increased cell migration. After 20 hours, hypoxic keratinocytes migrated into over 75% of the scratched area (H+Mock group and H+Vector group), while normoxic keratinocytes occupied only 54%. In addition, keratinocytes with CD9 overexpression migrated into only 39% of the area (Ad-CD9 group) under hypoxia, while CD9-silenced keratinocytes were present in over 95% of the scratched area (siCD9 group), compared with over 75% for the scramble-infected keratinocytes (H+Mock group and H+Vector group) (Movie 1, Fig. 2A and 2B). No difference in cell proliferation was detected in these groups (Supplementary Fig. S3A). Thus, cell proliferation can be excluded as a possible explanation for changes in migratory capacity of CD9-regulated keratinocytes under hypoxia. The regulatory role of CD9 in hypoxia-induced keratinocyte migration was further confirmed using the transwell migration assay in both HaCaT cells and MKs. The number of cells migrating through the porous membranes of the transwell chambers was increased by 64.7% in CD9-silenced HaCaT cells and 44.8% in CD9-silenced MKs, but decreased by 47.5% in CD9-overexpressed HaCaT cells and 51.3% in CD9-overexpressed MKs, compared with the scramble-infected keratinocytes (H+Mock group and H+Vector group) (Fig. 2C). Taken together, these results demonstrate that CD9 plays an important role in hypoxia-induced keratinocyte migration.
Effect of CD9 on hypoxia-induced keratinocyte migration.
(A) HaCaT cells infected with recombinant adenovirus vectors to overexpress CD9 (Ad-CD9), silence CD9 expression (siCD9) and negative vectors (Vector and Mock group) were scratch wounded with a micropipette tip and filmed for 20 hours using time-lapse video-microscopy under hypoxic conditions. Wound closure is illustrated by showing the wound size immediately and 20 hours after scratching. (B) The panel represents the quantification of the CD9 effect on the wound closure calculated by measuring the reduction of the wound bed surface over time using Image J software. (C and D) Recombinant adenovirus-infected HaCaT cells and MKs were seeded in the upper chambers of transwell inserts. After 20 hours, cells on the lower side of the filter were stained with 0.5% crystal violet and scored in five independent fields (n = 3). *P < 0.05 versus H+Mock group; #P < 0.05 versus H+Vector group.
Hypoxia activates p38/MAPK signaling pathway
To further elucidate the signaling events involved in the hypoxia-induced effects on CD9 expression and cell migration, we investigated the status of p38/MAPK in hypoxic HaCaT cells and MKs. Phosphorylated p38 (p-p38) and p38 were tested using immunoblotting (Fig. 3). Hypoxia-induced phosphorylation of p38/MAPK was significantly elevated by hypoxia for 12 hours with a 1.6-fold increase in HaCaT cells and 1.7-fold increase in MKs and remained elevated throughout the 36 hours of hypoxia, compared with normoxic keratinocytes. We also observed that the p38/MAPK pathway was markedly activated in the newly formed migrating epidermis post-wounding, where keratinocytes were under hypoxia conditions (Day 5, Supplementary Fig. S5).
Hypoxia activates p38/MAPK signaling pathway.
(A) Representative cropped blots and data summary of phospho-p38 (p-p38) and p38 in HaCaT cells and MKs under normoxic and hypoxic conditions. Representative western blots are shown for the two groups. (B) The graph represents the mean ± SD (n = 3) of the relative integrated signals. N, normoxia; *P < 0.05 versus N group.
Effect of p38/MAPK pathway on hypoxia-induced changes in CD9 expression
To test whether hypoxia-induced changes in CD9 expression depend on the p38/MAPK activity, we treated HaCaT cells and MKs with the p38/MAPK inhibitor, SB203580. SB203580 treatment resulted in a significant increase in CD9 protein expression under hypoxic conditions (2% O2 for 24 hours), while there was no change under normoxic conditions (Fig. 4A and Supplementary Fig. S1B). We then further overexpressed the p38 kinase activator MKK6 (Glu) in these cells. MKK6 is known to phosphorylate p38/MAPK on Thr-180 and Tyr-182, which leads to p38/MAPK activation31. The cells were transfected with MKK6 (Glu) recombinant adenoviruses. After infection for 48 hours, more than 90% of the HaCaT cells and MKs were determined to be infected by observing GFP expression using a fluorescent microscope (Fig. 4B). The effective activation of p38/MAPK was confirmed using western blot (data not shown). The effects of overexpressing MKK6 (Glu) on CD9 expression are shown in Fig. 4C and supplementary Fig. S1C. MKK6 (Glu) overexpression resulted in a reduction in the CD9 protein levels by 3.25-fold in HaCaT cells and 6.8-fold in MKs. These results suggest that the p38/MAPK pathway is involved in regulating CD9 expression.
Effect of p38/MAPK pathway on hypoxia-induced changes in CD9 expression.
(A) Western blot analysis of CD9 in HaCaT cells and MKs in the presence or absence of the p38/MAPK inhibitor SB203580 (5 μM, SB) under normoxic and hypoxic conditions. The graph represents the mean ± SD (n = 3) of the relative integrated signals. *P < 0.05 versus N group;#P < 0.05 versus H group. (B) HaCaT cells and MKs were infected with MKK recombinant adenovirus (MKK) or a negative vector (GFP) for 48 hours and then observed under a fluorescence microscope to determine the infection efficiency by visualizing GFP expression. Scale bar = 200 μm (C) HaCaT cells and MKs were divided into Normoxia, GFP transduction and MKK transduction groups. Cell lysates were immunoblotted with antibodies that recognize CD9 and GAPDH. The graph represents the mean ± SD (n = 3) of the relative integrated signals. *P < 0.05 versus the GFP group.
Involvement of the p38/MAPK pathway in hypoxia-induced keratinocyte migration
To study the p38/MAPK signaling mechanism involved in regulating hypoxia-induced keratinocyte migration, we assessed hypoxic keratinocyte migration with or without the p38/MAPK inhibitor, SB203580 and the MKK6 (Glu) recombinant adenovirus, using the cell scratch wound assay and the cell migration assay. Under hypoxic conditions (2% O2), SB203580 treatment significantly impaired HaCaT cell migration, while MKK6 (Glu) overexpression significantly increased the cell migration (Movie 2). After 20 hours, wound closure was reduced 1.5-fold in hypoxic HaCaT cells after addition of SB203580, while it was increased 1.6-fold in MKK6-infected cells (Fig. 5A and 5B). No difference in cell proliferation was also detected in these groups (Supplementary Fig. S3B). Moreover, the cell migration assay also showed that p38/MAPK inhibition significantly suppressed HaCaT cell migration (1.6-fold reduction) and MKs (1.5-fold reduction) under hypoxia and p38/MAPK activation promoted HaCaT cell migration (2.1-fold increase) and MKs (1.9-fold increase) (Figure 5C and 5D). Our findings suggest that the p38/MAPK pathway participates in hypoxia-induced keratinocyte migration.
Involvement of the p38/MAPK pathway in hypoxia-induced keratinocyte migration.
(A) HaCaT cells incubated with or without SB203580 (SB) or infected with MKK6 recombinant adenovirus (MKK) or a negative vector (GFP) were scratch wounded using a micropipette tip and filmed for 20 hours using time-lapse video-microscopy under hypoxic conditions (H). The wound closure is illustrated by showing the wound size immediately and 20 hours after scratching. (B) The wound closure area was calculated by measuring the reduction of the wound bed surface over time using Image J software. (C) and (D) HaCaT cells and MKs incubated with or without SB203580 (SB) or infected with MKK recombinant adenovirus (MKK) or a negative vector (GFP) were seeded in the upper chambers of the transwell inserts. After 20 hours, cells on the lower side of the filter were stained with 0.5% crystal violet and scored in five independent fields (n = 3). *P < 0.05 versus H+GFP group; #P < 0.05 versus H group.
Discussion
Wound healing is a complex process requiring coagulation, re-epithelialization, angiogenesis, fibroplasia, contraction and remodeling9,32. Keratinocyte migration is an early event in the process of re-epithelialization33. Our previous study revealed that downregulation of CD9 is critical to initiate keratinocyte migration during wound healing7,8. The change in CD9 expression is also implicated in the migration of other cells4,6,34. However, the mechanism that regulates CD9 expression remains unknown. In the current study, we demonstrated that hypoxia downregulates CD9 expression and promote keratinocyte migration via the p38/MAPK pathway.
It has been thought that wounds begin when physical damage to a tissue causes a disruption in oxygen supply, leading to a drop in oxygen tension in the wound tissue35,36. Following injury, vascular damage results in the loss of perfusion and low oxygen tension (hypoxia). This may be exacerbated by a rapid influx of inflammatory and mesenchymal cells with high metabolic demands for oxygen37. Hypoxia can be detected on re-epithelializing sheets during cutaneous wound healing in the early proliferative phase using pimonidazole adduct staining and the oxygen tension is less than 10 mmHg38,39. Hypoxic stress after acute injury activates genes and growth factor synthesis, leading to tissue repair/angiogenesis14. In addition, the use of semi-occlusive dressings in cutaneous wound healing promotes re-epithelialization and wound closure compared with wounds that are allowed to air-dry, but the dressings must be used early after wounding to induce rapid re-epithelialization in vivo40,41,42,43. Wounds allowed to air-dry have oxygen tensions between 1–4% at the surface where keratinocytes are migrating17,44. Our previous results showed that CD9 was downregulated after wound injury, followed by an increase to a normal level in normal skin epidermis when re-epithelialization is fully completed7, which correlates well with the appearance of hypoxia. In the present study, we found that hypoxia (2% O2) downregulated CD9 expression (Fig. 1) and induced keratinocyte migration, while CD9 overexpression reversed promoting effect of hypoxia on migration (Fig. 2). This indicates that hypoxia regulates CD9 expression in keratinocytes and CD9 is also involved in hypoxia-induced cell migration.
CD9 is a member of the tetraspanins family. The most peculiar feature of tetraspanins is their ability to associate with other tetraspanins, integrins and signaling receptors, thereby forming tetraspanin-enriched microdomains on the cell surface45. The accumulation of CD9, CD81 and CD151 has been found to locate in normal human keratinocytes and be involved in cell migration46. The best characterized interactions of tetraspanins are those with integrins, which are involved in signaling pathway47. CD9 forms a complex with integrin α6, α3, β1, β4 in keratinocytes, which plays an important role in cell migration48,49. Thus, CD9 may participate in keratinocyte migration through regulating the interaction with other molecules. Further work is required to elucidate the possible interaction of CD9 with other molecules in hypoxia-induced cell migration. In addition, our previously results showed that CD9 downregulation in keratinocytes contributes to cell migration via upregulation of matrix metalloproteinase-9 (MMP-9)8. MMP-9 degrades the extracellular matrix and is required for tubular network formation50 and the actin cytoskeletal organization51, which are essential for cell migration. O'Toole et al. revealed that hypoxia promotes keratinocyte migration and that hypoxia-induced MMP-9 secretion plays an important role in the process11,20. All these results suggest that CD9 plays a key role in keratinocyte migration.
CD9 has been implicated in cell migration and cancer invasion and metastasis34,52,53. Downregulation of CD9 protein expression is associated with aggressive behavior of oral squamous cell carcinoma54 and peritoneal dissemination of human ovarian carcinoma cells55. We previously found that CD9 downregulation is critical for initiating keratinocyte migration during wound healing7,8. In this study, we revealed that hypoxia is a key point that regulates CD9 expression. Carcinoma cells are also in a hypoxic microenvironment56,57 and down-regulation of CD9 expression during prostate carcinoma progression is associated with a change in CD9 expression58. These results indicate that hypoxia may regulate the CD9 expression level in carcinoma cells.
Interestingly, we found that hypoxia activates the p38/MAPK pathway, which is negatively related to CD9 levels in hypoxic HaCaT cells and MKs (Fig. 3). Additionally, accumulating data indicates that the p38/MAPK pathway is involved in the intracellular signaling events triggered by hypoxia. Thus, we pharmacologically inhibited p38/MAPK using SB203580 and overexpressed an endogenous p38/MAPK activator, MKK6, to observe the changes in CD9 levels in hypoxic HaCaT cells and MKs. We found that inhibition of p38/MAPK signaling increased CD9 expression in hypoxic keratinocytes, while p38/MAPK activation decreased it. These findings suggest that the p38/MAPK pathway is involved in the process of CD9 changes induced by hypoxia. It has been reported that the p38/MAPK pathway is activated under hypoxia26,59 and we found that hypoxia-activated p38/MAPK activation regulated CD9 expression, but the mechanism of how the p38/MAPK interacts with CD9 is not clear. Future studies are expected to reveal the details of this process.
In addition, our results also demonstrated that activation of p38/MAPK signaling accelerates keratinocyte migration under hypoxia, while inhibition of p38/MAPK results in slower cell migration (Fig 5). Activation of the p38/MAPK pathway was also implicated in promoting keratinocyte migration28,60,61, but there is no research showing that hypoxia-activated p38/MAPK signaling is involved in the keratinocyte migration. Moreover, it has been reported that wounding activates the p38/MAPK pathway which activates transcription factors in the leading keratinocytes27 and p38/MAPK pathway regulates cellular migration in epithelial wound healing29. Thus, during the early stage of wound repair, the hypoxic conditions around the wound tissue may induce keratinocyte migration through the activation of the p38/MAPK.
Taken together, we observed that hypoxia downregulates CD9 by activating the p38/MAPK pathway, which promotes keratinocyte migration in both hypoxic HaCaT cells and MKs. These findings provide new insights into the molecular mechanism of CD9 protein changes in keratinocytes that initiate cell migration during wound healing.
Methods
Ethics Statement
All animal-based investigations were designed and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Pub. No. 85–23, revised 1996). The entire project was reviewed and approved by the Animal Experiment Ethics Committee of the Third Military Medical University in Chongqing, China.
Cell culture and hypoxia treatment
HaCaT cells were obtained from Cell Bank of the Chinese Academy of Sciences in Beijing, China. Cells were cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum (Hyclone, USA). The cells were incubated at 37°C, 5% CO2 and 95% humidity. MKs were cultured using a modification of a described method62. Briefly, MKs were isolated from skin of newborn BALB/c mice (postnatal day 1–3) by 0.25% trypsin/0.04% EDTA solution (Invitrogen, USA) at 4°C overnight and primary cultures were established in keritinocyte serum-free medium (K-SFM medium) (Gibco, USA). The second-passage mouse keratinocytes were plated with the K-SFM medium and used in the subsequent experiments. The cells were incubated at 37°C, 5% CO2 and 95% humidity.
Hypoxic conditions of 2% O2, 5% CO2 and 93% N2 were created by a continuous flow of nitrogen by using a Forma Series II Water Jacket CO2 incubator (model: 3131; Thermo Scientific). The p38/MAPK inhibitor, SB203580 (Beyotime) (5 μmol/L), was added to these cultures and allowed to incubated at 37°C for 30 minutes before hypoxia treatment.
Western blot analysis
Cells were washed with ice-cold phosphate-buffered saline (PBS), harvested in 70–200 μL of 1× loading buffer on ice and homogenized. Lysates were sonicated for 4 seconds and separated by centrifugation at 4°C and 14000 g for 2 minutes. Protein concentration was determined by RCDC protein assay kit (Sigma, USA). The lysates containing 10 or 20 μg of proteins were separated on 10% SDS–PAGE gel and transferred electrophoretically to polyvinylidene difluoride (PVDF) membranes. All the following antibodies were used at 1:1000 dilution, the loading control anti-GAPDH at 1:5000 dilution and the secondary antibody at 1:4000 dilution. The blots were probed using primary antibodies: anti-CD9 (Millipore, USA), anti-p38, anti-phospho-p38 at Thr180/Tyr182 (Cell Signaling, USA). Horseradish peroxidase- conjugated IgG was used as a secondary antibody and GAPDH was used as loading control. The results were analysed with ChemiDoc imaging system (Bio-Rad, USA).
Real-time PCR of CD9 mRNA
Total RNA was extracted from HaCaT cells and MKs with TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. CD9 mRNA was subjected to RT-PCR with a Realtime 7500 PCR apparatus (Applied Biosystems) according to the instruction manual (SYBRII Green Realtime PCR; Toyobo). The results were analysed with Applied Biosystems 7500 system v1.4.0 software. The sense and antisense primers for human CD9 and GAPDH were as follows: CD9 (5′- CCTGCTGTTCGGATTTAACTTCA-3′; 5′-TGGTCTGA GAGTCGAATCGGA-3′) and GAPDH (5′-GGTGGTCTCCTCTGACTTCAACA- 3′; 5′-GTTGCTGTAGCCA AATTCGTTGT-3′). PCR conditions were: denaturing once at 95°C (5 minutes), then 40 cycles at 95°C (30 seconds), 62°C (1 minutes) and 72°C (1 minutes).
Recombinant adenovirus vector for overexpressing or silencing of CD9 expression
The recombinant adenovirus vectors for overexpressing CD9 (Ad-CD9), the negative control adenovirus vector (Vector) or for silencing of CD9 expression (siCD9) and the negative vector containing non-specific shRNA (Mock) were purchased from Shanghai GeneChem, Co. Ltd (Shanghai, China). All vectors contained the gene for green fluorescent protein (GFP), which served as a marker. HaCaT cells and MKs were infected with these vectors at a multiplicity of infection of 10 for 48 hours and these cells were used in the experiments.
MKK6(Glu) recombinant adenovirus construction and transduction
The recombinant adenovirus vectors Ad-MKK6(Glu)-GFP and the negative vector EGFP (GFP) were produced by Shanghai GeneChem, Co. Ltd (Shanghai, China). The transgene expression in HaCaT cells and MKs was observed under a fluorescence microscope (Leica Micro systems, Germany) to determine the infection efficiency by visualizing GFP expression.
Cell scratch wounding assay and time-lapse videomicroscopy
Scratch wounding assay was performed as described previously8. HaCaT cells uninfected or infected with recombinant adenoviruses were grown to confluence in the 24-well plates in serum conditioned RPMI 1640. Scratch wounds were created in confluent monolayers using a sterile p20 pipette tip and different fields were filmed for 20 hours under normoxic or hypoxic conditions using a Zeiss videomicroscope then analysed using the NIH ImageJ image software (http://rsb.info.nih.gov/ij/).
In vitro cell migration assays
Cell-migration assays were performed using polycarbonate filters (8 mm pore size, Transwell; Becton Dickinson, USA). The lower chamber was filled with 600 μL of RPMI 1640 medium supplemented with 10% FBS and the cells were plated at a density of 3 × 105 in 100 mL of migration buffer in the upper chamber of triplicate wells followed incubation at 37°C for 20 hours. Transwell inserts were then fixed with 10% formalin, stained using 0.5% crystal violet in 10% ethanol for 10 minutes and washed with PBS three times. Cells in the upper compartment were removed using a cotton wool swab and the filter was mounted onto glass slides. Cells from 5 random fields were counted under 100× magnification. Mean cell numbers for each sample were from triplicate inserts.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). SPSS 13.0 was used for statistical analysis and significance was evaluated by one-way ANOVA. P values < 0.05 were considered statistically significant.
References
Miyake, M. et al. Suppression of pulmonary metastasis using adenovirally motility related protein-1 (MRP-1/CD9) gene delivery. Oncogene 19, 5221–5226 (2000).
Garcia-Lopez, M. A., Barreiro, O., Garcia-Diez, A., Sanchez-Madrid, F. & Penas, P. F. Role of tetraspanins CD9 and CD151 in primary melanocyte motility. J Invest Dermatol 125, 1001–1009 (2005).
Powner, D., Kopp, P. M., Monkley, S. J., Critchley, D. R. & Berditchevski, F. Tetraspanin CD9 in cell migration. Biochem Soc Trans 39, 563–567 (2011).
Deissler, H., Kuhn, E. M. & Lang, G. E. Tetraspanin CD9 is involved in the migration of retinal microvascular endothelial cells. Int J Mol Med 20, 643–652 (2007).
Masellis-Smith, A. & Shaw, A. R. CD9-regulated adhesion. Anti-CD9 monoclonal antibody induce pre-B cell adhesion to bone marrow fibroblasts through de novo recognition of fibronectin. J Immunol 152, 2768–2777 (1994).
Kawano, N. et al. Absence of CD9 reduces endometrial VEGF secretion and impairs uterine repair after parturition. Sci Rep 4, 4701 (2014).
Zhang, J. P. et al. CD9 Is Critical for Cutaneous Wound Healing through JNK Signaling. J Invest Dermatol 132, 226–236 (2012).
Jiang, X. P. et al. Downregulation of CD9 in Keratinocyte Contributes to Cell Migration via Upregulation of Matrix Metalloproteinase-9. Plos One 8, e77806 (2013).
Martin, P. Wound healing--aiming for perfect skin regeneration. Science 276, 75–81 (1997).
Raja, Sivamani, K., Garcia, M. S. & Isseroff, R. R. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci 12, 2849–2868 (2007).
O'Toole, E. A. et al. Hypoxia increases human keratinocyte motility on connective tissue. J Clin Invest 100, 2881–2891 (1997).
Hunt, T. K., Niinikoski, J. & Zederfeldt, B. Role of oxygen in repair processes. Acta Chir Scand 138, 109–110 (1972).
Niinikoski, J., Hunt, T. K. & Dunphy, J. E. Oxygen supply in healing tissue. Am J Surg 123, 247–252 (1972).
Tandara, A. A. & Mustoe, T. A. Oxygen in wound healing--more than a nutrient. World J Surg 28, 294–300 (2004).
Moeller, B. J. et al. The relationship between hypoxia and angiogenesis. Semin Radiat Oncol 14, 215–221 (2004).
Ridgway, P. F., Ziprin, P., Peck, D. H. & Darzi, A. W. Hypoxia increases reepithelialization via an alphavbeta6-dependent pathway. Wound Repair Regen 13, 158–164 (2005).
Ninikoski, J., Heughan, C. & Hunt, T. K. Oxygen tensions in human wounds. J Surg Res 12, 77–82 (1972).
Xing, D. et al. Hypoxia and hypoxia-inducible factor in the burn wound. Wound Repair Regen 19, 205–213 (2011).
Xia, Y. P., Zhao, Y., Tyrone, J. W., Chen, A. & Mustoe, T. A. Differential activation of migration by hypoxia in keratinocytes isolated from donors of increasing age: implication for chronic wounds in the elderly. J Invest Dermatol 116, 50–56 (2001).
O'Toole, E. A., van Koningsveld, R., Chen, M. & Woodley, D. T. Hypoxia induces epidermal keratinocyte matrix metalloproteinase-9 secretion via the protein kinase C pathway. J Cell Physiol 214, 47–55 (2008).
Chien, C. W. et al. Regulation of CD151 by hypoxia controls cell adhesion and metastasis in colorectal cancer. Clin Cancer Res 14, 8043–8051 (2008).
Semenza, G. L. Does loss of CD151 expression promote the metastasis of hypoxic colon cancer cells? Clin Cancer Res 14, 7969–7970 (2008).
Iida, H., Suzuki, M., Goitsuka, R. & Ueno, H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int J Oncol 40, 71–79 (2012).
Fan, B., Wang, Y. X., Yao, T. & Zhu, Y. C. p38 Mitogen-activated protein kinase mediates hypoxia-induced vascular endothelial growth factor release in human endothelial cells. Sheng li xue bao 57, 13–20 (2005).
Zheng, M. et al. Intracellular acidosis-activated p38 MAPK signaling and its essential role in cardiomyocyte hypoxic injury. Faseb J 19, 109–111 (2005).
Haddad, J. J. & Hanbali, L. B. Hypoxia upregulates MAPK(p38)/MAPK(ERK) phosphorylation in vitro: neuroimmunological differential time-dependent expression of MAPKs. Protein Pept Lett 21, 444–451 (2014).
Harper, E. G., Alvares, S. M. & Carter, W. G. Wounding activates p38 map kinase and activation transcription factor 3 in leading keratinocytes. J Cell Sci 118, 3471–3485 (2005).
Li, W. et al. The p38-MAPK/SAPK pathway is required for human keratinocyte migration on dermal collagen. J Invest Dermatol 117, 1601–1611 (2001).
Sharma, G. D., He, J. & Bazan, H. E. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem 278, 21989–21997 (2003).
Stoll, S. W., Kansra, S. & Elder, J. T. Keratinocyte outgrowth from human skin explant cultures is dependent upon p38 signaling. Wound Repair Regen 11, 346–353 (2003).
Raingeaud, J., Whitmarsh, A. J., Barrett, T., Derijard, B. & Davis, R. J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16, 1247–1255 (1996).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Garlick, J. A., Parks, W. C., Welgus, H. G. & Taichman, L. B. Re-epithelialization of human oral keratinocytes in vitro. J Dent Res 75, 912–918 (1996).
Klein-Soyer, C., Azorsa, D. O., Cazenave, J. P. & Lanza, F. CD9 participates in endothelial cell migration during in vitro wound repair. Arterioscler Thromb Vasc Biol 20, 360–369 (2000).
Ninikoski, J., Heughan, C. & Hunt, T. K. Oxygen and carbon dioxide tensions in experimental wounds. Surg Gynecol Obstet 133, 1003–1007 (1971).
Goodson, W. H., 3rd, Andrews, W. S., Thakral, K. K. & Hunt, T. K. Wound oxygen tension of large vs small wounds in man. Surg Forum 30, 92–95 (1979).
Lokmic, Z., Musyoka, J., Hewitson, T. D. & Darby, I. A. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol 296, 139–185 (2012).
Haroon, Z. A., Raleigh, J. A., Greenberg, C. S. & Dewhirst, M. W. Early wound healing exhibits cytokine surge without evidence of hypoxia. Ann Surg 231, 137–147 (2000).
Lokmic, Z., Darby, I. A., Thompson, E. W. & Mitchell, G. M. Time course analysis of hypoxia, granulation tissue and blood vessel growth and remodeling in healing rat cutaneous incisional primary intention wounds. Wound Repair Regen 14, 277–288 (2006).
Eaglstein, W. H. Experiences with biosynthetic dressings. J Am Acad Dermatol 12, 434–440 (1985).
Falanga, V. Occlusive wound dressings. Why, when, which? Arch Dermato 124, 872–877 (1988).
Alper, J. C., Welch, E. A., Ginsberg, M., Bogaars, H. & Maguire, P. Moist wound healing under a vapor permeable membrane. J Am Acad Dermatol 8, 347–353 (1983).
Eaglstein, W. H., Davis, S. C., Mehle, A. L. & Mertz, P. M. Optimal use of an occlusive dressing to enhance healing. Effect of delayed application and early removal on wound healing. Arch Dermato 124, 392–395 (1988).
Mani, R., White, J. E., Barrett, D. F. & Weaver, P. W. Tissue oxygenation, venous ulcers and fibrin cuffs. J R Soc Med 82, 345–346 (1989).
Hemler, M. E. Targeting of tetraspanin proteins--potential benefits and strategies. Nat Rev Drug Discov 7, 747–758 (2008).
Penas, P. F., Garcia-Diez, A., Sanchez-Madrid, F. & Yanez-Mo, M. Tetraspanins are localized at motility-related structures and involved in normal human keratinocyte wound healing migration. J Invest Dermatol 114, 1126–1135 (2000).
Bassani, S. & Cingolani, L. A. Tetraspanins: Interactions and interplay with integrins. Int J Biochem Cell Biol 44, 703–708 (2012).
Baudoux, B., Castanares-Zapatero, D., Leclercq-Smekens, M., Berna, N. & Poumay, Y. The tetraspanin CD9 associates with the integrin alpha6beta4 in cultured human epidermal keratinocytes and is involved in cell motility. Eur J Cell Biol 79, 41–51 (2000).
Jones, P. H., Bishop, L. A. & Watt, F. M. Functional significance of CD9 association with beta 1 integrins in human epidermal keratinocytes. Cell Adhes Commun 4, 297–305 (1996).
Karroum, A. et al. Matrix metalloproteinase-9 is required for tubular network formation and migration of resistant breast cancer cells MCF-7 through PKC and ERK1/2 signalling pathways. Cancer Lett 295, 242–251 (2010).
Hsu, J. Y. C. et al. Matrix Metalloproteinase-9 Facilitates Glial Scar Formation in the Injured Spinal Cord. J Neurosci 28, 13467–13477 (2008).
Wang, H.-X., Li, Q., Sharma, C., Knoblich, K. & Hemler, M. E. Tetraspanin protein contributions to cancer. Biochem Soc Trans 39, 547–552 (2011).
Anton, E. S., Hadjiargyrou, M., Patterson, P. H. & Matthew, W. D. CD9 plays a role in Schwann cell migration in vitro. J Neurosci 15, 584–595 (1995).
Buim, M. E. et al. Downregulation of CD9 protein expression is associated with aggressive behavior of oral squamous cell carcinoma. Oral Oncol 46, 166–171 (2010).
Furuya, M. et al. Down-regulation of CD9 in human ovarian carcinoma cell might contribute to peritoneal dissemination: Morphologic alteration and reduced expression of beta 1 integrin subsets. Cancer Res 65, 2617–2625 (2005).
Bienes-Martinez, R. et al. Autocrine stimulation of clear-cell renal carcinoma cell migration in hypoxia via HIF-independent suppression of thrombospondin-1. Sci Rep 2, 788 (2012).
Welsh, S. J. et al. Inhibition of the hypoxia-inducible factor pathway by a G-quadruplex binding small molecule. Sci Rep 3, 2799 (2013).
Wang, J. C. et al. Down-regulation of CD9 expression during prostate carcinoma progression is associated with CD9 mRNA modifications. Clin Cancer Res 13, 2354–2361 (2007).
An, S. S. et al. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK- and Rho kinase-dependent pathways. Am J Physiol Cell Physiol 289, C521–530 (2005).
Qiao, H. et al. Collagen XVII participates in keratinocyte adhesion to collagen IV and in p38MAPK-dependent migration and cell signaling. J Invest Dermatol 129, 2288–2295 (2009).
Fitsialos, G. et al. Transcriptional signature of epidermal keratinocytes subjected to in vitro scratch wounding reveals selective roles for ERK1/2, p38 and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 282, 15090–15102 (2007).
Paladini, R. D. & Coulombe, P. A. Directed expression of keratin 16 to the progenitor basal cells of transgenic mouse skin delays skin maturation. J Cell Biol 142, 1035–1051 (1998).
Acknowledgements
This research was supported (in part) by National Natural Science Foundation of China (30973125) and the State Key Development Program for Basic Research of China (973 Program) (No. 2012CB518101).
Author information
Authors and Affiliations
Contributions
X.J. and J.Z. developed initial concept. X.J., C.H., J.Z., M.T. and Y.H. designed experiments. X.J., X.G., X.X. D.Z. and Q.Z. performed experiments and analysed data. X.J., X.G., X.X., M.T., J.Z. and Y.H. co-wrote the manuscript. J.Z. and Y.H. supervised the study. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supplementary information
Supplementary Information
Movie 1
Supplementary Information
Movie 2
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Jiang, X., Guo, X., Xu, X. et al. Hypoxia regulates CD9-mediated keratinocyte migration via the P38/MAPK pathway. Sci Rep 4, 6304 (2014). https://doi.org/10.1038/srep06304
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06304
This article is cited by
-
Involvement of autophagy in hypoxia-BNIP3 signaling to promote epidermal keratinocyte migration
Cell Death & Disease (2019)
-
ERK activating peptide, AES16-2M promotes wound healing through accelerating migration of keratinocytes
Scientific Reports (2018)
-
Notch1 Signaling Contributes to Hypoxia-induced High Expression of Integrin β1 in Keratinocyte Migration
Scientific Reports (2017)
-
Desmoglein 3-Dependent Signaling Regulates Keratinocyte Migration and Wound Healing
Journal of Investigative Dermatology (2016)
-
The Galvanotactic Migration of Keratinocytes is Enhanced by Hypoxic Preconditioning
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.