Abstract
Mn-doped SrTiO3.0, when synthesized free of impurities, is a paramagnetic insulator with interesting dielectric properties. Since delocalized charge carriers are known to promote ferromagnetism in a large number of systems via diverse mechanisms, we have looked for the possibility of any intrinsic, spontaneous magnetization by simultaneous doping of Mn ions and electrons into SrTiO3 via oxygen vacancies, thereby forming SrTi1−xMnxO3−δ, to the extent of making the doped system metallic. We find an absence of any enhancement of the magnetization in the metallic sample when compared with a similarly prepared Mn doped, however, insulating sample. Our results, thus, are not in agreement with a recent observation of a weak ferromagnetism in metallic Mn doped SrTiO3 system.
Similar content being viewed by others
Introduction
SrTiO3 crystallizes in the simplest possible cubic structure. It is a diamagnetic, high band-gap insulator and exhibits many technologically important properties, making it a promising material for various dielectric capacitor and DRAM applications1,2. Lots of effort have been devoted in making SrTiO3 a dilute magnetic semiconductor material by suitably doping various transition-metal ions into it3,4,5. Recently, Mn doped SrTiO3 has attracted increased attention due to claims of simultaneous presence of dielectric and magnetic glassiness6. Our earlier work on Mn doped SrTiO3, however, has shown that very small amounts of Mn-oxide impurity phase, which is often overlooked in commonly employed characterization tools, can give rise to extrinsic magnetic glassiness7. Our earlier work, thus, highlights the importance of characterizing phase-pure samples for highly sensitive magnetization studies. During our past efforts in establishing the elusive magnetic ordering in Mn-doped SrTiO3 samples, we had naturally attempted converting insulating SrTiO3 to a metallic state via doping of electrons attained through oxygen vacancies8, since the presence of charge carriers are known to give rise to ferromagnetic ordering by promoting magnetic interactions between localized magnetic moments by several different mechanisms, such as double exchange9, RKKY10,11,12 and kinetic stability13. All our phase-pure metallic samples of Mn doped SrTiO3−δ invariably exhibited only a paramagnetic behavior without any evidence of any M-H loop down to 10 K. This negative result attains particular significance in view of a recent claim in the literature14 that Mn-doped metallic SrTiO3 shows long-range magnetic ordering due to RKKY interaction. The importance of such a study to ascertain the true nature of magnetism in similar dilute magnetic semiconductors has been exemplified earlier in the literature in the face of claims of intrinsic magnetism in Mn-doped ZnO15.
Results
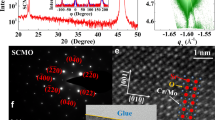
We have already reported our synthesis of SrTi1−xMnxO3.0 with x = 0.02 and its properties in our earlier publication7. In order to dope electrons as charge carriers in the system, we introduce oxygen vacancies in the sample, forming SrTi1−xMnxO3−δ. This is achieved by mixing SrTi1−xMnxO3.07 and SrTiO2.808 in required proportions to achieve targeted x and δ values and by repeated melting of the mixture in an arc furnace under high purity argon atmosphere. Additionally, we carried out thermogravimetric analysis (TGA) to quantify the oxygen content in each case, thereby defining the chemical formula of the samples. This way, two specific oxygen deficient samples, namely SrTi0.98Mn0.02O2.99 and SrTi0.99Mn0.01O2.96, were prepared for the present investigation. Room temperature X-ray diffraction of all three samples (Fig. 1a) establish phase pure cubic Pm-3m space group. Fig. 1b shows the XRD data for all samples, plotted in a log scale that accentuates the small signals from impurity phases, if present, as was shown in our earlier publication7. We specifically expand the region between 300 and 400 in the inset of Fig. 1b, where the magnetic imputiry phase arising from Mn3O4 is known to show a diffraction peak7; clearly, there is no trace of this magnetic impurity phase in any of our samples. SrTi0.98Mn0.02O2.99 sample was surprisingly found to be highly insulating in spite of the electron doping via oxygen vacancies; this highly insulating nature precluded any temperature dependent resistivity measurement on this sample, with the room temperature resistivity being larger than 106 ohm-cm. SrTi0.99Mn0.01O2.96 sample was found to be metallic, as evidenced by the plot of the resistivity as a function of the temperature, T, in Fig. 2. In order to understand the insulating nature of one sample and metallic property of the other, in spite of both samples being doped with oxygen vacancies, we have investigated the state of Mn using electron paramagnetic resonance (EPR).
XRD data of SrTi0.98Mn0.02O3.0 (O3.0), SrTi0.98Mn0.02O2.99 (O2.99) and SrTi0.99Mn0.01O2.96 (O2.96) plotted in linear scale (a) and in log scale (b) to illustrate the phase purity of the synthesized samples.
The inset of Fig. 1(b) highlights the absence of any Mn3O4 related magnetic impurity phase, which is expected to have a maximum XRD peak around 2θ = 36°.
Fig. 3 shows the EPR spectra of SrTi0.98Mn0.02O2.99 and SrTi0.99 Mn0.01O2.96 compared to that of SrTi0.98Mn0.02O3.0, taken from ref. 7. As discussed in ref. 7, 16, the EPR spectrum of SrTi0.98Mn0.02O3.0 sample in absence of any oxygen vacancy exhibits Landé g-factor, g = 1.997and a hyperfine splitting of 74 Oe, characteristic of Mn4+ in an octahedral [Ti] site. The sample with the same level of Mn doping, but with an oxygen vacancy, δ, equal to 0.01, clearly shows two sets of sextet lines, one set corresponding to Mn4+ ions in octahedral sites with a hyperfine splitting of 74 Oe. The other set of sextet structure has a Landé g-factor of 2.005 and a hyperfine splitting of 83.5 Oe, suggesting Mn2+ species at the octahedral site3. Combined with the insulating nature, this clearly suggests that doped electrons due to oxygen vacancies are effectively trapped by Mn4+ sites, converting Mn4+ to Mn2+ and retaining the insulating nature of the sample. Thus, it suggests that we need to have oxygen vacancy at least comparable to Mn doping (i.e. δ ≈ x) for a sample of SrTi1−xMnxO3−δ to be metallic after converting all Mn4+ to Mn2+ species. This interpretation is validated by the observation of SrTi0.99Mn0.01O2.96 sample being metallic. Additionally, the EPR spectrum of this sample shows exclusive presence of only Mn2+ ions in octahedral sites (see Fig. 3).
Electron Paramagnetic Resonance spectra of SrTi0.98Mn0.02O3.0 (a), SrTi0.98Mn0.02O2.99 (b) and SrTi0.99Mn0.01O2.96 (c).
The spectrum (a) is taken from ref. 7.
We have investigated the possibility of a magnetic order in our phase pure, high quality samples by carrying out magnetization (M) vs. applied magnetic field (B) at a low temperature of 10 K. The results for M(B) of SrTi0.98Mn0.02O2.99 and SrTi0.99Mn0.01O2.96 are plotted in Fig. 4, as we have already reported the properties of SrTi0.98Mn0.02O3.0 as paramagnetic in ref. 7. Fig. 4 makes the absence of spontaneous magnetic order in either of the samples evident by the absence of any hysteresis in the M-B plot. We have analyzed the M-B plot in terms of a Brillouin function fit to the lineshape, since the magnetic response of a paramagnetic system is described by a Brillouin function. The solid lines through the data points in Fig. 4, representing the best fits in each case, describe the experimental data very well. In the case of insulating SrTi0.98Mn0.02O2.99, the magnetization data in Fig. 4a required two Brillouin functions, one for Mn2+ with J = 5/2 and the other for Mn4+ with J = 3/2 spin-only values17 with abundances of Mn2+ and Mn4+ in one unit cell equal to 0.003 and 0.009, respectively, thereby giving rise to an average moment of 4.4 μB. The relative abundances of Mn2+ and Mn4+ are qualitatively consistent with the EPR spectrum of this compound in Fig. 3b, showing the presence of both species. The Mn2+ and Mn4+ contents per unit cell are also consistent with the average Mn composition of the sample within the experimental and synthetic uncertainties. The magnetization data of the metallic SrTi0.99Mn0.01O2.96 could be fitted with a single Brillouin function corresponding to Mn2+J = 5/2 state with the Mn2+ content being 0.005 per unit cell, once again consistent with the EPR data (Fig. 3c) and the composition. The effective magnetic moment of SrTi0.99Mn0.01O2.96 extracted from the slope of the low temperature Curie-Weiss plot is 3.8 μB per Mn ion, which is close to the expected value of 5.9 μB for ionic Mn2+ ions within the aforementioned uncertainties in determining the exact composition of dilute constituents, illustrating paramagnetic contribution from nearly all Mn ions in the sample. Interestingly, both metallic and insulating samples show a small negative intercept (in the range of ~−5 K) of the low temperature Curie-Weiss plot, suggesting only a weak antiferromagnetic interaction between the Mn sites.
Discusssion
The EPR spectra in Fig. 3 show dominantly the narrow sextet pattern of hyperfine splitting of isolated Mn ions, doped at octahedral Ti site, for both insulating and metallic samples, with negligible contribution from any broad Mn signal. Broad Mn signal in EPR is known to arise from Mn-Mn dipolar interaction and is indicative of clustering of Mn- ions. In our earlier studies7, we found a correlation between a significant presence of such a dipolar broadened EPR spectrum and the occurrence of extrinsic ferromagnetic signal in the system. It is important to note here that the insulating SrTi0.95Mn0.05O3 (termed OSTMN) of ref. 14 exhibiting a sharp sextet signal is paramagnetic, but the same sample, somewhat reduced, to form a metallic SrTi0.95Mn0.05O3−δ exhibits both a strong dipole broadened EPR component and ferromagnetism.
The Brillouin function analysis could very nicely explain the magnetization data of both insulating SrTi0.98Mn0.02O2.99 and metallic SrTi0.99Mn0.01O2.96 samples, as shown in Fig. 4 and the extracted paramagnetic Mn dopings from the fits to the experimental data were found to be consistent with corresponding nominal Mn doping levels, as discussed in the earlier section. This internal consistency check may be contrasted against the results presented in ref. 14, claiming ferromagnetism in their nominally SrTi0.95Mn0.05O2.85 sample. The saturation magnetization of the M-H loop reported there corresponds to a moment of ~0.04 μB/Mn ion [or 0.002 μB per unit cell]; in other words, roughly one Mn ion out of a hundred doped ones contributes to the ferromagnetic signal in that sample. Even if we consider a significant loss in Mn content in the reported sample and compare the paramagnetic moment from the slope of χ−1 vs. T plot at the high temperature of that sample to estimate the actual Mn content, we find that the unit cell has about 0.03 Mn2+ ions. Even with this correction, it would appear that only a small fraction (~1%) of the total Mn content gives rise to the ferromagnetic signal reported in ref. 14, while the remaining 99% of Mn presumably remains paramagnetic. This interpretation, consistent with the absence of any signature of a ferromagnetic transition in the M vs. T plot, together with the observed dipole broadened EPR signal only in the metallic sample, points to an extrinsic origin of the ferromagnetic signal in that report.
In conclusion, we have synthesized Mn doped SrTiO3 samples, with electron doping through generation of varying amounts of oxygen vacancies in these samples. We find that for a small oxygen vacancy, doped electrons are trapped by Mn4+ sites, converting them partially to Mn2+, while the sample remains a strong insulator. However, for larger oxygen vacancy, namely SrTi0.99Mn0.01 O2.96, the sample becomes metallic, accompanied by a complete reduction of Mn4+ ions to Mn2+. We find that our phase pure Mn doped SrTiO3 samples, both in insulating and metallic ground states, are paramagnetic down to the lowest measurement temperatures. Unlike recent literature reporting weak ferromagnetism in metallic Mn doped SrTiO3 samples, we do not find any enhancement of magnetic couplings in the metallic sample compared to the insulating samples.
Methods
To achieve targeted electron and Mn doping levels into SrTiO3, stoichiometric amounts of SrTi1−xMnxO3.07 and SrTiO2.80 were mixed thoroughly and arc melted repeatedly in high-purity Ar gas atmosphere. SrTiO2.80 was prepared by arc melting stoichiometric amounts of Sr2TiO4, TiO2 and Ti8. EPR experiments were performed at 9.8 GHz in a ER 200D X-band Bruker Spectrometer. Resistivity measurements were performed using van-der Pauw geometry18. TGA experiments were performed by heating the sample in air and monitoring the weight gain on complete oxidation. Magnetization measurements were performed in a Quantum-design SQUID magnetometer.
References
Haeni, J. H. et al. Room-temperature ferroelectricity in strained SrTiO3 . Nature 430, 758–761 (2004).
Streiffer, S. K. & Kingon, A. I. New recipe for a wee DRAM. Nature 377, 194 (1995).
Azzoni, C. B. et al. Magnetic evidence of different environments of manganese ions in Mn-substituted strontium titanate. Solid State Commun. 114, 617–622 (2000).
Inaba, J. & Katsufuji, T. Large magnetoresistance in spin- and carrier-doped SrTiO3 . Phys. Rev. B 72, 052408 (2005).
Savinov, M. et al. Dielectric properties of Mn doped SrTiO3 . J. Phys. Condens. Matter 20, 095221 (2008).
Shvartsman, V. V. et al. (Sr,Mn)TiO3: A Magnetoelectric Multiglass. Phys. Rev. Lett. 101, 165704 (2008).
Choudhury, D. et al. Tuning of dielectric properties and magnetism of SrTiO3 by site-specific doping of Mn. Phys. Rev. B 84, 125124 (2011).
Sarma, D. D., Barman, S. R., Kajueter, H. & Kotliar, G. Spectral functions in doped transition metal oxides. Eur. Phys. Lett. 36, 307 (1996).
Anderson, P. W. & Hasegawa, H. Considerations on Double Exchange. Phys. Rev. 100, 675–681 (1955).
Ruderman, M. A. & Kittel, C. Indirect Exchange Coupling of Nuclear Magnetic Moments by Conduction Electrons. Phys. Rev. 96, 99–102 (1954).
Kasuya, T. A Theory of Metallic Ferro- and Antiferromagnetism on Zener's Model. Prog. Theor. Phys. 16, 45–57 (1956).
Yosida, K. Magnetic Properties of Cu-Mn Alloys. Phys. Rev. 106, 893–898 (1957).
Sarma, D. D., Mahadevan, P., Saha-Dasgupta, T., Ray, S. & Kumar, A. Electronic Structure of Sr2FeMoO6. . Phys. Rev. Lett. 85, 2549–2552 (2000).
Middey, S., Meneghini, C. & Ray, S. Evidence of oxygen-vacancy-induced ferromagnetic order in single crystal Mn-doped SrTiO3 . Appl. Phys. Lett. 101, 042406 (2012).
Kundaliya, D. C. et al. On the origin of high-temperature ferromagnetism in the low-temperature-processed Mn–Zn–O system. Nat. Mater. 3, 709–714 (2004).
Muller, K. A. Electron Paramagnetic Resonance of Manganese IV in SrTiO3 . Phys. Rev. Lett. 2, 341–343 (1959).
Ashcroft, N. W. & Mermin, N. D. Solid State Physics. Table 31.4, Brooks/Cole (1976).
Van der Pauw, L. J. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Res. Rep. 13, 1–9 (1958).
Acknowledgements
Authors thank Department of Science and Technology, Board of Research in Nuclear Sciences and Centre for Scientific and Industrial Research, Government of India for funding and support. Authors also thank Dr. A. Sundaresan of Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India for the M(T) data of SrTi0.99Mn0.01O2.96 sample.
Author information
Authors and Affiliations
Contributions
D.D.S. and D.C. planned the project. D.C. performed all resistivity and magnetic measurements. B.P. performed TGA experiments. A.S. and S.V.B. measured the EPR data. D.D.S. and D.C. wrote the manuscript and all authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Choudhury, D., Pal, B., Sharma, A. et al. Magnetization in electron- and Mn- doped SrTiO3. Sci Rep 3, 1433 (2013). https://doi.org/10.1038/srep01433
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01433
This article is cited by
-
Effects of Mn-doping on the thermoelectric properties of SrTi0.9Nb0.1O3−δ perovskite oxide
Journal of Materials Science: Materials in Electronics (2023)
-
Influence of O2 Adsorption on Ferromagnetism of Fe- and Mn-Doped SrTiO3 (001) Surfaces with Oxygen Vacancy
Journal of Superconductivity and Novel Magnetism (2022)
-
Site-specific doping, tunable dielectric properties and intrinsic paramagnetism in Mn-doped SrTiO 3
Pramana (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.