Abstract
Microparticle and nanoparticle formulations are widely used to improve the bioavailability of low-solubility drugs and as vehicles for organ- and tissue-specific targeted drug delivery. We investigated the effect of a novel, controlled-release form of a bioactive lipid, cyclic phosphatidic acid (cPA), on human colon cancer cell line functions. We encapsulated cPA in gelatin-based hydrogels and examined its ability to inhibit the viability and migration of HT-29 and DLD-1 cells in vitro and the LPA-induced activity of the transcription factor peroxisome proliferator-activated receptor gamma (PPARγ). The hydrogel delivery system prolonged cPA release into the culture medium. Accordingly, cPA-hydrogel microspheres substantially inhibited LPA-induced PPARγ activity and cell growth and migration compared with that of cells cultured with cPA alone. Thus, hydrogel microspheres are a potential system for stable and efficient delivery of bioactive lipids such as cPA and may offer a new strategy for targeted colon cancer treatment.
Similar content being viewed by others
Introduction
Metastatic colon cancer is associated with high mortality1. Although the outcome of advanced colon cancer has been improved by combining the first-line chemotherapy agent 5-fluorouracil with new drugs such as bevacizumab and cetuximab2,3, the prognosis for metastatic colon cancer remains very poor and novel therapeutic strategies are needed to reduce the mortality of this disease. Recent reports suggest that peroxisome proliferator-activated receptor γ (PPARγ) is an important molecular target for the development of anticancer treatments4,5,6. PPARγ is a member of a family of nuclear transcription factors that regulate the expression of genes controlling a number of key metabolic processes, including lipid and glucose homeostasis7,8, cell proliferation9 and inflammation10. The PPARs are activated by fatty acids, leukotrienes and prostaglandins and these molecules initiate the transcription of an array of genes involved in energy homeostasis. Upon ligand binding, PPARγ heterodimerizes with the retinoid X receptor, RXRα and the heterodimer then recruits additional proteins such as coactivators (SRC-1, CBP and TRAP220) and corepressors (SMRT and NCoR) to modulate gene transcription11. Although PPARγ is highly expressed in the mucosa of the colon and rectum12, the potential benefits of modulating PPARγ in colon cancer remain controversial. We recently reported that PPARγ is required for the growth inhibition of colon cancer cells induced by cyclic phosphatidic acid (cPA), a naturally occurring structural analog of lysophosphatidic acid (LPA)13. cPA is known to be generated via phospholipase D-catalysed transphosphatidylation of LPC14 and has been shown to be present in plasma15. cPA is a stable lipid, with 75% of the molecule remaining intact after incubation in tissue culture medium for up to 24 h16,17. cPA can be converted to LPA by opening the ring structure at the sn-2 and sn-3 positions of the glycerol backbone via the action of endogenous and exogenous phosphatases18,19.
Bioactive lipids, particularly lysophospholipids, are a group of molecules that have multiple biological functions, including regulation of cell proliferation, morphology and migration. One of the most bioactive lipids is water-soluble LPA, which has been previously shown to enhance cancer cell proliferation, migration and survival20. LPA was initially found at considerable levels in the ascites of ovarian cancer patients21. However, it has recently been reported that LPA levels are increased not only in the body fluid of ovarian cancer patients, but also in the plasma of patients with colon cancer22,23. These reports suggest that LPA also plays important roles in the progression of colon cancer. These cellular effects are elicited through cell surface G protein-coupled receptors24,25,26,27; and can also directly interact with intracellular targets such as PPARγ28,29,30. To date, 7 LPA-specific cell surface receptors have been identified. cPA is a weak agonist of many LPA receptors31 and it has been reported that cPA has antimitogenic effects32 and prevents cancer cell invasion in vitro33 and metastasis in vivo34,18. cPA inhibits the LPA-induced invasion of many cancer cell lines by 70–95%16,33. Furthermore, cPA inhibits LPA-induced RhoA activation, suggesting that the anti-metastatic effect of cPA is achieved by the inhibition of RhoA activation35. These reports suggest that the cellular effects of cPA are remarkably different from those of LPA.
Recently, biocompatible hydrogels and delivery methods have been developed with the aim of achieving controlled release of therapeutic drugs36. Hydrogels are thermodynamically compatible with water, which allows them to swell in aqueous media37. The hydrogels form a reservoir-based, swelling-controlled, release system in which the drug is incorporated into the hydrophilic hydrogel matrix and diffuses out in a controlled fashion as the hydrogel swells. One of the newest approaches to drug delivery uses gelatin-based hydrogels38. Gelatin is a natural, biodegradable, non-toxic macromolecule and these properties have led to its wide application in drug delivery. In this study, we formulated cPA-containing gelatin hydrogels and we showed that binding within the hydrogel microspheres is essential to prevent degradation of cPA and maintain long-term biological activity in the aqueous environment.
Results
Synthesis and analysis of fluorescent NBD-cPA
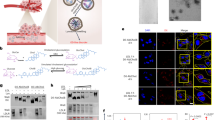
Fluorescent 7-nitro-2-1,3-benzoxadiazole-4-yl (NBD)-labelled lipids are commonly used as analogs of native lipids and closely related probes have been used to monitor the intracellular localization and spatial dynamics of lipid molecules39. In this study, we synthesised NBD-labelled cPA from NBD-tagged lysophosphatidylcholine (LPC) (Fig. 1a). As shown in Fig. 1b, reaction progress was monitored by the appearance of a cPA spot on thin layer chromatography (TLC), visualised by fluorescence imaging (Fig. 1b) and 0.01% primuline staining (data not shown). The TLC analysis confirmed the purity of authentic NBD-cPA. In a chloroform:methanol:28% ammonium hydroxide solvent (6:4:1), purified NBD-cPA had with a retention factor (Rf) value of 0.92, whereas NBD-LPC had with an Rf of 0.14. Murakami-Murofushi et al.18 examined the formation of cPA from LPC by phospholipase D (PLD) derived from Actinomadura sp. No. 362 and found that under certain conditions, this enzyme preferentially catalyzes the transphosphatidylation of LPC to cPA rather than hydrolysis to LPA and thus produces cPA almost exclusively (Fig. 1b)18. We found the procedure described here to be useful for the purification of NBD-cPA from the reaction mixture.
Visualization of hydrogel-encapsulated lipids by fluorescence microscopy and ultraviolet light.
(a) Structural formulae of compounds examined in this study. (b) Efficient synthesis of NBD-cPA from NBD-LPC using Actinomadura PLD. (c) Light micrograph of pure microspheres in the swollen state, scale bar = 80 μm. (d-g) Buffer diffusion into an NBD-labelled hydrogel was monitored by confocal microscopy. Light and fluorescent microscopic images of hydrogel microspheres loaded with NBD-cPA. Scale bar = 50 μm. (h) Distribution of cPA-hydrogel microspheres in the growth medium after 24 and 72 h of incubation. Scale bar = 50 μm. NBD-cPA from the supernatant fractions (Sup.) or NBD-cPA incorporated in microspheres (Hydrogel) was then quantified by the molybdenum blue method. The data is shown as a percentage of the input NBD-cPA present in supernatants and NBD-cPA microspheres.
Characterization of hydrogel microspheres
Fig. 1c shows a typical light micrograph of the hydrogel microspheres before loading with NBD-cPA in PBS. The red colour of the NBD-cPA surrounding the microspheres in Fig. 1f becomes lighter after incorporation of NBD-cPA into the core of the hydrogel microsphere. NBD-cPA distribution (up to 72 h) can also be visualised by fluorescence microscopy, which shows NBD-cPA fluorescing strongly in the centre of the hydrogel microsphere (Fig. 1g and h; excitation 470 nm/emission 530 nm). This finding confirmed that NBD-cPA had successfully penetrated the microsphere surface. To investigate further, we examined the release of cPA 17:0 (a chemically synthesised, non-physiological cPA) from the hydrogel microspheres by electrospray ionization liquid chromatography-mass spectrometry (ESI-LC-MS) and TLC analysis. In Fig. 2a and b, HT-29 cells were incubated with free cPA (Fig. 2a) or cPA-hydrogel (Fig. 2b) at 37°C. We previously reported that cPA inhibits the growth of colon cancer cells13, which raised the possibility of using cPA for cancer therapy. However, hydrolytic cleavage of the cyclic phosphate ring of cPA by lipid phosphate phosphatases leads to the formation of LPA, which is a growth stimulator40. Indeed, in long-term cultures of HT-29 cells incubated with cPA alone, we observed the production of LPA (Fig. 2a). TLC analysis (Fig. 2c) after 72 h of incubation confirmed that >85% of cPA was converted into LPA and an unknown fatty acid (FA). In contrast, when HT-29 cells were treated with cPA-hydrogel microspheres, the cPA was released into the culture medium, but almost no conversion of cPA to LPA was observed (Fig. 2d). These results suggest that cPA bound within the hydrogel microspheres was protected from degradation during long-term incubation (>72 h) in cell culture medium.
Intracellular cPA content from gelatin hydrogels in vitro.
(a and b) Time course of the degradation of cPA to LPA (a), or the release of adsorbed cPA from hydrogel microspheres (b) in HT-29 cultures at 37°C. Cells were harvested by scraping and lipids were extracted using the method reported by Bligh and Dyer. The extracted lipids were dissolved in 0.1 mL of methanol:chloroform:28% NH4OH (90:10:1) and were immediately analysed by ESI-LC-MS. (c and d) TLC analysis of degradation products from cPA alone (c) or cPA-hydrogel microspheres (d) in the medium after 72 h of incubation. Conditioned medium was harvested and extracted in chloroform:methanol (1:1) solvent system. Lipids were extracted from the cells, separated by thin-layer chromatography and visualised with 0.1% primuline.
cPA-hydrogel microspheres inhibit LPA-induced activation of PPARγ
We next examined the ability of cPA-hydrogel microspheres to inhibit LPA-induced PPARγ activation in HT-29 and DLD-1 cells. Our previous work showed that the cPA-induced growth inhibition of HT-29 cells is mediated by inhibition of the PPARγ pathway13; therefore, we investigated whether the same effect could be observed with cPA-hydrogel microspheres. For this purpose, HT-29 and DLD-1 cells were plated in 96-well plates (5 × 103/well) in DMEM supplemented with 10% FBS. The next day, cells were transiently transfected with a luciferase reporter (125 ng) containing 3 copies of PPRE driven by the promoter for acyl CoA oxidase (pGL3-PPRE-acyl-CoA oxidase-luciferase), pcDNA3.1-PPARγ (62.5 ng) and pSV-β-galactosidase (12.5 ng) (Promega) using Lipofectamine 2000 (Invitrogen). Transfected cells were incubated for 20 h or 48 h with combinations of vehicle, free cPA, cPA-hydrogel microspheres and the PPARγ agonist LPA 18:1. As shown in Fig. 3a, luciferase activity increased upon the addition of 1 and 10 μM LPA 18:1; however, as expected, no increase was observed in response to cPA alone or cPA-hydrogel microspheres. In contrast, when cPA, either alone or as cPA-hydrogel microspheres, was added to cultures containing 10 μM LPA 18:1, luciferase activity was inhibited and cPA-hydrogel microspheres inhibited luciferase activity more strongly than did cPA alone, at both 20 h and 48 h of incubation. Therefore, LPA-induced PPARγ activity, as measured by luciferase reporter activity, was efficiently suppressed by co-treatment with cPA-hydrogel microspheres.
Hydrogel microspheres protected cPA from degradation and suppress PPARγ activation.
(a) HT-29 and DLD-1 cells were transfected with PPARγ expression and PPRE-luc reporter plasmids and treated with the vehicle (DMSO), cPA, LPA, cPA-hydrogel, or hydrogel microspheres for 20 or 48 h. Luciferase activity was measured in the lysates of treated cells. Data are presented as mean ± s.e.m.; n = 3; **P < 0.01.
cPA-hydrogel microspheres inhibit the growth and migration of HT-29 and DLD-1 cells
To ensure that the hydrogel microspheres had no detrimental effects on cell function, we examined the viability, growth and migration of HT-29 cells in the presence of control and cPA-containing hydrogel microspheres. As shown in Fig. 4a, the viability of cultured HT-29 and DLD-1 cells was not affected by incubation with various concentrations of control hydrogel microspheres (up to 30 μg/mL). However, similar to the antitumor effects observed for cPA, the viability of HT-29 and DLD-1 cells decreased when incubated with cPA and the cPA-hydrogel microspheres had a much greater effect on cell viability than cPA alone. Tumor cell migration across the endothelial barrier and penetration into the underlying basement membrane is an important step in cancer metastasis41. To investigate the potential anti-metastatic effects of cPA-hydrogel microspheres, we determined their effect on the migration of HT-29 cells in a wound-healing assay. A wound was inflicted in the HT-29 cell monolayers and the widths were recorded (Fig. 4b, t = 0). Cells were then treated with vehicle or cPA-hydrogel microspheres and photographs were taken over the next 72 h to measure wound widths. Representative results are shown in Fig. 4b and c, which demonstrate that HT-29 cell migration was substantially inhibited by incubation with the cPA-hydrogel microspheres. In Fig. 4d, a cell invasion assay using a modified Boyden chamber showed that co-treatment with cPA-hydrogel microspheres resulted in a substantial decrease in both HT-29 and DLD-1 cell invasion. Therefore, the cPA-hydrogel microspheres not only controlled the release and stability of cPA, but also inhibited HT-29 cell proliferation and migration, suggesting the cPA hydrogels might be useful for inhibiting metastasis. Our proposed mechanism of action for the cPA-hydrogel microspheres is summarised in Fig. 5a.
Effect of cPA-hydrogel microspheres on viability and migration of HT-29 and DLD-1 cells.
(a) Cells were treated with cPA hydrogels for 2 days and cell viability and proliferation was then determined by alamarBlue assay. Data are presented as mean ± s.e.m.; n = 4; **P < 0.01. Similar results were obtained in 3 independent experiments. (b) Migration of HT-29 cells co-incubated with cPA or cPA-hydrogel microspheres was measured with a wound-healing assay. Images were taken after 0, 48 and 72 h of incubation using an Olympus IX70 microscope (100× magnification). (c) Data from the wound-healing assays are presented as mean ± s.e.m.; n = 3; **P < 0.01. Data are expressed as percentages of wound closure relative to the wound width at time 0. Wound closure with vehicle-treated cells was regarded as 100%. (d) Cells were seeded in 24-well plates containing a polycarbonate filter with 8-μm pores (Chemotaxicell CH8-24; Kurabo, Osaka, Japan) with 200 μL of serum-free DMEM and incubated for 6 h at 37°C in the presence of 1 μM LPA with or without cPA on the lower side of the filter. At least 4 fields per sample were counted and tabulated; values are expressed as mean (s.e.m.); n = 4; **P < 0.01.
Graphical representation of the release of cPA from hydrogel microspheres for intracellular delivery.
(a) In the tested cell lines, lipid phosphate phosphatases (intracellular or extracellular) lead to the formation of LPA by hydrolytic cleavage of the cyclic phosphate ring of cPA. LPA-induced PPARγ activity was efficiently suppressed by co-treatment with cPA-hydrogel microspheres. Hydrogel microspheres are thus essential to prevent the degradation of cPA to LPA.
Discussion
Drug bioavailability is defined as the rate and extent at which the ingredients or active moiety is absorbed from the drug product and becomes available at the site of action. Ensuring adequate bioavailability is a key concern in drug development, particularly for poorly water-soluble drugs and novel entities for which there may be limited information on biodistribution. We prepared a resorbable bioactive lipid using a gelatin-based hydrogel and evaluated its potential for controlled release of cPA in vitro. Hydrogels have many potential applications in the field of medicine and biodegradable, injectable hydrogels might provide important delivery system options for bioactive molecules. We showed that the cPA-hydrogel microspheres inhibited the growth and migration of a colon cancer cell line, suggesting the potential use of cPA-hydrogel microspheres in the development of PPARγ-targeted drug delivery. Our recent work identified cPA as a naturally occurring PPARγ antagonist19,42 and exogenous cPA might also enter cells and inhibit PPARγ because cPA can bind to and displace the PPARγ agonist, rosiglitazone14. cPA suppresses PPARγ activation both by preventing the binding of exogenous agonists to PPARγ and inducing a specific conformational change that suppresses PPARγ activation. Using the hydrogel delivery system, cPA can be released for extended periods at 37°C while maintaining its structural and functional integrity. The complete resistance of cPA to phosphatase degradation was a key contributor to its stability following release from the hydrogel (Fig. 2c). We expect that novel preventive and therapeutic cancer treatments can be developed using cPA hydrogels that have a more favourable pharmacological impact than cPA alone. Over the past 2 decades, our understanding of the action of cPA has progressed to the point where it is now considered a potential drug candidate for cancers mediated through the PPARγ signalling pathway13. These studies have shown that cPA-hydrogel microspheres ensure steady and sustained release, which should improve bioavailability in vivo compared with cPA alone. Many pharmaceutical companies are exploiting the hydrogel technology to re-examine the potential of pharmacologically active molecules that were previously abandoned at the formulation stage because of poor solubility and bioavailability. Even though further research is needed on most biomolecule-containing hydrogels, including ligand-mediated delivery, they are likely to become important biomaterials in the near future. Therefore, it will be of great interest to confirm this novel anticancer strategy with further in vivo studies.
Methods
Reagents and antibodies
Biodegradable gelatin-based hydrogel (MedGel®-PI5 microsphere; Kyoto, Japan) was purchased from WAKO Chemical (Tokyo, Japan). PLD from Actinomadura sp. No. 362 was purchased from Seikagaku Corporation (Tokyo, Japan). LPA (18:1) and fluorescent NBD-LPC were purchased from Avanti Polar Lipids Inc. (Alabaster, AL, USA). cPA 17:0 was chemically synthesised as previously described13. The purity of LPA and cPA was confirmed by TLC. LPA and cPA were quantified by the molybdenum blue method43 and stored under an argon atmosphere at -85°C until use.
Plasmids and vectors
The pSV40-β-galactosidase and pcDNA3.1 plasmids were purchased from Promega (Madison, WI, USA) and Invitrogen (Carlsbad, CA, USA), respectively. The pcDNA3.1-PPARγ expression plasmid and pGL3b-PPRE-acyl-CoA oxidase-renilla luciferase (pGL3b-PPRE-Luc) reporter plasmid were constructed as previously described29.
Cell culture and transfection
The human colon cancer cell line HT-29 was obtained from American Type Culture Collection (Manassas, VA, USA). DLD-1 human adenocarcinoma cells were obtained from Health Science Research Resources Bank (Osaka, Japan). Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich Co., St Louis, MO, USA) containing 10% (v/v) foetal bovine serum (FBS) at 37°C in a humidified 5% CO2atmosphere. Transfection of cells with plasmid DNA was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Synthesis and purification of NBD-cPA
NBD-cPA was prepared from 12:0 NBD-LPC as follows: NBD-LPC was dissolved in 10 mM HEPES-NaOH (pH 7.9) and incubated with Actinomadura PLD at 37°C for 2 h to generate NBD-cPA through transphosphatidylation. Water-saturated butanol was added to stop the reaction and extract the lipids. TLC analysis of lipid extracts was performed on Merck Silica gel 60 HPTLC plates by developing with a mobile phase of chloroform:methanol:28% ammonium hydroxide (6:4:1). NBD-cPA was visualised using a ChemiDoc XRS+ imager (Bio-Rad, Hercules, CA, USA) and the spot was scraped and extracted in chloroform:methanol (1:1). Purified NBD-cPA was then evaporated under argon and quantified by the molybdenum blue method43.
Preparation of cPA-hydrogel microspheres and analysis of cPA release
Gelatin hydrogel (MedGel®-PI5 microsphere, 1 mg) was added to 20 μL of phosphate-buffered saline (0.1× PBS, pH 7.4) with or without 100 nmol of cPA 17:0 (dissolved in DMSO) and was allowed to swell to equilibrium for 24 h at 4°C. The hydrogel matrices were then washed in PBS 5 times to remove excess cPA. The amount of cPA encapsulated within the microsphere was quantified by the molybdenum blue method. HT-29 cells were plated at 2 × 104 cells/well in 0.5 mL of DMEM-1% FBS in a 24-well plate and incubated at 37°C for 12 h. Hydrogel matrices were then added to the wells in 0.5 mL of DMEM-1% FBS and incubation was continued at 37°C for the indicated times. Cells were harvested by scraping and lipids were extracted using the method reported by Bligh and Dyer44. The extracted lipids were dissolved in 0.1 mL of methanol:chloroform:28% NH4OH (90:10:1) and immediately analysed by ESI-LC-MS as previously described13.
Measurement of PPARγ activity by a luciferase reporter assay
To determine PPARγ activation, HT-29 cells were co-transfected with the pcDNA3.1-PPARγ expression plasmid and the pGL3b-PPRE-Luc and pSV40-β-galactosidase reporter plasmids and analysed in a luciferase reporter assay, as previously described29. Twenty-four hours after transfection, cells were treated with vehicle (hydrogel), cPA, LPA, or cPA-hydrogel microspheres in DMEM without FBS and cultured for an additional 20 h. Luciferase activity was measured with the Bright-Glo Luciferase Assay System (Promega, Madison, WI, USA) using a SpectraMax plate reader (Molecular Devices, Sunnyvale, CA, USA). The results shown are representative data from 3 independent experiments. Both activities were measured according to the manufacturer's protocol.
Effect of hydrogel-released cPA on cell proliferation
HT-29 cells were plated at 1 × 104 cells/well in a 96-well plate. Growth medium was removed and replaced with fresh serum-free DMEM containing the indicated concentrations of hydrogel and/or free cPA. Cells were then incubated for 2 days at 37°C. To measure cell viability, 10 μL of alamarBlue reagent (Invitrogen, Carlsbad, CA, USA) was added to each well and the assay was performed according to the manufacturer's instructions. In this assay, the substrate is actively metabolised to the coloured water-soluble product, which is excreted into the culture medium and is measured as the change in absorbance at 570 nm. The assay measures metabolic activity as an indicator of cell proliferation and/or cell viability.
Wound healing assay
Cell migration assays were performed using μ-Dish 35-mm culture inserts (Ibidi, Martinsried, Germany) according to the manufacturer's protocols. In brief, HT-29 cells were seeded into each well of the culture inserts and incubated at 37°C. After the cells were attached, the culture inserts were gently removed and the cells were treated with fresh DMEM containing 0.1% FBS along with the vehicle, cPA, or cPA-hydrogel microspheres. Immediately after removing the inserts (t = 0) and at 48 and 72 h, cultures were photographed by phase contrast microscopy, the width was determined and the mean percent closure was calculated for each culture condition.
Modified boyden chamber cell invasion assay
Cells were seeded in 24-well plates at 5 × 104 cells/well, containing a polycarbonate filter with 8-μm pores (Chemotaxicell CH8-24, Kurabo, Osaka, Japan) with 200 μL of serum-free DMEM (Nacalai, Kyoto, Japan) and incubated for 6 h at 37°C in the presence of 3 μM LPA with or without cPA (500 μL) in the lower side of the filter. All non-migrant cells were removed from the upper face of the membrane with a cotton swab, whereas migrant cells on the lower face were fixed with absolute methanol and stained with Giemsa stain solution (Wako, Osaka, Japan). The number of cells that had migrated to the lower side of the filter was manually counted under a microscope. At least 4 fields of cells per sample were counted and tabulated.
Statistical analysis
Results were analysed by one-way Student's t-test and are expressed as mean ± s.e.m. P values less than 0.05 were considered statistically significant.
References
Denlinger, C. S. & Engstrom, P. F. Colorectal cancer survivorship: movement matters. Cancer Prev. Res. (Phila) 4, 502-511 (2011).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335-2342 (2004).
Tol, J. et al. Chemotherapy, bevacizumab and cetuximab in metastatic colorectal cancer. N. Engl. J. Med. 360, 563-572 (2009).
Schafer, C., Schott, M., Brandl, F., Neidhart, S. & Carle, R. Identification and quantification of epsilon-(gamma-glutamyl)lysine in digests of enzymatically cross-linked leguminous proteins by high-performance liquid chromatography-electrospray ionization mass spectrometry (HPLC-ESI-MS). J. Agric. Food Chem. 53, 2830-2837 (2005).
Burton, J. D., Goldenberg, D. M. & Blumenthal, R. D. Potential of peroxisome proliferator-activated receptor gamma antagonist compounds as therapeutic agents for a wide range of cancer types. PPAR Res. 2008, 494161 (2008).
Zaytseva, Y. Y., Wallis, N. K., Southard, R. C. & Kilgore, M. W. The PPARgamma antagonist T0070907 suppresses breast cancer cell proliferation and motility via both PPARgamma-dependent and -independent mechanisms. Anticancer Res. 31, 813-823 (2011).
Evans, R. M. The nuclear receptor superfamily: A Rosetta Stone for physiology. Mol. Endocrinol. 19, 1429-1438 (2005).
Tontonoz, P. & Spiegelman, B. M. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77, 289-312 (2008).
Lim, S. et al. PPARgamma gene transfer sustains apoptosis, inhibits vascular smooth muscle cell proliferation and reduces neointima formation after balloon injury in rats. Arterioscler. Thromb. Vasc. Biol. 26, 808-813 (2006).
Duval, C., Chinetti, G., Trottein, F., Fruchart, J. C. & Staels, B. The role of PPARs in atherosclerosis. Trends. Mol. Med. 8, 422-430 (2002).
Yu, C. et al. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. J. Biol. Chem. 280, 13600-13605 (2005).
Hawk, E. T., Umar, A. & Viner, J. L. Colorectal cancer chemoprevention--an overview of the science. Gastroenterology 126, 1423-1447 (2004).
Tsukahara, T., Hanazawa, S., Kobayashi, T., Iwamoto, Y. & Murakami-Murofushi, K. Cyclic phosphatidic acid decreases proliferation and survival of colon cancer cells by inhibiting peroxisome proliferator-activated receptor gamma. Prostaglandins Other Lipid Mediat. 93, 126-133 (2010).
Tsukahara, T. et al. Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARgamma by cyclic phosphatidic acid. Mol. Cell 39, 421-432 (2010).
Murakami-Murofushi, K. et al. A novel lipid mediator, cyclic phosphatidic acid (cPA) and its biological functions. Ann. N. Y. Acad. Sci. 905, 319-321 (2000).
Fujiwara, Y. Cyclic phosphatidic acid - a unique bioactive phospholipid. Biochim. Biophys. Acta 1781, 519-524 (2008).
Fujiwara, Y. et al. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J. Biol. Chem. 280, 35038-35050 (2005).
Murakami-Murofushi, K. et al. Biological functions of a novel lipid mediator, cyclic phosphatidic acid. Biochim. Biophys. Acta 1582, 1-7 (2002).
Uchiyama, A. et al. Inhibition of transcellular tumor cell migration and metastasis by novel carba-derivatives of cyclic phosphatidic acid. Biochim. Biophys. Acta 1771, 103-112 (2007).
Mills, G. B. & Moolenaar, W. H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 3, 582-591 (2003).
Xu, Y. et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 280, 719-723 (1998).
Lee, S. J. & Yun, C. C. Colorectal cancer cells - Proliferation, survival and invasion by lysophosphatidic acid. Int. J. Biochem. Cell Biol. 42, 1907-1910 (2010).
Sun, H. et al. Effects of lysophosphatidic acid on human colon cancer cells and its mechanisms of action. World J. Gastroenterol. 15, 4547-4555 (2009).
Fukushima, N., Kimura, Y. & Chun, J. A single receptor encoded by vzg-1/lpA1/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc. Natl. Acad. Sci. U. S. A. 95, 6151-6156 (1998).
Contos, J. J. et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol. Cell. Biol. 22, 6921-6929 (2002).
Noguchi, K., Ishii, S. & Shimizu, T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem. 278, 25600-25606 (2003).
Yamada, T. et al. Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J. Biol. Chem. 279, 6595-6605 (2004).
McIntyre, T. M. et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. U. S. A. 100, 131-136 (2003).
Tsukahara, T. et al. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 281, 3398-3407 (2006).
Tsukahara, T. et al. Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARgamma by cyclic phosphatidic acid. Mol. Cell 39, 421-432 (2010).
Tigyi, G. Aiming drug discovery at lysophosphatidic acid targets. Br. J. Pharmacol. 161, 241-270 (2010).
Fischer, D. J. et al. Naturally occurring analogs of lysophosphatidic acid elicit different cellular responses through selective activation of multiple receptor subtypes. Mol. Pharmacol. 54, 979-988 (1998).
Mukai, M. et al. Inhibition of tumor invasion and metastasis by a novel lysophosphatidic acid (cyclic LPA). Int. J. Cancer 81, 918-922 (1999).
Ishihara, R. et al. Attenuation by cyclic phosphatidic acid of peritoneal metastasis of azoxymethane-induced intestinal cancers in Wistar rats. Int. J. Cancer 110, 188-193 (2004).
Mukai, M. et al. Hepatoma cell migration through a mesothelial cell monolayer is inhibited by cyclic AMP-elevating agents via a Rho-dependent pathway. FEBS Lett. 484, 69-73 (2000).
Weng, L., Le, H. C., Lin, J. & Golzarian, J. Doxorubicin loading and eluting characteristics of bioresorbable hydrogel microspheres: in vitro study. Int. J. Pharm. 409, 185-193 (2011).
Lee, P. I. & Kim, C. J. Probing the mechanisms of drug release from hydrogels. J. Control. Release 16, 229-236 (1991).
Einerson, N. J., Stevens, K. R. & Kao, W. J. Synthesis and physicochemical analysis of gelatin-based hydrogels for drug carrier matrices. Biomaterials 24, 509-523 (2003).
Mukherjee, S., Raghuraman, H., Dasgupta, S. & Chattopadhyay, A. Organization and dynamics of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids: a fluorescence approach. Chem. Phys. Lipids 127, 91-101 (2004).
Fang, X. et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim. Biophys. Acta 1582, 257-264 (2002).
Beerling, E., Ritsma, L., Vrisekoop, N., Derksen, P. W. & van Rheenen, J. Intravital microscopy: new insights into metastasis of tumors. J. Cell Sci. 124, 299-310 (2011).
Tsukahara, T. The role of PPARgamma in the transcriptional control by agonists and antagonists. PPAR Res. 2012, 362361 (2012).
Lowry, R. R. & Tinsley, I. J. A simple, sensitive method for lipid phosphorus. Lipids 9, 491-492 (1974).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911-917 (1959).
Acknowledgements
This work was supported by grants from the Astellas Foundation for Research on Metabolic Disorders (to Tamotsu Tsukahara) and the Takeda Science Foundation (to Tamotsu Tsukahara) and by a Grant-in-Aid for Scientific Research (C) 22591482 (to Tamotsu Tsukahara) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Contributions
TT and KM conceived and designed the experiments. TT performed the experiments. TT analysed the data and wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Tsukahara, T., Murakami-Murofushi, K. Release of Cyclic Phosphatidic Acid from Gelatin-based Hydrogels Inhibit Colon Cancer Cell Growth and Migration. Sci Rep 2, 687 (2012). https://doi.org/10.1038/srep00687
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00687
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.