Abstract
Neurodevelopmental conditions such as attention deficit hyperactivity disorder (ADHD) vary among individuals. For instance, variation exists in clinical response to methylphenidate (MPH) treatment, especially in adults, but the biological basis of this variability is poorly understood. In this longitudinal structural imaging study, we showed that pre-treatment neuroanatomical measures were associated with response to MPH at two months in 60 adults with ADHD. We compared anatomy with that of 23 controls and examined whether differences were enriched for genes linked to MPH dynamics and brain cells. Individuals with ADHD differed from controls in cortical volume and thickness, predominantly in temporo-parietal regions. Treatment non-responders differed from responders and controls in fronto-temporo-parieto-occipital regions and such differences were associated with reduced improvement on inattentive symptoms. These novel findings suggest that variation in neuroanatomy is associated with varying treatment responses. Group differences in cortical thickness were enriched for biologically plausible genes, including those supporting noradrenaline transport, a target of MPH.
Similar content being viewed by others
Main
Attention deficit hyperactivity disorder (ADHD) is a common neurodevelopmental condition characterized by inattentive and/or hyperactive-impulsive symptoms. These typically manifest in childhood but persist in up to 65% of adults1. The prevalence of adult ADHD is approximately2 4%. Stimulants such as methylphenidate (MPH) are the first line of treatment. They modulate dopamine and noradrenaline transmission in striato-cortical regions3,4,5. Specifically, MPH blocks both the dopamine transporter (DAT) and noradrenaline (norepinephrine) transporter (NET) through allosteric binding (that is, at a different site from that of the endogenous neurotransmitter), and thus inhibits both catecholamines reuptake and increases their availability in the synaptic cleft3,6,7. This is turn increases the endogenous stimulation of dopamine and noradrenaline receptors, which optimizes striato-cortical function (for example, top-down regulation of attention, response inhibition and motivation)8,9,10. Methylphenidate is generally effective in improving ADHD symptoms, but randomized controlled trials have reported that more than one-third of adults do not respond11. A recent meta-analysis also confirmed lower response rates in adults than in children12. This has been associated with an increased risk of negative outcomes, including occupational failure, criminal behavior and substance misuse13,14. Yet the biological mechanisms underlying variation in adult treatment response are poorly understood.
Different neurobiological characteristics have been suggested to contribute to the varying treatment response in ADHD, including variants of genes encoding DAT (SLC6A3) and NET (SLC6A2)15; DAT epigenetic alterations16; theta oscillations17,18; and resting-state fronto-striatal functional connectivity19. However, the studies were primarily focused on children20 and thus the findings may not apply to the adult population21. Here we test whether heterogeneity in brain anatomy may contribute to variation in adult treatment response. Most structural magnetic resonance imaging (MRI) studies have so far focused on ADHD versus control comparisons without investigating the treatment response, and mainly included children. For instance, meta-analyses have highlighted lower total and regional grey matter volumes in the basal ganglia and in fronto-parieto-occipital regions in young people with ADHD compared with neurotypical controls22,23,24,25,26. They also suggested that increasing age is associated with less evident volumetric alterations24,25. Recent large multicentre studies also confirmed diffuse but subtle morphometric alterations in cortico-subcortical regions in children with ADHD, but could not identify group differences in adults27,28. The former study focused on the total intracranial volume and subcortical structures, and reported smaller total and subcortical volumes, including in the amygdala, accumbens, putamen, caudate and hippocampus. The latter study reported lower surface area measures in fronto-temporo-cingulate regions, and lower cortical thickness in the temporal pole and fusiform gyrus. Findings were not affected by current stimulant treatment27,28. These observations are in line with the hypothesis that ADHD symptoms are related to a maturational delay of brain regions involved in cognitive and motor functions27,29. Nevertheless, ADHD is a highly heterogeneous condition and past studies have suggested that anatomical variation exists (even in adults), and that more pronounced alterations may be associated with co-morbidities or symptom persistence30,31. In summary, most neuroanatomical investigations have focused on group comparisons with controls, yielding inconsistent or no results, especially in adults. There is therefore an increasing need to parse neuroanatomical heterogeneity, perhaps especially regarding variation in treatment response.
There are limited structural imaging studies on individuals with ADHD that compare treatment responders and non-responders. A small study in children reported that the volume of the caudate and nucleus accumbens were reduced in non-responders compared with responders32. Similarly, a more recent retrospective MRI study including both children and adults observed smaller left putamen and larger precuneus volumes in non-responders than responders33. These studies represent valuable first steps and suggest that anatomical differences may underpin the variation in treatment response. We hypothesize that this may also apply to adults with ADHD because, although case-control brain anatomical differences decrease with age—perhaps especially in the basal ganglia24,25—cortical alterations have even been reported in adults. For instance, a meta-analysis of whole brain voxel-based morphometry studies indicated lower volumes of the ventromedial prefrontal cortex in both children and adults with ADHD34. Unfortunately, the study by Hoogman and colleagues27 did not investigate case-control differences in regional cortical volumes, and thus these cannot be excluded in adults27. In line with this observation, a past study from our group suggested that anatomical connectivity among fronto-parietal cortical regions—but not fronto-striatal connectivity—was associated with treatment response in adults with ADHD35. Finally, there is preliminary evidence that biological factors implicated in treatment response may at least partly differ from those underlying differences between ADHD and neurotypical controls20,36. Unfortunately, anatomical studies comparing responders and non-responders so far: (1) include only children, or a mixed sample of children and adults; (2) did not include neurotypical controls for comparison; and (3) have mostly been based on volumetric measures. Cortical volume (CV) is a product of two distinct parameters, cortical thickness (CT) and surface area (SA), that, in turn, have distinct genetic and developmental origins37,38. However, no study has yet investigated the relationship between regional differences in CV, SA and CT, and adult treatment outcome. Hence, it remains unclear what the brain anatomical associates of adult treatment response are, whether these involve surface-based cortical measures, and how these relate to potential underpinning cellular mechanisms.
To address these questions, this prospective study uses structural MRI to first compare CV, CT and SA in adult neurotypical controls and adults with ADHD, and then separately in ADHD responders and non-responders to two months of MPH treatment. It also tested whether differences between responders and non-responders were associated with improvement on inattentive and/or hyperactive-impulsive symptoms. Finally, we performed a virtual histology analysis to aid interpretation of the group morphometric differences. Specifically, following a previously published approach39,40, we tested whether regional group differences were enriched for genes involved in MPH pharmacodynamics or expressed by different brain cell types.
Results
Sample
The sample included 60 adults with ADHD and 23 matched neurotypical controls. At follow-up, 42 individuals with ADHD were identified as responders and 18 as non-responders to MPH. The two subgroups did not significantly differ in clinical presentation, baseline severity and MPH dose at follow-up (refer to page 2 of the Supplementary Information). All of the participants were included in the following analyses.
Structural MRI analysis
Global brain measures
Non-responders had significantly lower total intracranial volume (t = 2.51, P = 0.015) and mean SA (t = 3.146, P = 0.003) than responders. We did not observe other significant group differences (Supplementary Table 1).
Vertex-wise comparison between ADHD and controls
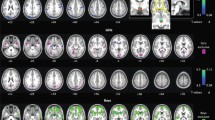
When compared with the controls, individuals with ADHD (combined responders and non-responders) had significantly smaller CV in three clusters in the left hemisphere (random field theory (RFT) P < 0.05). These clusters included: (1) the posterior cingulate cortex, precuneus and superior parietal lobule; (2) the pars triangularis and middle/superior frontal gyri; (3) and the fusiform gyrus and inferior/middle temporal gyri (Fig. 1 and Table 1a). Furthermore, adults with ADHD had significantly lower CT in two clusters (RFT P < 0.05): (1) the left fusiform gyrus, temporal pole and gyri; (2) and the right supramarginal gyrus and parietal lobules (Fig. 1 and Table 1a). No significant differences in SA were observed.
Vertex-wise comparisons between the whole ADHD group and neurotypical controls identified significant CV reductions in the ADHD group in three clusters, and lower CT in the ADHD group in two clusters. Compared with ADHD responders, ADHD non-responders had significant reductions in CV across five clusters and significantly lower cortical SA in a further five clusters. For each contrast, uncorrected t-maps of effects are shown on the left, cluster maps after RFT correction are shown on the right. Cluster coordinates are reported in Table 1a,b.
Vertex-wise comparison between responders and non-responders
Non-responders showed much smaller CV across five clusters (RFT P < 0.05) than ADHD treatment responders. These clusters included: (1) the left temporo-parietal-insular regions; (2) the right precuneus and parietal lobules; (3) the right orbitofrontal cortex and middle frontal gyrus; (4) the right insula, temporal pole and middle/superior temporal gyri; and (5) the fusiform, lingual and parahippocampal gyri, and occipital cortex (Fig. 1 and Table 1b). Non-responders also had significantly lower cortical SA (RFT P < 0.05) in five clusters partially overlapping with those showing significant group differences in CV. These included the right fronto-parietal and temporal regions, and the left temporal, occipital and parietal regions (Fig. 1 and Table 1b). No significant differences in CT were observed between responders and non-responders.
Secondary statistical analysis
Subgroup comparisons
We first considered cortical regions that significantly differed between the whole ADHD group and controls. To determine the separate contribution of the ADHD subgroups to these significant differences, we used independent-sample t-tests to compare morphometric measures in the above five clusters between controls, and (separately) between responders and non-responders. We observed that both ADHD non-responders and responders showed significantly lower CV and CT than the controls in these five clusters. The two clinical groups only significantly differed in the volume of cluster 3 (that is, the left fusiform gyrus and inferior/middle temporal gyri), which was smaller in non-responders (t = −2.337, P = 0.023; Fig. 2 and Supplementary Table 2).
a,b, Subgroup comparisons for CV (a) and CT (b). Both responders and non-responders significantly differed from the controls in all of the five clusters that were significantly different between the whole ADHD sample and controls. Furthermore, non-responders had lower CV in cluster 3 (that is, the left fusiform gyrus and inferior and middle temporal gyri) than responders. Individual participants’ data plotted as dots. Bounds of upper and lower whiskers represent maxima and minima, respectively; the center boxes represents the interquartile range, with the median represented by the middle horizontal lines. Significance: *P < 0.05; **P < 0.01; ***P < 0.001. Full statistical results are reported in Supplementary Table 2. Con, controls; Nre, non-responders; Re, responders.
We then considered the cortical regions that significantly differed between responders and non-responders. Independent-sample t-tests revealed no differences in these ten clusters between ADHD responders and controls; however, significant differences in CV and SA were noted between non-responders and controls in all ten clusters (Fig. 3 and Supplementary Table 3).
Only non-responders significantly differed from the controls in the ten clusters significantly different between ADHD responders and non-responders. Responders did not significantly differ from controls in these clusters. a,b, Display comparisons for CV (a) and SA (b). Individual participants’ data plotted as dots. Significance: *P < 0.05; **P < 0.01; ***P < 0.001. Full statistical results are reported in Supplementary Table 3.
Associations with symptom improvement
We used linear regression to determine whether the observed cortical differences between ADHD treatment responders and non-responders were associated with individual improvement on either inattentive or hyperactive-impulsive symptoms after two months of treatment. After correcting for multiple comparisons (P ≤ 0.005), out of the ten clusters in which significant differences between responders and non-responders were observed, one-fifth of the volumetric and three-fifths of the SA clusters showed significant positive associations with improvement on inattentive symptoms (Fig. 4 and Supplementary Table 4).
After correcting for multiple comparisons, the morphometric differences between responders (N = 42) and non-responders (N = 18) in four clusters were significantly associated with an improvement on inattentive symptoms at two months. Full statistical results are reported in Supplementary Table 4. BAARS-ina, Barkley-inattentive score.
Genomic analyses (virtual histology)
Finally, we investigated the genomic associates of the observed morphometric differences between individuals with ADHD and neurotypical controls, and between ADHD responders and non-responders to MPH. Differences in CT between the whole ADHD group and controls were enriched for genes associated with noradrenaline transport (CT odds ratio (OR) = 13.012, pFDR = 0.028), and for genes expressed by astrocytes (CT OR = 2.93, pFDR = 0.014). We did not observe any significant enrichment of the cortical differences between responders and non-responders. See Supplementary Figs. 1 and 2, and Supplementary Tables 5 and 6 for the complete results.
Discussion
Compared with neurotypical controls, individuals with ADHD (responders and non-responders combined) had significantly smaller CV in the left fronto-temporo-parietal regions, and CT in the left temporal and right parietal regions. These group differences were driven by both ADHD treatment responders and non-responders, although the latter had more prominent temporo-occipital volumetric alterations. When directly compared, non-responders exhibited lower total and regional CV and SA than responders, especially in fronto-temporal regions; however, only the former significantly differed from the controls in these measures, and only the volumetric differences in the left temporo-occipital cortex partially overlapped with those observed in the ADHD versus controls comparison. Thus, non-responders had more pronounced volumetric differences and additional morphometric alterations not related to an ADHD diagnosis per se than responders.
The fact that the identified cortical areas are related to lower treatment response is in line with their role in supporting brain functions implicated in ADHD. For instance, the superior and inferior parietal cortices are part of the dorsal and ventral fronto-parietal networks, which support attentive functions41,42. The right middle prefrontal cortex is also important to reorient attention to relevant stimuli43, whereas the orbitofrontal cortex—together with anterior temporal regions—has been related to decision-making, emotion regulation and reward processing44,45,46. The inferior and middle temporal gyri are involved in several cognitive processes, including memory, visual perception and multimodal integration47. Furthermore, these brain regions have been implicated in functional MRI studies. For instance, functional MRI meta-analyses have identified consistently reduced activation in fronto-parietal areas during response inhibition, attention, and timing in adults and children with ADHD compared with controls48,49. Finally, there is evidence that MPH modulates the activity of these brain areas. For example, past meta-analyses reported upregulation of fronto-parieto-temporo-occipital areas during cognitive tasks after MPH administration in youths with ADHD50,51. In summary, the observed fronto-parieto-temporo-occipital morphometric differences between responders and non-responders are in line with the functional roles of the identified regions and the modulatory action of MPH.
This work adds to past findings from structural MRI studies. These have mostly focused on comparisons between ADHD and neurotypical individuals, and have reported reduced total and regional cortico-subcortical volumes, especially in children with ADHD26. However, heterogeneity exists even in adults, and neuroanatomical alterations have been associated with ADHD persistence and co-morbidities30,31. We observed that morphometric heterogeneity is also associated with variation in treatment response. Past structural MRI studies comparing ADHD responders and non-responders to treatment are limited. One such recent study in a mixed pediatric and adult sample reported reduced left putamen and increased bilateral precuneus volumes in non-responders compared with responders, and that these (and additional volumetric differences in fronto-parieto-occipital cortical regions) could differentiate between responders and non-responders using a machine learning approach33. These cortical areas partially overlap with those identified in our study, albeit we did not observe regions of increased volume. Inconsistencies may be related to differences in both the sample and study characteristics. For instance, they included both children and adults, but increasing age is associated with less evident volumetric alterations24,25,27,28. Further, in this previous retrospective study, individuals with ADHD were classified as responders or non-responders on the basis of a global clinical improvement after a variable treatment duration, whereas, in our prospective interventional study, we classified individuals with ADHD as responders or non-responders on the basis of an ADHD symptom scale after a fixed period of treatment (two months). Our results add to these previous findings, as we observed that differences in cortical structure between responders and non-responders were associated with variation in improvement on inattentive symptoms.
Finally, to aid the interpretation of the observed morphometric group differences, we used a virtual histology approach to map our neuroanatomical results to putative biological processes involved in MPH pharmacodynamics and brain cell types. This exploratory analysis built on previous work showing that morphometric measures rely on different developmental processes37,38, which, in turn, are regulated by a complex gene–environment interplay52,53,54. We observed that ADHD versus controls differences in CT were enriched for genes involved in the transport of noradrenaline. These preliminary results corroborate previous evidence that links noradrenaline dysregulation to ADHD pathophysiology, and that substantiates the therapeutic use of MPH55. Animal studies suggest that negative early life events may impact on brain development partly through disrupting noradrenaline signaling56. For instance, exposure to nicotine during pregnancy, which is a known risk factor for ADHD57, has been associated with increased levels of cortical noradrenaline in infant mice58. Furthermore, animal studies suggested that therapeutic doses of stimulants mainly affect catecholamine levels at a cortical level, and especially those of NA4,59. Taken together, our current work and previous findings suggest that variation in noradrenaline transport may contribute to altered brain development and heterogeneity in the treatment response.
We also observed an enrichment for genes expressed by astrocytes. These are glial cells that contribute to neurotransmitter homeostasis, synaptic development and modulation, and the blood brain barrier60. Astrocyte integrity is also required to support myelination during development61. Accordingly, alterations to astrocytes may hinder myelination and affect white matter tract development. In line with this, meta-analyses of diffusion imaging studies have identified several brain connectivity alterations in individuals with ADHD, especially in the splenium and body of the corpus callosum, which connect posterior cortical areas such as those identified in this structural analysis62. Thus, altered astrocyte development might potentially represent a shared mechanism underlying both grey and white matter pathology in ADHD. Furthermore, as they support optimal synaptic functioning, an alteration at this level may potentially contribute to lower treatment response to MPH. In summary, our virtual histology approach suggested that differences in CT between ADHD and controls were enriched for genes involved in noradrenaline transport and expressed by astrocytes. Further studies are needed to elucidate the biological pathways linking variation in genomic expression to neuroanatomy and this to treatment response. For instance, the single nucleotide polymorphism rs28386840 of the SLC6A2 gene has been associated with increased noradrenaline transporter binding in the thalamus of individuals with ADHD compared with that of controls63, and also with response to MPH in a meta-analysis15. Unfortunately, this variant was not tested in the only imaging genetic study investigating the relationship between SLC6A2 polymorphism and cerebral volume or thickness64, but it warrants further investigation.
This study has several strengths. We specifically investigated the morphometric differences between responders and non-responders to MPH in a sample of adults with ADHD. The interventional study design allowed us to overcome limitations of naturalistic studies in ADHD, which mainly include patients on treatment and thus are enriched in responders. The study also benefitted from the homogeneity in treatment formulation and duration; the inclusion of controls for comparative analyses; and the investigation of both volumetric and surface-based cortical differences. However, limitations should be taken into consideration. We included a small percentage of individuals with ADHD who had previously been exposed to medication. Previous exposure to medication has been suggested to have a normalizing effect on brain structure24, although other reports suggest that this may not be the case65. Our findings suggest that the observed normalizing effect of long-term treatment on brain anatomy may be at least in part dependent on a selection bias, as responders to treatment have less severe/fewer pre-treatment brain alterations. We only recruited males because ADHD is more commonly diagnosed in males66, and there is preliminary evidence of sex differences in brain anatomy and biological response to stimulants67,68,69. Further, it is not known whether sex differences in brain morphometry may relate to sex differences in treatment response. We therefore only included males to avoid potential sex-related confounding on our morphometric analyses. Nonetheless, we encourage future studies to extend our analyses to the female population with ADHD. Similarly, we only included participants without current co-morbid disorders because there is evidence that differences in neuroanatomy exist between individuals with/without co-morbidities70. However, our results should be validated in clinical samples also including participants with co-morbidities. Furthermore, we focused our analyses on cortical morphometric characteristics. We made this choice because a previous structural connectivity study from our group identified cortico-cortical but not fronto-striatal anatomical characteristics related to treatment response in adult ADHD35. This is also in line with prior meta-analytic studies showing that increasing age was associated with less evident structural abnormalities in the basal ganglia24,25. Nevertheless, future studies would benefit from investigating subcortical regions in relation to adult treatment response. Moreover, although we recruited a relatively large sample of adults with ADHD and included controls for comparative analyses, findings need to be validated in larger samples to ensure generalizability. For instance, our prospective study identified a limited number of non-responders (N = 18). The observed proportion of non-responders is in line with previous randomized clinical trials11 and thus representative of the actual ADHD clinical population. However, the small sample size together with the limited number of genes identified during the coding phase may have limited our power to explore genetic enrichment within ADHD subgroups. Furthermore, despite offering a unique opportunity to map neuroanatomy to neurobiology, virtual histology approaches remain limited in their spatial coverage and resolution (especially compared with structural MRI data). Consequently, future studies should validate our analysis once high-resolution gene expression datasets become available and in larger samples. We considered a two-month follow-up as this is the median duration of randomized controlled trials of extended-release MPH in adults with ADHD71. This timeframe is usually sufficient for dose optimization, nevertheless, studies with longer follow-ups are encouraged. They may also clarify how treatment-related changes may be potentially related to varying response over time. Finally, we established associations between brain anatomical characteristics and known genetic expression maps, however, future longitudinal imaging genetic studies are needed to clarify potential causal mechanisms.
Conclusion
We provide evidence that adults with ADHD who do not respond to MPH have significant differences in cortical morphometry compared with those who respond. Responders and non-responders may therefore represent different biological subgroups within the heterogenous population with ADHD. Further, ADHD versus control group differences in cortical regions were enriched for genes expressed by astrocytes and involved in the transport of noradrenaline. Parsing neuroanatomic heterogeneity is critically important to better understand the mechanisms underlying variation in clinical presentation and outcome31. These results need to be replicated and extended in independent samples and, preferably, confirmed by meta-analyses72. In the future, this knowledge may help advance the development of clinical interventions, for example, by identifying treatment resistant individuals in the context of clinical trials of new treatments.
Methods
Sample and research protocol
This study is part of a single-blind placebo-controlled cross-over study, followed by a longitudinal open-label phase (NCT 03709940), which was conducted at the South London and Maudsley NHS Foundation Trust (United Kingdom). Details on the sample and study protocol (NCT 03709940) have been described in a previous work35. In this report we focused on the structural MRI data. We included 60 participants with a clinical diagnosis of ADHD according to the DSM-5 criteria73, aged 18–45 years, with no current co-morbid disorders, and a full-scale IQ of above 70. The sample size was determined based on a power calculation (Supplementary Information). Attention deficit hyperactivity disorder is more commonly diagnosed in males66, and there is preliminary evidence of sex differences in brain morphometry and biological response to stimulants67,68,69, we therefore only included males to enhance sample homogeneity (see the 'Discussion' section for potential limitations). Recruitment focused on the inclusion of medication-naive adults, but we also included a small minority of individuals who were previously medicated and had stopped treatment at least one year before the start of the study (see the 'Results' section). The severity of the symptoms was measured at the baseline and also at follow-up using BAARS-IV74, which provided three scores (ADHD total score, ADHD inattention and ADHD hyperactivity-impulsivity). IQ was measured using the Wechsler Abbreviated Scale of Intelligence75, whereas handedness was measured using a modified version of the Edinburgh Handedness Inventory76.
Participants with ADHD completed MRI scanning before starting routine treatment with the same long-acting formulation of MPH (Concerta XL, titrated up to 54 mg). During titration, side effects were monitored, and the dose was adjusted if needed. The dose was considered as a co-variate. Treatment response was measured at two months. According to previously published criteria, we used a categorical definition of treatment response based on a symptomatic improvement of at least 30%, as measured by the BAARS-IV total score at follow-up as compared with the baseline11,77. We identified a subgroup with a high average and a subgroup with a low average treatment response, which we labelled as responders and non-responders, respectively. We also included 23 neurotypical adults matched for sex, age and IQ for comparative analyses. All participants gave written informed consent. We assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Participants received £50 after the scanning sessions as a compensation for their time. Ethical approval was obtained by Camden and Islington Research Ethics Committee (12/LO/0630).
Structural MRI data acquisition and analysis
MRI acquisition parameters
High-resolution T1-weighted structural images were acquired on a 3 T MR750 GE scanner (General Electric, Milwaukee) for each patient with the following parameters: 196 slices, 1.2 mm thickness with 1.2 mm gap, repetition time (TR) = 7.312 ms, time to echo (TE) = 3.016 ms and flip angle = 11°.
Cortical reconstruction using FreeSurfer
FreeSurfer v.5.3.0 software78 was used to derive tessellated models of the cortical surface for each T1-weighted image. These well-validated and fully automated procedures have been extensively described elsewhere79,80,81,82,83. Resulting surface models were visually inspected for reconstruction errors. Surface reconstructions with visible inaccuracies were further excluded from the statistical analysis and are not described in this study. Three separate vertex based morphometric cortical features were calculated from the individual surface reconstructions: (1) the cortical volume, which is composed of (2) CT83 and (3) pial SA84. Before further analysis, all features were smoothed using a 15 mm surface-based full-width at half-maximum Gaussian kernel.
Vertex-wise between-group comparison of cortical measures
Vertex-wise statistical analyses of each cortical feature were conducted using the SurfStat toolbox85 for MATLAB (R2014a, The Mathworks).
For the comparisons between the controls and participants with ADHD, parameter estimates were obtained by regression of a generalised liner model at each vertex i, with age as a continuous co-variate, that is,
where εi is the residual error.
To examine differences between ADHD treatment responders and non-responders in each morphometric measure, we also included age, full-scale IQ, BAARS-IV baseline total score, MPH dose and weight as continuous co-variates, and handedness as a categorical variable, that is,
where εi is the residual error. Effects of interest were estimated from coefficient β1-normalized by the corresponding standard error. Co-variates were selected as previously associated with treatment response35. Corrections for multiple comparisons were performed using a RFT-based cluster analysis for non-isotropic images at a cluster-threshold of P < 0.05 (two-tailed)86. Imaging files are publicly available on OSF (https://osf.io/mw4y3/).
Secondary statistical analysis
Subgroup comparisons
To test whether significant vertex-wise differences between the whole ADHD group and controls were driven by either responders or non-responders (or both), we ran independent-sample t-tests (two-tailed) using SPSS (v28, IBM) and compared the morphometric measures in the identified clusters separately between controls and responders, and between controls and non-responders. For completeness, we also compared CT, CV and SA between clinical subgroups. Similarly, where we observed significant vertex-wise differences between responders and non-responders, we ran independent sample t-tests to compare morphometric measures in these identified clusters separately between responders and controls and non-responders and controls. We considered these secondary analyses significant at P < 0.05.
Associations with symptom improvement
We ran linear regressions to determine whether differences between responders and non-responders were associated with individual improvement on either inattentive or hyperactive-impulsive symptoms. Morphometric measures were the independent variables, whereas the BAARS-IV inattentive and hyperactive-impulsive scores at follow-up, as compared with the baseline, were the dependent variables. We applied Bonferroni correction for multiple comparisons (number of clusters = 10, P ≤ 0.005).
Genomic analyses (virtual histology)
Finally, we conducted an exploratory analysis to map our neuroanatomical results to putative biological processes and cell-types involved in MPH treatment response and brain development. This analysis included two steps: decoding and enrichment.
Decoding
First, we identified those genes whose spatial expression was significantly similar to our neuroanatomical patterns of interest (neuroanatomical differences between the participants with ADHD and the controls, and between the responders and non-responders). Following a previously published approach39,40, we leveraged the Allen Human Brain Atlas of gene expression87, which is currently the most comprehensive publicly available atlas of gene-expression in the brain, and Neurovault (https://neurovault.org), a Python code embedded within Neurovault and Neurosynth (https://neurosynth.org).
Enrichment
Next we tested how the resulting genes were enriched for: (1) genes previously linked to the regulation of the metabolism and signaling of key neurotransmitters potentially involved in MPH pharmacodynamics; and (2) genes expressed in brain cell types during development.
Specifically, in line with the mechanism of action of MPH, we focused on genes involved in the synthesis and degradation of dopamine and noradrenaline, as well as their receptors and transporters55. We also included genes expressed within serotoninergic pathways, as there is some evidence that MPH may also bind the 5-HT1A receptor; however, its biological significance is unclear88. Finally, we tested genes regulating nitric oxide signaling, which has been suggested to be affected by MPH treatment89. The tested genes were based on gene-set annotations listed in the human gene ontology database (http://www.gsea-msigdb.org/)90. The gene list overlap was computed using a specific R (v.4.3) code written by M.V. Lombardo (github.com/mvlombardo/utils/blob/master/genelistOverlap.R)39.
Finally, due to the developmental nature of ADHD, we explored if the identified genes overlapped with those expressed by brain cell types during development, including progenitor cells, microglia, astrocytes and neuronal cells (excitatory and inhibitory). The cell type-specific gene lists were based on a past work91 and are available at (http://www.gsea-msigdb.org/). We corrected each of these enrichment analyses for multiple comparisons across all contrasts and gene sets (PFDR < 0.05).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Imaging files are publicly available via OSF at https://doi.org/10.17605/OSF.IO/MW4Y3 (ref. 92).
Code availability
Magnetic resonance imaging data analysis was performed using the FreeSurfer v.5.3.0 software78, which was used to derive tessellated models of the cortical surface for each T1-weighted image. Vertex-wise statistical analyses of each cortical feature were conducted using the SurfStat toolbox92 for MATLAB (R2014a, The Mathworks). Following a previously published approach39,40, we leveraged the Allen Human Brain Atlas (AHBA) of gene expression87, currently the most comprehensive publicly available atlas of gene-expression in the brain, and Neurovault (https://neurovault.org), a Python code embedded within Neurovault and Neurosynth (https://neurosynth.org), for decoding. For the enrichment analysis, the tested genes were based on gene-set annotations listed in the human gene ontology database (http://www.gsea-msigdb.org/)90; the gene list overlap was computed using a specific R (v.4.3) code written by M.V. Lombardo (github.com/mvlombardo/utils/blob/master/genelistOverlap.R)39,40.
References
Faraone, S. V., Biederman, J. & Mick, E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol. Med. 36, 159–165 (2006).
Kessler, R. C. et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am. J. Psychiatry 163, 716–723 (2006).
Arnsten, A. F. & Pliszka, S. R. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 99, 211–216 (2011).
Berridge, C. W. et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry 60, 1111–1120 (2006).
Faraone, S. V. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 87, 255–270 (2018).
Kuczenski, R. & Segal, D. S. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J. Neurosci. 22, 7264–7271 (2002).
Seu, E. et al. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology 202, 505–519 (2009).
Arnsten, A. F. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology 31, 2376–2383 (2006).
Arnsten, A. F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422 (2009).
Arnsten, A. F. & Rubia, K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry 51, 356–367 (2012).
Biederman, J. et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 59, 829–835 (2006).
Cortese, S. et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 5, 727–738 (2018).
Chang, Z. et al. Risks and benefits of attention-deficit/hyperactivity disorder medication on behavioral and neuropsychiatric outcomes: a qualitative review of pharmacoepidemiology studies using linked prescription databases. Biol. Psychiatry 86, 335–343 (2019).
Pitts, M., Mangle, L. & Asherson, P. Impairments, diagnosis and treatments associated with attention-deficit/hyperactivity disorder (ADHD) in UK adults: results from the lifetime impairment survey. Arch. Psychiatr. Nurs. 29, 56–63 (2015).
Myer, N. M., Boland, J. R. & Faraone, S. V. Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Mol. Psychiatry 23, 1929–1936 (2018).
Ding, K., et al. DAT1methylation is associated with methylphenidate response on oppositional and hyperactive-impulsive symptoms in children and adolescents with ADHD. World J. Biol. Psychiatry 18, 291–299 (2017).
Sari Gokten, E. et al. Predictive value of slow and fast EEG oscillations for methylphenidate response in ADHD. Clin EEG Neurosci 50, 332–338 (2019).
Ogrim, G. et al. Predicting the clinical outcome of stimulant medication in pediatric attention-deficit/hyperactivity disorder: data from quantitative electroencephalography, event-related potentials, and a go/no-go test. Neuropsychiatr Dis. Treat. 10, 231–242 (2014).
Hong, S. B. et al. Functional dysconnectivity of corticostriatal circuitry and differential response to methylphenidate in youth with attention-deficit/hyperactivity disorder. J. Psychiatry Neurosci. 40, 46–57 (2015).
Michelini, G. et al. Treatment biomarkers for ADHD: taking stock and moving forward. Transl. Psychiatry 12, 444 (2022).
Pagerols, M. et al. Integrative genomic analysis of methylphenidate response in attention-deficit/hyperactivity disorder. Sci. Rep. 8, 1881 (2018).
Valera, E. M. et al. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry 61, 1361–1369 (2007).
Ellison-Wright, I., Ellison-Wright, Z. & Bullmore, E. Structural brain change in attention deficit hyperactivity disorder identified by meta-analysis. BMC Psychiatry 8, 51 (2008).
Nakao, T. et al. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am. J. Psychiatry 168, 1154–1163 (2011).
Frodl, T. & Skokauskas, N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr. Scand. 125, 114–126 (2012).
Albajara Saenz, A., Villemonteix, T. & Massat, I. Structural and functional neuroimaging in attention-deficit/hyperactivity disorder. Dev. Med. Child Neurol. 61, 399–405 (2019).
Hoogman, M. et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 4, 310–319 (2017).
Hoogman, M. et al. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am. J. Psychiatry 176, 531–542 (2019).
Shaw, P. et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl Acad. Sci. USA 104, 19649–19654 (2007).
Shaw, P. et al. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol. Psychiatry 74, 599–606 (2013).
Li, T. et al. Characterizing neuroanatomic heterogeneity in people with and without ADHD based on subcortical brain volumes. J. Child Psychol. Psychiatry 62, 1140–1149 (2021).
Moreno, A. et al. Striatal volume deficits in children with ADHD who present a poor response to methylphenidate. Eur. Child Adolesc. Psychiatry 23, 805–812 (2014).
Chang, J. C. et al. Regional brain volume predicts response to methylphenidate treatment in individuals with ADHD. BMC Psychiatry 21, 26 (2021).
Norman, L. J. et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry 73, 815–825 (2016).
Parlatini, V. et al. Poor response to methylphenidate is associated with a smaller dorsal attentive network in adult attention-deficit/hyperactivity disorder (ADHD). Transl. Psychiatry 13, 303 (2023).
Hegvik, T. A. et al. Druggable genome in attention deficit/hyperactivity disorder and its co-morbid conditions. New avenues for treatment. Mol. Psychiatry 26, 4004–4015 (2021).
Rakic, P. Specification of cerebral cortical areas. Science 241, 170–176 (1988).
Hazlett, H. C. et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry 68, 467–476 (2011).
Pretzsch, C. M. et al. Cross-sectional and longitudinal neuroanatomical profiles of distinct clinical (adaptive) outcomes in autism. Mol. Psychiatry 28, 2158–2169 (2023).
Pretzsch, C. M. et al. Neurobiological correlates of change in adaptive behavior in autism. Am J Psychiatry 179, 336–349 (2022).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002).
Shulman, G. L. et al. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J. Neurosci. 30, 3640–3651 (2010).
Japee, S. et al. A role of right middle frontal gyrus in reorienting of attention: a case study. Front. Syst. Neurosci. 9, 23 (2015).
Bechara, A. & Van Der Linden, M. Decision-making and impulse control after frontal lobe injuries. Curr. Opin. Neurol. 18, 734–739 (2005).
Rempel-Clower, N. L. Role of orbitofrontal cortex connections in emotion. Ann. N. Y. Acad. Sci. 1121, 72–86 (2007).
Remijnse, P. L. et al. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage 26, 609–618 (2005).
Cabeza, R. & Nyberg, L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12, 1–47 (2000).
Hart, H. et al. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD). Neurosci. Biobehav. Rev. 36, 2248–2256 (2012).
Hart, H. et al. Pattern classification of response inhibition in ADHD: toward the development of neurobiological markers for ADHD. Hum Brain Mapp. 35, 3083–3094 (2014).
Rubia, K. et al. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol. Psychiatry 76, 616–628 (2014).
Czerniak, S. M. et al. Areas of the brain modulated by single-dose methylphenidate treatment in youth with ADHD during task-based fMRI: a systematic review. Harv Rev Psychiatry 21, 151–162 (2013).
Quinn, J. C. et al. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev. Biol. 302, 50–65 (2007).
Land, P. W. & Monaghan, A. P. Expression of the transcription factor, tailless, is required for formation of superficial cortical layers. Cereb Cortex 13, 921–931 (2003).
Yun, K. et al. Id4 regulates neural progenitor proliferation and differentiation in vivo. Development 131, 5441–5448 (2004).
Markowitz, J. S. & Melchert, P. W. The pharmacokinetics and pharmacogenomics of psychostimulants. Child Adolesc. Psychiatr. Clin. N. Am. 31, 393–416 (2022).
Saboory, E., Ghasemi, M. & Mehranfard, N. Norepinephrine, neurodevelopment and behavior. Neurochem. Int. 135, 104706 (2020).
Faraone, S. V. et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 128, 789–818 (2021).
Peters, D. A. Prenatal stress: effect on development of rat brain adrenergic receptors. Pharmacol. Biochem. Behav. 21, 417–422 (1984).
Hannestad, J. et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol. Psychiatry 68, 854–860 (2010).
Endo, F. et al. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 378, eadc9020 (2022).
Traiffort, E. et al. Astrocytes and microglia as major players of myelin production in normal and pathological conditions. Front. Cell Neurosci. 14, 79 (2020).
Parlatini, V. et al. White matter alterations in attention-deficit/hyperactivity disorder (ADHD): a systematic review of 129 diffusion imaging studies with meta-analysis. Mol. Psychiatry 28, 4098–4123 (2023).
Sigurdardottir, H. L. et al. Effects of norepinephrine transporter gene variants on NET binding in ADHD and healthy controls investigated by PET. Hum Brain Mapp 37, 884–895 (2016).
Bobb, A. J. et al. Support for association between ADHD and two candidate genes: NET1 and DRD1. Am. J. Med. Genet. B 134B, 67–72 (2005).
Greven, C. U. et al. Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry 72, 490–499 (2015).
Young, S. et al. Females with ADHD: an expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/ hyperactivity disorder in girls and women. BMC Psychiatry 20, 404 (2020).
Carucci, S. et al. Clinical characteristics, neuroimaging findings, and neuropsychological functioning in attention-deficit hyperactivity disorder: sex differences. J. Neurosci. Res. 101, 704–717 (2023).
Manza, P. et al. Sex differences in methylphenidate-induced dopamine increases in ventral striatum. Mol. Psychiatry 27, 939–946 (2022).
Duffy, K. A. & Epperson, C. N. Evaluating the evidence for sex differences: a scoping review of human neuroimaging in psychopharmacology research. Neuropsychopharmacology 47, 430–443 (2022).
Kangarani-Farahani, M., Izadi-Najafabadi, S. & Zwicker, J. G. How does brain structure and function on MRI differ in children with autism spectrum disorder, developmental coordination disorder, and/or attention deficit hyperactivity disorder? Int. J. Dev. Neurosci. 82, 681–715 (2022).
Boesen, K. et al. Extended-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2, CD012857 (2022).
Fusar-Poli, P. et al. The science of prognosis in psychiatry: a review. JAMA Psychiatry 75, 1289–1297 (2018).
Diagnostic and Statistical Manual of Mental Disorders. DSM-5 (American Psychiatric Association, 2013).
Barkley, R. A. Barkley Adult ADHD Rating Scale-IV (BAARS-IV) (Guilford, 2011).
Wechsler, D. Wechsler Abbreviated Scale of Intelligence (Pearson, 1999).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Rosler, M. et al. A randomised, placebo-controlled, 24-week, study of low-dose extended-release methylphenidate in adults with attention-deficit/hyperactivity disorder. Eur. Arch. Psychiatry Clin. Neurosci. 259, 120–129 (2009).
FreeSurfer Software Suite (FreeSurfer, 2020); https://surfer.nmr.mgh.harvard.edu/
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207 (1999).
Ségonne, F. et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage 22, 1060–1075 (2004).
Jovicich, J. et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30, 436–443 (2006).
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA 97, 11050–11055 (2000).
Winkler, A. M. et al. Measuring and comparing brain cortical surface area and other areal quantities. NeuroImage 61, 1428–1443 (2012).
Worsley, K. J. SurfStat: A Matlab Toolbox for the Statistical Analysis of Univariate and Multivariate Surface and Volumetric Data Using Linear Mixed Effects Models and Random Field Theory (McGill University, 2008); www.math.mcgill.ca/keith/surfstat/
Worsley, K. et al. Detecting changes in nonisotropic images. Human Brain Mapp. 8, 98–101 (1999).
Hawrylycz, M. J. et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399 (2012).
Markowitz, J. S. et al. The psychostimulant d-threo-(R,R)-methylphenidate binds as an agonist to the 5HT(1A) receptor. Pharmazie 64, 123–125 (2009).
Jansen, K. et al. Enhanced nitric oxide (NO) and decreased ADMA synthesis in pediatric ADHD and selective potentiation of NO synthesis by methylphenidate. J. Clin. Med. 9, 175 (2020).
The Gene Ontology Consortium The Gene Ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 47, D330–D338 (2019).
Zhong, S. et al. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature 555, 524–528 (2018).
Parlatini, V. et al. Alterations in cortical anatomy are associated with lower response to methylphenidate in adults with ADHD. OSF https://doi.org/10.17605/OSF.IO/MW4Y3 (2024).
Acknowledgements
This research project was funded by Shire (project no. IST-ALB-000217 to D.G.M.) and included support from the NIHR Maudsley Biomedical Research Centre. Further, D.G.M. was funded by two IMI initiatives (EU AIMS and AIMS-2-TRIALS) under grant agreement nos.115300 and 777394. The design of the study, data collection and analysis, and decision to publish were not influenced by the funding bodies. We thank all of the study participants, the Pharmacy Team and the Adult ADHD Clinic at the Maudsley Hospital (London, UK). We thank A. Hye and M. Metha (King’s College London) for their support. We greatly appreciate M.V. Lombardo’s input as he wrote the code used in the enrichment analysis. We would like to thank the Department of Forensic and Neurodevelopmental Sciences, King’s College London, for helpful discussion.
Author information
Authors and Affiliations
Contributions
All of the authors made a substantial contribution to the study. V.P. was responsible for the recruitment of participants, data acquisition and analysis, and writing the paper. D.S.A. was responsible for the data analysis and writing. C.M.P. and M.A. were responsible for the genomic analysis and writing. E.D. and C.E. contributed to study design and supervision. D.G.M. contributed to study design, supervision and critical revision of the paper.
Corresponding author
Ethics declarations
Competing interests
D.M. was funded by two IMI initiatives (EU AIMS and AIMS-2-TRIALS) under grant agreement nos.115300 and 777394. The other authors have no conflicts of interest to declare.
Peer review
Peer review information
Nature Mental Health thanks Claiton Dotto Bau, Pin-Yang Yeh and the other, anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–6 and Figs. 1 and 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parlatini, V., Andrews, D.S., Pretzsch, C.M. et al. Cortical alterations associated with lower response to methylphenidate in adults with ADHD. Nat. Mental Health (2024). https://doi.org/10.1038/s44220-024-00228-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44220-024-00228-y