Abstract
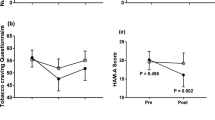
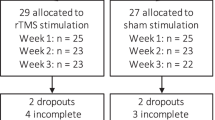
Abstinence from cigarettes is associated with withdrawal and anxiety symptoms, which are barriers to smoking cessation, although they may respond to treatment using repetitive transcranial magnetic stimulation (rTMS). We hypothesized that (1) withdrawal symptoms and state anxiety were related and (2) rTMS-related reductions in anxiety would mediate reductions in withdrawal. Participants received one session of rTMS applied to the dorsolateral prefrontal cortex and one session to a control region, and reported their withdrawal and anxiety before and after rTMS. Withdrawal and state anxiety were significantly related (P < 0.001). Stimulation of the dorsolateral prefrontal cortex significantly reduced craving (P < 0.05). However, state anxiety did not mediate this reduction in craving (P > 0.05). Exploratory post-hoc analyses indicated that rTMS had no direct effect on anxiety or affect, and larger amygdala volumes were related to greater craving reductions. This study is a step towards elucidating the psychological mechanisms of rTMS on nicotine withdrawal and craving.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The study design, hypotheses and analysis plan were pre-registered with the Open Science Framework (OSF) data repository, available at https://osf.io/82s6p. The ROIs depicted in Extended Data Fig. 3 and the raw data for the planned and exploratory analyses, including cortical thickness and subcortical volume values, are available for download from the OSF repository at https://osf.io/z3rd5/files/osfstorage. Source data are provided with this paper.
Code availability
The MRI preprocessing and analysis code is available upon request; the tools used to analyse the data are openly available through FSL and Freesurfer.
References
WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025, Fourth Edition (WHO, 2021).
Babb, S., Malarcher, A., Schauer, G., Asman, K. & Jamal, A. Quitting smoking among adults—United States, 2000–2015. Morb. Mortal. Wkly Rep. 65, 1457–1464 (2017).
Lightwood, J. M. & Glantz, S. A. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation 96, 1089–1096 (1997).
Samet, J. M. Health benefits of smoking cessation. Clin. Chest Med. 12, 669–679 (1991).
Jha, P. et al. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 368, 341–350 (2013).
Diagnostic and Statistical Manual of Mental Disorders: DSM-5 5th edn (American Psychiatric Association Publishing, 2013).
West, R., Hajek, P. & Belcher, M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol. Med. 19, 981–985 (1989).
Morrell, H. E. & Cohen, L. M. Cigarette smoking, anxiety, and depression. J. Psychopathol. Behav. Assess. 28, 281–295 (2006).
Smith, P. H., Homish, G. G., Giovino, G. A. & Kozlowski, L. T. Cigarette smoking and mental illness: a study of nicotine withdrawal. Am. J. Public Health 104, e127–e133 (2014).
Giulietti, F. et al. Pharmacological approach to smoking cessation: an updated review for daily clinical practice. High Blood Press. Cardiovasc. Prev. 27, 349–362 (2020).
Perkins, K. A. & Karelitz, J. L. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob. Res. 17, 443–448 (2015).
Xu, J. et al. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob. Res. 10, 1653–1661 (2008).
Faulkner, P. et al. Sex differences in tobacco withdrawal and responses to smoking reduced-nicotine cigarettes in young smokers. Psychopharmacology 235, 193–202 (2018).
McLaughlin, I., Dani, J. A. & De Biasi, M. In The Neuropharmacology of Nicotine Dependence (eds Balfour, D. J. K. & Munafò, M. R.) 99–123 (Springer, 2015).
Westman, E. C., Behm, F. M., Simel, D. L. & Rose, J. E. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Arch. Intern. Med. 157, 335–340 (1997).
Buckner, J. D., Langdon, K. J., Jeffries, E. R. & Zvolensky, M. J. Socially anxious smokers experience greater negative affect and withdrawal during self-quit attempts. Addict. Behav. 55, 46–49 (2016).
Chen, Y., Dhingra, I., Chaudhary, S., Fucito, L. & Li, C.-S. R. Overnight abstinence is associated with smaller secondary somatosensory cortical volumes and higher somatosensory-motor cortical functional connectivity in cigarette smokers. Nicotine Tob. Res. 24, 1889–1897 (2022).
Watson, N. L., DeMarree, K. G. & Cohen, L. M. Cigarette craving and stressful social interactions: the roles of state and trait social anxiety and smoking to cope. Drug Alcohol Depend. 185, 75–81 (2018).
Johnson, K. A., Stewart, S., Rosenfield, D., Steeves, D. & Zvolensky, M. J. Prospective evaluation of the effects of anxiety sensitivity and state anxiety in predicting acute nicotine withdrawal symptoms during smoking cessation. Psychol. Addict. Behav. 26, 289–297 (2012).
Hendricks, P. S., Ditre, J. W., Drobes, D. J. & Brandon, T. H. The early time course of smoking withdrawal effects. Psychopharmacology 187, 385–396 (2006).
Langdon, K. J. et al. Anhedonia and anxiety sensitivity: prospective relationships to nicotine withdrawal symptoms during smoking cessation. J. Stud. Alcohol Drugs 74, 469–478 (2013).
Piper, M. E., Cook, J. W., Schlam, T. R., Jorenby, D. E. & Baker, T. B. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction 106, 418–427 (2011).
Weinberger, A. H., Desai, R. A. & McKee, S. A. Nicotine withdrawal in US smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug Alcohol Depend. 108, 7–12 (2010).
Cahill, K., Lindson-Hawley, N., Thomas, K. H., Fanshawe, T. R. & Lancaster, T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD006103.pub7 (2016).
Silagy, C., Lancaster, T., Stead, L. F., Mant, D. & Fowler, G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD000146.pub2 (2004).
Richmond, R. & Zwar, N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 22, 203–220 (2003).
Spears, C. A. et al. Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. J. Consult. Clin. Psychol. 85, 1029–1040 (2017).
Hill, K. P. et al. Cognitive behavioral therapy and the nicotine transdermal patch for dual nicotine and cannabis dependence: a pilot study. Am. J. Addict. 22, 233–238 (2013).
Stillman, F. A., Bone, L. R., Rand, C., Levine, D. M. & Becker, D. M. Heart, body, and soul: A church-based smoking-cessation program for urban African Americans. Prev. Med. 22, 335–349 (1993).
Lupton, J. R. & Townsend, J. L. A systematic review and meta-analysis of the acceptability and effectiveness of university smoke-free policies. J. Am. Coll. Health 63, 238–247 (2015).
Bryant, J., Bonevski, B., Paul, C., McElduff, P. & Attia, J. A systematic review and meta-analysis of the effectiveness of behavioural smoking cessation interventions in selected disadvantaged groups. Addiction 106, 1568–1585 (2011).
Vogeler, T., McClain, C. & Evoy, K. E. Combination bupropion SR and varenicline for smoking cessation: a systematic review. Am. J. Drug Alcohol Abuse 42, 129–139 (2016).
Koegelenberg, C. F. et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA 312, 155–161 (2014).
Ebbert, J. O., Wyatt, K. D., Hays, J. T., Klee, E. W. & Hurt, R. D. Varenicline for smoking cessation: efficacy, safety, and treatment recommendations. Patient Prefer. Adherence 4, 355–362 (2010).
Gómez-Coronado, N., Walker, A. J., Berk, M. & Dodd, S. Current and emerging pharmacotherapies for cessation of tobacco smoking. Pharmacotherapy 38, 235–258 (2018).
Caponnetto, P., Russo, C. & Polosa, R. Smoking cessation: present status and future perspectives. Curr. Opin. Pharmacol. 12, 229–237 (2012).
Hartmann-Boyce, J., Chepkin, S. C., Ye, W., Bullen, C. & Lancaster, T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD000146.pub5 (2018).
Moreno-Ortega, M. et al. Parcel-guided rTMS for depression. Transl. Psychiatry 10, 283 (2020).
Perera, T. et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 9, 336–346 (2016).
McClintock, S. M. et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J. Clin. Psychiatry 79, 3651 (2017).
Amiaz, R., Levy, D., Vainiger, D., Grunhaus, L. & Zangen, A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 104, 653–660 (2009).
Li, X. et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol. Psychiatry 73, 714–720 (2013).
Li, X. et al. Two weeks of image-guided left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation improves smoking cessation: a double-blind, sham-controlled, randomized clinical trial. Brain Stimul. 13, 1271–1279 (2020).
Chang, D. et al. Smoking cessation with 20 Hz repetitive transcranial magnetic stimulation (rTMS) applied to two brain regions: a pilot study. Front. Hum. Neurosci. 12, 344 (2018).
Parikh, T. K., Strawn, J. R., Walkup, J. T. & Croarkin, P. E. Repetitive transcranial magnetic stimulation for generalized anxiety disorder: a systematic literature review and meta-analysis. Int. J. Neuropsychopharmacol. 25, 144–146 (2022).
Bystritsky, A. et al. A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder. J. Clin. Psychiatry 69, 15243 (2008).
Tuinstra, D. et al. Treatment of anxiety symptoms in patients receiving rTMS for treatment resistant depression. Psychiatry Res. Commun. 2, 100014 (2022).
Abdelrahman, A. A. et al. A double-blind randomized clinical trial of high frequency rTMS over the DLPFC on nicotine dependence, anxiety and depression. Sci. Rep. 11, 1640 (2021).
Zangen, A. et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 20, 397–404 (2021).
Boes, A. D. et al. Rostral anterior cingulate cortex is a structural correlate of repetitive TMS treatment response in depression. Brain Stimul. 11, 575–581 (2018).
Tabibnia, G., Ghahremani, D. G., Pochon, J.-B. F., Diaz, M. P. & London, E. D. Negative affect and craving during abstinence from smoking are both linked to default mode network connectivity. Drug Alcohol Depend. 249, 109919 (2023).
Pripfl, J., Tomova, L., Riecansky, I. & Lamm, C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 7, 226–233 (2014).
Wing, V. C., Bacher, I., Wu, B. S., Daskalakis, Z. J. & George, T. P. High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr. Res. 1, 264–266 (2012).
Su, H. et al. Neuroplastic changes in resting-state functional connectivity after rTMS intervention for methamphetamine craving. Neuropharmacology 175, 108177 (2020).
Camprodon, J. A., Martınez-Raga, J., Alonso-Alonso, M., Shih, M.-C. & Pascual-Leone, A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 86, 91–94 (2007).
Liu, X. et al. The effects of DLPFC-targeted repetitive transcranial magnetic stimulation on craving in male methamphetamine patients. Clin. Transl. Med. 10, e48 (2020).
MacKinnon, D. P., Fairchild, A. J. & Fritz, M. S. Mediation analysis. Annu. Rev. Psychol. 58, 593–614 (2007).
Eichhammer, P. et al. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J. Clin. Psychiatry 64, 951–953 (2003).
Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391, 1683–1692 (2018).
Bakker, N. et al. rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. 8, 208–215 (2015).
Ekhtiari, H. et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: a consensus paper on the present state of the science and the road ahead. Neurosci. Biobehav. Rev. 104, 118–140 (2019).
Donse, L., Padberg, F., Sack, A. T., Rush, A. J. & Arns, M. Simultaneous rTMS and psychotherapy in major depressive disorder: clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 11, 337–345 (2018).
Dinur-Klein, L. et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76, 742–749 (2014).
Pell, G. S. et al. Efficacy of deep TMS with the H1 coil for anxious depression. J. Clin. Med. 11, 1015 (2022).
Kaster, T. S. et al. Differential symptom cluster responses to repetitive transcranial magnetic stimulation treatment in depression. EClinicalMedicine 55, 101765 (2023).
Teo, E. W., Lee, Y. Y., Khoo, S. & Morris, T. Translation and validation of the Malay version of Shiffman–Jarvik withdrawal scale and cessation self-efficacy questionnaire: a review of psychometric properties. Health Qual. Life Outcomes 13, 45 (2015).
Tiffany, S. T. & Drobes, D. J. The development and initial validation of a questionnaire on smoking urges. Br. J. Addict. 86, 1467–1476 (1991).
Okun, A., Stein, R. E., Bauman, L. J. & Silver, E. J. Content validity of the Psychiatric Symptom Index, CES-depression Scale, and State-Trait Anxiety Inventory from the perspective of DSM-IV. Psychol. Rep. 79, 1059–1069 (1996).
Eisenegger, C., Treyer, V., Fehr, E. & Knoch, D. Time-course of “off-line” prefrontal rTMS effects—a PET study. Neuroimage 42, 379–384 (2008).
al’Absi, M., Hatsukami, D., Davis, G. L. & Wittmers, L. E. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 73, 267–278 (2004).
VanderVeen, J. W., Cohen, L. M., Cukrowicz, K. C. & Trotter, D. R. The role of impulsivity on smoking maintenance. Nicotine Tob. Res. 10, 1397–1404 (2008).
Shiffman, S. M. & Jarvik, M. E. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology 50, 35–39 (1976).
Speilberger, C. D., Gorsuch, R., Lushene, R., Vagg, P. & Jacobs, G. Manual for the State-Trait Anxiety Inventory (Consulting Psychologists Press, 1983).
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C. & Fagerström, K.-O. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict. 86, 1119–1127 (1991).
Watson, D., Clark, L. A. & Tellegen, A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070 (1988).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
Jenkinson, M. Pechaud, M., & Smith, S. BET2: MR-based estimation of brain, skull and scalp surfaces. In Proc. Eleventh Annual Meeting of the Organization for Human Brain Mapping 167 (OHBM, 2005).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Andersson, J. L. R., Jenkinson, M. & Smith, S. Non-linear Registration, aka Spatial Normalisation FMRIB Technial Report TR07JA2 (Univ. Oxford, 2007).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Xia, M., Wang, J. & He, Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE 8, e68910 (2013).
Westin, G. G., Bassi, B. D., Lisanby, S. H. & Luber, B. Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: safety implications. Clin. Neurophysiol. 125, 142–147 (2014).
Hayes, A. F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach (Guilford Press, 2017).
Yu, Z. et al. Beyond t test and ANOVA: applications of mixed-effects models for more rigorous statistical analysis in neuroscience research. Neuron 110, 21–35 (2021).
Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Rosen, A. F. et al. Quantitative assessment of structural image quality. Neuroimage 169, 407–418 (2018).
Acknowledgements
This work was supported by a National Institute on Drug Abuse grant R00DA045749 to N.P. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We would like to thank A. Sun, G. Liu, G. Haase, L. Kim, M. Belnap and S. Ewing for their assistance with table/figure creation, data cleaning and proofreading; C. Wells and A. Lin for statistical consulting; J. Gilbert for assistance with MRI data collection; and D. Ngo, N. Vince-Cruz and B. Ramesh for assistance in collecting the TMS data. This work used computational and storage services associated with the Hoffman2 Shared Cluster provided by the UCLA Institute for Digital Research and Education Research Technology Group.
Author information
Authors and Affiliations
Contributions
M.R.A. and N.P. conceptualized the research question and analysis plan. M.R.A., T.J., A.F.L. and N.P. collected the data. M.R.A. analysed the behavioral data. T.J. and N.P. performed the neuroimaging data preprocessing. All authors interpreted the results. M.R.A. and N.P. prepared the original draft of the manuscript. A.F.L. contributed to the neuromodulation protocol design, safety and interpretation of the findings. All authors contributed to the critical evaluation of the manuscript. N.P. gave critical supervision and guidance.
Corresponding authors
Ethics declarations
Competing interests
M.R.A., T.J. and N.P. have no conflicts of interest to disclose. A.F.L. has received research support from National Institutes of Health, Department of Defense, and Neuroptics. He serves as a consultant for ElMindA. These organizations were not involved with the conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript. These organizations will not gain or lose financially through publication of this manuscript.
Peer review
Peer review information
Nature Mental Health thanks Abraham Zangen and the other, anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Smoking Characteristics.
Smoking behavior and characteristics of participants at baseline (non-abstinent) and pre-rTMS (abstinent).
Extended Data Fig. 2 Scatterplot.
State anxiety and withdrawal were significantly correlated before (black) and after (gray) rTMS.
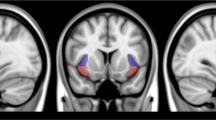
Extended Data Fig. 3 Regions of Interest.
The DLPFC (top panel) and V5 (bottom panel) ROIs were generated by thresholding the left DLPFC and V5, respectively, as defined by neurosynth.org.
Extended Data Fig. 4 Demographics.
Sociodemographic characteristics of participants.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, Discussion and Figures.
Source data
Source Data Fig. 2
File of coordinates.
Source Data Extended Data Fig. 1 and 4
Datasheet.
Source Data Extended Data Fig. 2
Datasheet.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Apostol, M.R., Jordan, T., Leuchter, A.F. et al. Effects of transcranial magnetic stimulation to the dorsolateral prefrontal cortex on craving and state anxiety in tobacco use disorder. Nat. Mental Health 1, 1001–1012 (2023). https://doi.org/10.1038/s44220-023-00154-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00154-5