Abstract
Currently available therapies for smoking cessation have limited efficacy, and potential treatments that target specific brain regions are under evaluation, with a focus on the insula. The ventral and dorsal anterior subregions of the insula serve distinct functional networks, yet our understanding of how these subregions contribute to smoking behavior is unclear. Resting-state functional connectivity (RSFC) provides a window into network-level function associated with smoking-related internal states. The goal of this study was to determine potentially distinct relationships of ventral and dorsal anterior insula RSFC with cigarette withdrawal after brief abstinence from smoking. Forty-seven participants (24 women; 18–45 years old), who smoked cigarettes daily and were abstinent from smoking overnight (~12 h), provided self-reports of withdrawal and underwent resting-state fMRI before and after smoking the first cigarette of the day. Correlations between withdrawal and RSFC were computed separately for ventral and dorsal anterior insula seed regions in whole-brain voxel-wise analyses. Withdrawal was positively correlated with RSFC of the right ventral anterior insula and dorsal anterior cingulate cortex (dACC) before but not after smoking. The correlation was mainly due to a composite effect of craving and physical symptoms of withdrawal. These results suggest a role of right ventral anterior insula-dACC connectivity in the internal states that maintain smoking behavior (e.g., withdrawal) and present a specific neural target for brain-based therapies seeking to attenuate withdrawal symptoms in the critical early stages of smoking cessation.
Similar content being viewed by others
Introduction
Smoking is one of the leading causes of preventable death worldwide, causing over 7 million deaths per year [1]. The ability of people who have high dependence on smoking to maintain abstinence on the first day of a cessation period is a strong predictor of their ability to achieve long-term abstinence [2]. Withdrawal symptoms that arise during brief abstinence and their relief by resumption of smoking pose a major challenge to long-term smoking cessation [3, 4]. Given the impact of the initial period of abstinence on cessation outcomes, understanding the neural systems underlying withdrawal during this initial period of cessation may help provide important insights in guiding brain-based treatments for smoking cessation.
The insula has been a primary neural target of interest for brain-based treatments [5, 6], partly due to clinical findings that have highlighted its relevance for smoking behavior. Studies of patients who smoked prior to sustaining a stroke involving the insula identified this region as important [7,8,9,10]. In stroke survivors, right insular damage predicted smoking cessation when assessed 1 year after discharge from the hospital [10], and even more so when combined with damage to the basal ganglia [8]. Stroke patients who smoked prior to damage of the insula also exhibited fewer and less intense withdrawal symptoms than their counterparts without insula damage [7].
Neuroimaging studies starting two decades ago have shown associations of insula function and structure with features of smoking behavior. Cue-induced cigarette craving was positively correlated with glucose metabolism in the insula [11], and functional MRI studies have confirmed that the insula exhibits activation during presentation of cigarette-related cues [12]. Among treatment-seeking participants who smoked cigarettes, those who later relapsed exhibited greater cue-induced activation of the insula than their counterparts who successfully maintained abstinence [13]. Studies of brain structure have shown negative relationships between thickness of insular subregions and smoking-related variables, including nicotine dependence, craving, and lifetime cigarette exposure [14, 15], confirming the importance of the insula in maintenance of cigarette smoking. Resting-state functional connectivity (RSFC) is another neuroimaging measure with potential for therapeutically relevant biomarkers [16, 17], and RSFC of the insula has been associated with craving, withdrawal, and nicotine dependence [18,19,20,21,22,23].
The insula has been subdivided into three subregions: dorsal anterior, ventral anterior, and posterior [24,25,26] with distinct functions segregating particularly along the anterior/posterior axis. While the posterior portion primarily connects with sensorimotor integration areas (e.g., premotor cortex, supplementary motor cortex), the anterior portion is functionally associated with limbic areas and regions comprising the “salience network” (e.g., dorsal ACC) [27, 28] and serves cognitive and affective functions [29, 30]. Further functional distinctions have been made between dorsal and ventral anterior insula connectivity along cognitive and affective domains, respectively [29, 31], as illustrated by positive correlations between dorsal anterior insula RSFC and performance on attention tasks, and ventral anterior insula connectivity with intensity of affective experience [32]. Distinctions between connectivity of the dorsal and ventral regions have also been framed according to externally and internally oriented networks—specifically, the frontoparietal attention network and the default mode network, respectively [33].
RSFC studies have attempted to capture distinctions between connectivity of insula subregions and smoking-related behavioral variables to clarify their contributions to maintenance of cigarette smoking [20, 34, 35]. One study examined connectivity of the three insular subregions in participants deprived of smoking (48-h abstinence) and also after ad lib smoking [20]; they observed that smoking increased connectivity between right ventral anterior insula and the left dorsolateral prefrontal cortex (DLPFC) and decreased connectivity of the right dorsal anterior insula and a region within the right inferior parietal lobule. Withdrawal was negatively correlated with right ventral anterior insula-left DLPFC connectivity. The findings highlighted the heterogeneity of relationships between insula connectivity and smoking-related variables that index withdrawal symptoms across insular subregions. However, a relatively small sample size (N = 20) left open the possibility of unreliable correlational statistics [36].
Examining the hypothesis that dorsal and ventral anterior insula connectivity may correspond to externally generated craving (via cue-induction) and internally generated craving (baseline craving without cue-induction), respectively, Janes et al. determined that baseline craving in nondeprived participants (having smoked 1 h prior to scanning) was associated with ventral anterior insula connectivity with components of the default mode network, including the rostral ACC, and that cue-induced craving was associated with dorsal anterior insula connectivity with mostly the frontoparietal attention network and with cue-induced activation of the rostral ACC [35]. These results suggested that cue-induced and spontaneous craving are linked to functions of different anterior insula subregions. However, further studies are required to determine how connectivity of these subregions differs while participants are in a state of abstinence (nicotine deprived).
Neural dynamics during brief cessation from smoking (e.g., after overnight abstinence) are likely very different from those immediately after smoking, yet except for a few studies [e.g., 20, 37, 38], most studies either only examine one state or the other, or more problematically, do not control for smoking state. In a group of 47 participants who smoked cigarettes daily, we examined changes in the relationship between withdrawal and RSFC of ventral and dorsal anterior insula subregions after overnight abstinence, before and after smoking the first cigarette of the day. Considering the literature, we hypothesized that connectivity between the right ventral anterior insula and the ACC would be correlated positively with withdrawal symptoms (i.e., participants with greater connectivity would exhibit more withdrawal), and that this relationship would not persist after the participants smoked a cigarette and withdrawal symptoms abated.
Materials and methods
Overview of experimental design
Functional magnetic resonance imaging (fMRI) data were collected during the resting state. Both fMRI data and self-report measures of cigarette craving and withdrawal were collected twice on the same day after overnight (~12 h) abstinence from smoking: once before and once after participants smoked their first cigarette of the day (pre and post smoking, respectively). Functional connectivity of the insular subregions was computed from both pre and post smoking fMRI scans. The study took place between September 2017 and February 2020 and included adults who endorsed daily smoking. The study was conducted at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles (UCLA). All study procedures were approved by the UCLA Internal Review Board.
Participants
One hundred and seventy nine participants were screened via online and print advertisements. They attended an intake session where they received a detailed explanation of the study procedures, provided written informed consent, and were screened for eligibility. Fifty-one participants met all study criteria and completed all procedures. Inclusion criteria were: age of 18–45 years, generally good health, self-report of smoking at least four cigarettes per day for at least 1 year, and urinary cotinine ≥ 100 ng/mL. Recent smoking history was verified during the intake session using a urine cotinine test (ACCUTEST Urine Cotinine Test, Jant Pharmacal Corp., Encino, CA, score ≥ 3, cotinine ≥ 100 ng/mL). Exclusion criteria were positive urine tests for drugs of abuse other than nicotine or tetrahydrocannabinol, consuming ≥10 alcoholic drinks per week, any current psychiatric disorder other than tobacco use disorder as assessed via the Mini International Neuropsychiatric Interview for DSM-5 [39, 40, DSM-5 update], history of neurological injury, and using electronic cigarettes, cigars, snuff, or chewing tobacco > 3 times a month.
Verification of drug and alcohol abstinence
On the testing day, overnight (~12 h) abstinence from smoking was verified with the Micro+ Smokerlyzer® breath CO monitor (Bedford Scientific Ltd., Maidstone, Kent, UK), with a required CO level of <10 ppm. Abstinence from cocaine, opiates, benzodiazepines, and amphetamines was also verified with a five-panel urine drug test (Drugs of Abuse Test Instant-view®, Alfa Scientific Designs Inc., Poway, CA). Alcohol abstinence was verified using a breathalyzer (Alco-Sensor FST®, Intoximeters, Inc., St. Louis, MO). Recent abstinence from cannabis use was verified with the Dräger DrugTest® 5000 saliva test (Dräger, Inc., Houston, TX).
Self-report measures
Nicotine dependence was measured during the intake session using the Fagerström test for nicotine dependence [41]. Withdrawal symptoms were measured using the Shiffman–Jarvik Withdrawal Scale [SJWS, 42] on the testing day before and after the participants smoked their first cigarette of the day (pre and post smoking, respectively). The SJWS total score was examined as were the subscales (i.e., in post hoc analyses).
Scanning protocol
Participants took part in two consecutive MRI sessions on the same day after overnight abstinence from smoking. After the first session, participants were allowed to smoke one cigarette. Between 15 and 20 min after smoking, they were scanned again.
MRI data acquisition
All images were acquired on a 3-Tesla PRISMA (Siemens) MRI scanner using a 32-channel head coil receiver. The resting-state imaging protocol consisted of the continuous acquisition of 738 echo-planar image (EPI) volumes over a period of 9 min and 50 s. A multiband accelerated EPI pulse sequence (factor 8) was used allowing us to acquire 72 axial slices during a repetition time (TR) of 800 ms with a 104 × 104 matrix. Resolution was 2 × 2 × 2 mm3, echo time (TE) was 37 ms, and the flip angle was 52°. During the resting-state scan, participants were asked to keep their eyes open and to look at a black screen. The structural T1-weighted images were obtained using a magnetization prepared rapid gradient echo sequence with the following parameters: isovoxel 0.8 mm3, FOV = 240 × 256 mm2, TE = 2.24 ms, TR = 2400 ms, flip angle = 8°, and 208 sagittal slices.
MRI data preprocessing
Image preprocessing was mostly conducted with FSL (5.0.9). The initial stages included rigid body realignment to correct for head movements within each scanning run, skull removal, and nonlinear registration to the Montreal Neurological Institute (MNI) template. A first motion cleaning and noise reduction were performed using a 24-parameter linear regression model that included six motion parameters (three translational dimensions along X, Y, and Z axes and rotational dimensions: “pitch,” “roll,” and “yaw”), the temporal derivatives of these parameters and the quadratic of all parameters [43]. Mean frame displacement (FD) and the variance of signal change from the average signal (DVARS) of the raw images were estimated. A null sampling distribution of DVARS was used to identify frames with excessive variance at P < 0.05 [44]; frames with FD exceeding 0.45 mm were also flagged. Those frames as well as the one located in time just prior (t − 1) and two just after (t + 1 and t + 2) each were included in a censoring temporal mask for data interpolation: a least squares spectral decomposition of the uncensored data was performed to reconstitute data of the censored timepoints [see methods in ref. 45]. The uncensored data defined the frequency characteristics of signals that then replaced the censored data. This step aimed at minimizing the contamination of the signal from the censored frames during frequency filtering. The interpolated signal was then demeaned, detrended, and filtered using an ideal bandpass filter (0.009–0.08 Hz). Following bandpass filtering, the interpolated timepoints were finally censored. Participants with more than 50% frames censored (i.e., those with less than 5 min of remaining resting-state data) were excluded from the analysis. To reduce the contribution from nonneuronal noise in the data, the minimal number of principle components that explained at least 50% of the variance of mean signal extracted from white matter and cerebrospinal fluid were evaluated and regressed out from the signal [aCompCor50, 46]. Volumes were then spatially smoothed with a Gaussian filter using a 5-mm FWHM kernel. Each voxel was normalized to a mean value of 100 (SD = 1) to transform the data to Pearson’s correlation coefficients (r). All analyses were performed on Linux (CentOS release 6.10) using FSL 5.0.9, MATLAB 8.6, R (version 3.6.0).
Resting-state fMRI seed-based analysis
To minimize bias, we used the statistically conservative approach of voxel-wise whole-brain analyses rather than restricting to a priori-selected target regions or networks. Four insula seeds were defined for RSFC analyses: two seeds on the left and two on the right anterior insula separately encompassing the ventral aspect and the dorsal aspects (Fig. 1). To define the anterior insula, we compared anatomical landmarks from a probabilistic atlas [47] to functional connectivity-based parcellations of the insula [24, 26]. From these studies, we defined the ventral anterior insula parcel as the anterior inferior insular cortex (which includes the apex, the limen, and the transverse gyrus). The dorsal anterior insula was defined as the anterior and middle short gyri. The precentral sulcus was used to segment the anterior from the posterior insula. Using these landmarks, we manually determined the four anterior insular subdivisions from the MNI152 template.
To evaluate the functional connectivity between the insula seeds and other brain regions during the resting state, the time series from each seed was extracted, and its first normalized eigenvector (mean = 100, SD = 1; to facilitate computation of Pearson’s r) was used as a regressor in an ordinary least squares linear regression analysis on every voxel (as implemented in film_gls in FEAT). The parameter estimates of the model, corresponding to the Pearson’s correlation coefficient (since data were previously normalized), were z-transformed to improve data normality. The resulting z-transformed images were used in multilevel mixed effects models for group analyses (FLAME1, FEAT). First, analyses were performed on pre and post smoking scans separately, testing for effects of withdrawal (SJWS total score) on functional connectivity for each. Then, a mixed model with participant as random effect was used to test for an interaction between withdrawal and smoking condition (pre/post smoking). To account for movement differences between sessions and participants, the mean FD value was included as a covariate in all models, in addition to age. Results were cluster-corrected for multiple comparisons as using a voxel-height threshold of p < 0.001 (Z > 3.1) and cluster threshold of p < 0.05 as recommended per Eklund et al. [48]. Post hoc tests of the SJWS subscales using iterative backwards hierarchical regression were conducted using R [49].
Results
Participant characteristics
Fifty-one participants completed self-report measures of cigarette withdrawal after overnight (~12 h) abstinence from smoking, before and after smoking their first cigarette of the day (pre and post smoking, respectively). Of those tested, four were excluded for excessive motion during fMRI, as revealed by the number of flagged volumes exceeding DVARS and/or FD thresholds after data preprocessing (>50%). Two participants had missing withdrawal symptom data and were excluded from analyses involving withdrawal measures. The final sample included 47 individuals with a mean age of 33.3 (SD = 7.2) years. They smoked a mean of 11.4 (SD = 4.5) cigarettes per day with a smoking exposure history of 8.1 (SD = 6.0) pack years. They had moderate levels of nicotine dependence (FTND scores of 4.0, SD = 2.0). Overall, 24 of the 47 participants had one or more alcoholic drinks per week (M = 4.33, SD = 1.99). None of the participants met criteria for cannabis- or alcohol-use disorder (or any other substance-use disorder other than tobacco use disorder). The cigarette withdrawal scores (SJWS total scores) differed significantly from before to after smoking: 56.4 (SD = 16.3) pre smoking and 38.4 (SD = 12.5) post smoking (t(44) = 7.99, p < 0.001). Mean time from last cigarette (night before) to the pre smoking resting-state fMRI scan was 13 h (SD = 2.54) and to the first cigarette of the day (after pre smoking scan) was 14.56 h (SD = 2.44). Mean time between smoking a cigarette and the beginning of the post smoking resting-state fMRI scan was 30 min (SD = 7.36). Sex was balanced with 24 females and 23 males.
Nicotine withdrawal
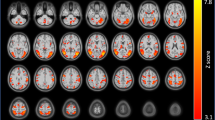
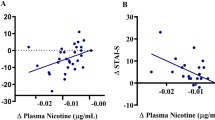
In the pre smoking condition, connectivity between the right ventral anterior insula and the posterior aspect of the dorsal anterior cingulate cortex (dACC; peak MNI coordinates: x = 4, y = 12, z = 32) was significantly correlated with the SJWS total score (Fig. 2A, B). The correlation was positive and was not observed in the post smoking condition (Fig. 2B). The interaction of smoking condition (pre/post smoking) and SJWS total score on right ventral anterior insula connectivity did not survive multiple comparison correction in the mixed effects model. However, the peak voxel within the dACC cluster observed for the correlation of SJWS total score and right ventral anterior insula during the pre smoking condition revealed a significant SJWS total score-by-smoking condition interaction (p < 0.05, uncorrected). No significant relationships were observed between SJWS total score and the other three anterior seed regions examined either pre or post smoking.
A Thresholded rvaInsula connectivity statistical map (cluster-corrected, height threshold: Z > 3.10, cluster threshold: p < 0.05) from scan before participants smoked the first cigarette of the day (pre smoking). The rvaInsula seed is shown in light red on the coronal section. Montreal Neurological Institute (MNI) coordinates (X, Y, and Z) are shown above each brain image. The brain is presented in neurological orientation (left = left). Scatterplots presenting B SJWS total score, C SJWS craving score, and D SJWS physical withdrawal score (abscissa axes) and mean extracted values of posterior dorsal anterior cingulate cortex from rvaInsula connectivity map (ordinate axes). Data presented are from before and after smoking the first cigarette of the day (pre and post smoking). SJWS Shiffman–Jarvik Withdrawal Scale. Statistics associated with linear fitting are available in Tables S1–S6 in the Supplementary Materials.
Post hoc analyses of nicotine withdrawal
To determine which factors of the SJWS contributed to the correlation between SJWS total scores and right ventral anterior insula connectivity with dACC, we conducted post hoc tests on the five subscales of the SJWS, which characterize: (1) physical symptoms, (2) psychological symptoms, (3) craving, (4) sedation, and (5) appetite. We used a linear model with scores for the five subscales as independent variables. The results suggested that sedation and psychological symptoms did not contribute to the observed effect (p = 0.79 and p = 0.60, respectively; see Supplementary Materials, Table S9). Therefore, the correlation observed between SJWS total score and right ventral anterior insula-dACC connectivity most likely reflected a composite effect of craving (Fig. 2C) and physical symptoms (Fig. 2D).
The direct comparison of functional connectivity before and after smoking a cigarette did not reveal any significant differences for any of the four anterior insula seeds examined.
Post hoc analyses of biological sex
Considering the emerging literature on sex differences in the acute effects of smoking, behavioral states related to smoking [50,51,52] and respective neural measures [53,54,55,56,57,58,59], we conducted post hoc assessments of the effect of biological sex on self-reported withdrawal and rvaInsula-dACC connectivity. We did not find a significant relationship between biological sex and SJWS total score, either as main effect or as an interaction with smoking status (pre or post smoking) (ps > 0.9). An additional post hoc test of biological sex on our main RSFC results (i.e., rvaInsula-dACC connectivity) also revealed no significant effects (p > 0.87). These findings are presented in Supplementary Tables S7 and S8.
Discussion
A distinct functional connectivity pattern of the right ventral anterior insula was associated with withdrawal in participants after overnight abstinence from cigarette smoking. Specifically, connectivity between the right ventral anterior insula and the posterior segment of the dorsal ACC was positively correlated with withdrawal, particularly the craving and physical symptoms of withdrawal, consistent with the view that the insula supports an “embodied” experience of addiction through integration of interoceptive signals [60]. These effects were mitigated after participants smoked the first cigarette of the day (see Fig. 2), illustrating that this pattern of connectivity depends on the participant’s state of abstinence.
The positive correlation between connectivity of the right ventral anterior insula and posterior dACC with cigarette withdrawal, especially craving and physical symptoms, suggests that greater connectivity with networks that include the dACC as a hub (e.g., salience network) may underlie tobacco withdrawal, promoting the maintenance of smoking. This finding is consistent with findings of attenuated craving and withdrawal symptoms in patients following damage to the insula [7,8,9,10], and implies that at least part of this attenuation may be attributable to disruption of right ventral anterior insula-dACC connectivity.
The dACC is involved in numerous functions and has been conceptualized as a hub for integration of heterogenous signals, behavioral flexibility, and cognitive control [61,62,63,64]. The location of the dACC cluster we observed is within an area described as the anterior mid-cingulate cortex, which has been considered an integration hub for negative affect, pain, and cognitive control with connections to subcortical regions involved both in reward (e.g., nucleus accumbens) and negative affect (e.g., amygdala) [63]. Along with the anterior insula, the region is a primary component of the salience network [27, 28], which has a role in detecting behaviorally relevant stimuli and is thought to regulate interactions between other large scale networks, such as the executive control network and default mode network [65]. Indeed, the salience network has been implicated in nicotine deprived states, showing weaker connectivity with the default mode network [37], and compared to individuals who do not smoke, those who do show greater intrinsic connectivity of the salience network (i.e., greater anterior insula-dACC connectivity) [66]. Given the existing literature and taking into account the complexity of interpreting the function of dACC [67], our findings suggest that increases in functional connectivity of the salience network with greater withdrawal symptoms may reflect a combination of intensified interoceptive and cognitive/behavioral regulatory signaling that emerges from homeostatic disturbance related to the state of brief abstinence.
Our results are only partially consistent with previous RSFC findings on individuals who smoked cigarettes. Although a study of 40 nondeprived young participants (15–24 years of age) found a relationship between right, but not left, anterior insula-ACC connectivity and craving [18], those correlations were negative, whereas we observed a positive relationship, during abstinence, and only for the right ventral anterior insula. However, direct comparisons between the studies are difficult as the study participants in the Bi et al.’s study were only asked to refrain from smoking 30 min prior to the scan (i.e., nondeprived) to avoid taking measurements while in a state of withdrawal; also, ventral and dorsal subregions of the right anterior insula were combined for analysis [18]. Moreover, their focus on the ACC was based on a prior contrast of anterior insula connectivity in individuals who smoke vs. those that do not, biasing their ACC selection based on group effects. In contrast, we studied participants after overnight abstinence, segregated the right dorsal and ventral insula, and determined in an unbiased whole-brain analysis that connectivity of the ventral anterior portion of the insula with dACC is related to withdrawal symptoms, especially craving and physical symptoms. Given our findings that relationships between withdrawal and functional connectivity are likely to depend significantly on recency of smoking and insular subregion, greater specificity in findings is required to produce generalizable conclusions. Further studies, especially those with larger, well-characterized samples that precisely control deprivation conditions, may provide greater clarity.
Our results resonate with previous work that established functional distinctions between dorsal and ventral anterior insula functional connectivity. Meta-analyses have distinguished dorsal and ventral anterior regions as having differential roles in cognition/attention and affect, respectively [29, 31]. For example, the right dorsal anterior insula connectivity is correlated with performance on attention tasks while connectivity of the right ventral anterior insula increases with the intensity of emotional experience when viewing arousing pictures [32]. Another distinction has been made between externally oriented (dorsal) and internally oriented (ventral) networks [33, 35], with ventral internally oriented connectivity shown to be related to baseline craving [35]. These results are consistent with our finding that right ventral, and not right dorsal, anterior insula connectivity is correlated with intensity of withdrawal symptoms, given its links with intensity of negative affective states [68] and that withdrawal is an internally oriented state (not elicited by external cues).
Our observation that the relationship between withdrawal and functional connectivity was primarily due to craving and physical symptoms, with psychological and stimulation/sedation having less of a role, suggests that distinct functional connectivity patterns may explain different aspects of withdrawal. As such, given our results, psychological and stimulation/sedation aspects may be related to connectivity patterns that are less inclusive of the right ventral anterior insula. Future studies may examine how distinct network patterns associated with different aspects of withdrawal integrate to give rise to the overall experience of withdrawal.
The lack of mean differences observed in insula connectivity from before to after smoking a cigarette may reflect various factors. Participants only smoked one cigarette after overnight abstinence, whereas participants in other studies that report effects of smoking on ventral and dorsal anterior insula connectivity smoked ad lib [e.g., 20, 38]. One cigarette may not have sufficiently changed the mean difference in RSFC across participants even though craving was reduced by 28%. Moreover, we used a relatively stringent, but recommended, statistical threshold (Z > 3.1) for whole-brain cluster-corrected voxel-wise analyses [48]. The effect of smoking a single cigarette after overnight abstinence may not have produced a strong enough effect to exceed this threshold.
Brain stimulation has been considered as a promising therapy for smoking cessation [69, 70], with preclinical studies showing attenuation of nicotine intake with stimulation of the insula [71]. However, human studies in which the insula was the target showed mixed success in affecting smoking-related variables, such as craving and withdrawal [5, 6, 72]. By indicating the relevance of ventral anterior insula connectivity for withdrawal symptoms, our study provides an additional level of anatomical specificity for targeting specific regions of the insula that may increase the efficacy of such studies.
Most stimulation studies target the insula indirectly via stimulation of the cortical surface (e.g., DLPFC) above the region using repetitive transcranial magnetic stimulation (rTMS), which acts primarily at the surface. Stimulating the DLPFC impacts intrinsic insula connectivity [73], but not in the anterior portion, where our results suggest that greater efficacy would be found. Alternatively, disruption of right ventral anterior insula-dACC connectivity may be more accurately accomplished by rTMS by targeting the dACC, which is located closer to the surface, as has been previously suggested [70]. Further testing is needed to determine the feasibility and efficacy of dACC stimulation. More recent stimulation technologies (e.g., low intensity focused ultrasound pulsation) [74] that can target deep structures offer the ability to target insular subregions more precisely. This technology will enable future examinations of the effects of focal stimulation of the ventral anterior insula to determine how disrupting its connectivity with the dACC may reduce withdrawal symptoms to impact smoking behavior.
This study is not without limitations. Although its design allowed for assessments after overnight abstinence and again immediately after smoking within the same day, it inherently did not afford the possibility of counterbalancing to eliminate potential order and time-of-day effects. As such, the comparison of pre and post smoking connectivity may be influenced by such effects; however, their attribution would be difficult as no suprathreshold comparison effects were observed. Our study contained a moderate sample size of 47 participants, which is fairly large relative to most published studies of RSFC in individuals who smoke. Nevertheless, larger scale studies are required to confirm these results. We tested four insula seeds in the current study, and did not apply a statistical correction for comparison across the four seeds as they were selected a priori. As with many ACC results found in fMRI studies, the dACC cluster we found was along the midline spanning from one hemisphere through the longitudinal fissure across to the other. Due to our rigorous motion cleaning procedures, which included removal of signal from cerebral spinal fluid, our results are unlikely due to motion artifact, but smoothing and spatial registration into standard space may have contributed to the positioning of this cluster along the midline. Moreover, the current study tested nontreatment-seeking individuals, and it is unclear whether the same effects would be observed in individuals from a treatment-seeking population, which may include candidates for brain-based therapies.
Overall, the current study provides evidence for functional connectivity of a specific anterior insula-ACC circuit (ventral anterior insula-dACC) that may facilitate the negative reinforcement mechanisms [75] underlying maintenance of smoking. Specifically, our demonstration that the ventral, and not dorsal, anterior insula connectivity is related to withdrawal highlights the heterogeneity of the insula with respect to addiction-related states and pinpoints a ventral anterior target for brain-based therapies.
Funding and disclosure
This research was supported, in part, by a grant from the National Institute on Drug Abuse (NIDA) (R37 DA044467, EDL) and endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies and the Marjorie M. Greene Trust (EDL). MPD is supported by a Ruth L. Kirschstein Postdoctoral Individual National Research Award from NIDA (F32 DA049500-01A1). We acknowledge the Canada Research Chairs program (RFT, the Canada Research Chair in Pharmacogenomics). RFT has consulted for Quinn Emanuel and Ethismos Research Inc. All other authors declare no conflict of interest.
Data availability
All self-report, toxicology, and summary fMRI data discussed in this manuscript, as well as the code used for statistical analyses, are publicly available from the Open Science Framework web site under project title, “Functional connectivity of the anterior insula during withdrawal from cigarette smoking” (https://osf.io/89ph5).
References
Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–94.
Westman EC, Behm FM, Simel DL, Rose JE. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Arch Intern Med. 1997;157:335–40.
Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res. 2008;10:35–45.
Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215.
Ibrahim C, Rubin-Kahana DS, Pushparaj A, Musiol M, Blumberger DM, Daskalakis ZJ, et al. The insula: a brain stimulation target for the treatment of addiction. Front Pharmacol. 2019;10:720.
Regner MF, Tregellas J, Kluger B, Wylie K, Gowin JL, Tanabe J. The insula in nicotine use disorder: functional neuroimaging and implications for neuromodulation. Neurosci Biobehav Rev. 2019;103:414–24.
Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, et al. Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction. 2015;110:1994–2003.
Gaznick N, Tranel D, McNutt A, Bechara A. Basal ganglia plus insula damage yields stronger disruption of smoking addiction than basal ganglia damage alone. Nicotine Tob Res. 2014;16:445–53.
Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4.
Suñer-Soler R, Grau A, Gras ME, Font-Mayolas S, Silva Y, Dávalos A, et al. Smoking cessation 1 year poststroke and damage to the insular cortex. Stroke. 2012;43:131–6.
Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–72.
Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–62.
Janes A, Gilman J, Radoman M, Pachas G, Fava M, Evins A. Revisiting the role of the insula and smoking cue-reactivity in relapse: a replication and extension of neuroimaging findings. Drug Alcohol Depend. 2017;179:8–12.
Lin F, Wu G, Zhu L, Lei H. Region-specific changes of insular cortical thickness in heavy smokers. Front Hum Neurosci. 2019;13:265.
Morales AM, Ghahremani D, Kohno M, Hellemann GS, London ED. Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology. 2014;39:1816–22.
Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci. 2015;1349:64.
Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage. 2012;62:2281–95.
Bi Y, Yuan K, Guan Y, Cheng J, Zhang Y, Li Y, et al. Altered resting state functional connectivity of anterior insula in young smokers. Brain Imaging Behav. 2017;11:155–65.
Moran LV, Sampath H, Stein EA, Hong LE. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophrenia Res. 2012;142:223–9.
Fedota JR, Ding X, Matous AL, Salmeron BJ, McKenna MR, Gu H, et al. Nicotine abstinence influences the calculation of salience in discrete insular circuits. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:150–9.
Li Y, Yuan K, Guan Y, Cheng J, Bi Y, Shi S, et al. The implication of salience network abnormalities in young male adult smokers. Brain Imaging Behav. 2017;11:943–53.
Wilcox CE, Calhoun VD, Rachakonda S, Claus ED, Littlewood RA, Mickey J, et al. Functional network connectivity predicts treatment outcome during treatment of nicotine use disorder. Psychiatry Res Neuroimaging. 2017;265:45–53.
Zhou S, Xiao D, Peng P, Wang SK, Liu Z, Qin HY, et al. Effect of smoking on resting-state functional connectivity in smokers: an fMRI study. Respirology. 2017;22:1118–24.
Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–49.
Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23.
Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–506.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Uddin, L. Q. Salience Network of the Human Brain. San Diego: Academic Press; 2017.
Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–34.
Gu X, Liu X, Van Dam NT, Hof PR, Fan J. Cognition-emotion integration in the anterior insular cortex. Cereb Cortex. 2013;23:20–7.
Wager TD, Barrett LF. From affect to control: functional specialization of the insula in motivation and regulation. 2017;102368. https://doi.org/10.1101/102368.
Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage. 2012;60:1947–58.
Wang Y, Zhu L, Zou Q, Cui Q, Liao W, Duan X, et al. Frequency dependent hub role of the dorsal and ventral right anterior insula. Neuroimage. 2018;165:112–17.
Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ. Increased functional connectivity in an insula-based network is associated with improved smoking cessation outcomes. Neuropsychopharmacology. 2015;40:2648–56.
Janes AC, Krantz NL, Nickerson LD, Frederick BB, Lukas SE. Craving and cue reactivity in nicotine-dependent tobacco smokers is associated with different insula networks. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:76–83.
Yarkoni T. Big correlations in little studies: inflated fmri correlations reflect low statistical power-commentary on Vul et al. (2009). Perspect Psychol Sci. 2009;4:294–8.
Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71:523–30.
Yip SW, Lichenstein SD, Garrison K, Averill CL, Viswanath H, Salas R, et al. Effects of smoking status and state on intrinsic connectivity. Biol Psychiatry: Cogn Neurosci. Neuroimaging. 2021. https://www.sciencedirect.com/science/article/pii/S2451902221000501?casa_token=cWIGPLn67zAAAAAA:AMSDb7r70T9bcV4UoXw3UqK2dzJEo4mhMjK0EVQurqOgAhyXLSNqJdw9p91Kon90hqrTvORpItw.
Hergueta T, Weiller E. Evaluating depressive symptoms in hypomanic and manic episodes using a structured diagnostic tool: validation of a new Mini International Neuropsychiatric Interview (MINI) module for the DSM-5’ With Mixed Features’ specifier. Int J Bipolar Disord. 2013;1:21.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clinical Psychiatry. 1998.
Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerström test for cigarette dependence. Nicotine Tob Res. 2011;14:75–78.
Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology. 1976;50:35–39.
Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56.
Afyouni S, Nichols TE. Insight and inference for DVARS. NeuroImage 2018;172:291–312.
Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–41.
Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage. 2014;96:22–35.
Faillenot I, Heckemann RA, Frot M, Hammers A. Macroanatomy and 3D probabilistic atlas of the human insula. NeuroImage. 2017;150:88–98.
Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA. 2016;113:7900–05.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2020. https://www.R-project.org/.
Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–15.
Faulkner P, Petersen N, Ghahremani DG, Cox CM, Tyndale RF, Hellemann GS, et al. Sex differences in tobacco withdrawal and responses to smoking reduced-nicotine cigarettes in young smokers. Psychopharmacology. 2018;235:193–202.
Perkins KA, Karelitz JL. Sex Differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17:443–48.
Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, et al. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol. 2012;15:989–94.
Cosgrove KP, Wang S, Kim S-J, McGovern E, Nabulsi N, Gao H, et al. Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci. 2014;34:16851–55.
Staley JK, Krishnan‐Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, et al. Sex differences in [123I] β‐CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–84.
Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addictive Behav. 2014;39:789–92.
Beltz AM, Berenbaum SA, Wilson SJ. Sex differences in resting state brain function of cigarette smokers and links to nicotine dependence. Exp Clin Psychopharmacol. 2015;23:247.
McCarthy JM, Dumais KM, Zegel M, Pizzagalli DA, Olson DP, Moran LV, et al. Sex differences in tobacco smokers: executive control network and frontostriatal connectivity. Drug Alcohol Depend. 2019;195:59–65.
Dumais KM, Franklin TR, Jagannathan K, Hager N, Gawrysiak M, Betts J, et al. Multi-site exploration of sex differences in brain reactivity to smoking cues: consensus across sites and methodologies. Drug Alcohol Depend. 2017;178:469–76.
Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76 Pt B:342–50.
Heilbronner SR, Hayden BY. Dorsal anterior cingulate cortex: a bottom-up view. Annu Rev Neurosci. 2016;39:149–70.
Kolling N, Wittmann MK, Behrens TEJ, Boorman ED, Mars RB, Rushworth MFS. Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci. 2016;19:1280–85.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67.
Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016;19:1286–91.
Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67.
Janes AC, Gilman JM, Frederick BB, Radoman M, Pachas G, Fava M, et al. Salience network coupling is linked to both tobacco smoking and symptoms of attention deficit hyperactivity disorder (ADHD). Drug Alcohol Depend. 2018;182:93–97.
Ebitz RB, Hayden BY. Dorsal anterior cingulate: a Rorschach test for cognitive neuroscience. Nat Neurosci. 2016;19:1278–79.
Piper ME. Withdrawal: expanding a key addiction construct. Nicotine Tob Res. 2015;17:1405–15.
Grall-Bronnec M, Sauvaget A. The use of repetitive transcranial magnetic stimulation for modulating craving and addictive behaviours: a critical literature review of efficacy, technical and methodological considerations. Neurosci Biobehav Rev. 2014;47:592–613.
Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, et al. Brain stimulation methods to treat tobacco addiction. Brain Stimulation. 2013;6:221–30.
Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, et al. Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology. 2013;38:690–8.
Antonelli M, Fattore L, Sestito L, Di Giuda D, Diana M, Addolorato G. Transcranial magnetic stimulation: a review about its efficacy in the treatment of alcohol, tobacco and cocaine addiction. Addictive Behav. 2021;114:106760.
Li X, Du L, Sahlem GL, Badran BW, Henderson S, George MS. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend. 2017;174:98–105.
Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, et al. A review of low-intensity focused ultrasound pulsation. Brain Stimulation. 2011;4:125–36.
Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013;23:559–63.
Acknowledgements
The authors would like to thank Ms. Andrea Donis, Ms. Diana Paez, Ms. Citlaly Cahuantzi, Ms. Tinisha Sakhrani, and Mr. Hector Diaz, whose contribution to data collection helped make this work possible.
Author information
Authors and Affiliations
Contributions
All authors were involved in designing the study and contributed to writing the manuscript and have reviewed and approved the final version of this manuscript. DGG and MPD acquired the data. J-BP, DGG, and MPD were primarily responsible for data analysis. DGG, J-BP, and EDL drafted the manuscript. As principal investigator of the study, EDL is accountable for all aspects of the work, including its accuracy and integrity.
Corresponding authors
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ghahremani, D.G., Pochon, JB., Perez Diaz, M. et al. Functional connectivity of the anterior insula during withdrawal from cigarette smoking. Neuropsychopharmacol. 46, 2083–2089 (2021). https://doi.org/10.1038/s41386-021-01036-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01036-z
This article is cited by
-
Nicotine dependence and insula subregions: functional connectivity and cue-induced activation
Neuropsychopharmacology (2023)
-
Smoking, tobacco dependence, and neurometabolites in the dorsal anterior cingulate cortex
Molecular Psychiatry (2023)