Abstract
A growing number of studies show an association between attention deficit hyperactivity disorder and physical abuse in childhood. We examined temporal associations of physical abuse risk with methylphenidate treatment in children with attention deficit hyperactivity disorder. Using Hong Kong electronic medical records, we conducted a self-controlled case series study in 1,064 children (5–16 years old) who were treated with methylphenidate and also experienced physical abuse. Compared with non-medicated periods, a higher risk of abuse was observed shortly before treatment initiation (incidence rate ratio = 4.49; 95% confidence interval = 3.76–5.36). After treatment initiation, the risk was comparable to that in non-medicated periods (incidence rate ratio = 0.90; 95% confidence interval = 0.63–1.29), followed by a 37% reduction during subsequent treatment. These findings are consistent with the hypothesis that methylphenidate treatment in children with attention deficit hyperactivity disorder is associated with a reduced risk of becoming a victim of physical abuse.
Similar content being viewed by others
Main
Physical abuse in childhood is common, with about 25% of adults reporting that they were physically abused as children1,2. The consequences of child abuse include impairments to physical and mental health that can extend into adulthood, ultimately affecting social and economic development2. Childhood physical abuse is considered an important risk factor for depressive disorders in adulthood3. Previous research has shown that abuse resulted in a 2.3-fold increase in hospitalization between 2001 and 2010 in Hong Kong, with recorded cases in 2010 at 7.3 per 10,000 children under 19 years old4.
Children with attention deficit hyperactivity disorder (ADHD) are at higher risk than their peers of being victims of abuse, particularly physical abuse5,6,7,8,9. Multiple factors may contribute to this increased risk. As ADHD is highly heritable and has shared genes with other psychopathologies10,11, many parents of children with ADHD also suffer from ADHD and other psychopathologies including depression, which could potentially increase the risk for negative and suboptimal parenting practices as well as perpetrating abuse10. Harsh parenting is also associated with an increased interactive aggravation of ADHD and oppositional symptoms in the child. In addition, many parents find parenting a child with ADHD challenging, particularly when ADHD is untreated12. Children with untreated ADHD may push boundaries laid down by adults, and such behaviours may be viewed as disobedient and wilful, further increasing parental stress and creating a cycle of escalating negative parent and child behaviours8,13 with serious consequences including domestic violence/abuse and child abuse14. Here, it is important to note that when one individual perpetrates abuse on another the responsibility sits with the perpetrator. Furthermore, it is a societal issue that requires assistance from the whole society, including but not limited to family-15,16, school-17,18 and community-based interventions19 to provide mental health support for both children and caregivers. However, such a comprehensive network of social support is not available in many countries, including Hong Kong20,21.
Direct training and support can help parents to become more competent in dealing with ADHD children, and to adopt a more supportive, empathetic and positive parenting style. This can improve parent–child relationships and reduce parental stress, thereby potentially leading to improved wellbeing and reduce rates of abuse for children with ADHD22,23,24.
In addition, reducing ADHD symptoms in childhood may also be associated with reduced risk of abuse. Previous studies have suggested that medications for ADHD, such as the psychostimulant methylphenidate (MPH)25,26, may lower the risk of physical injury27,28,29. This was hypothesized to be due to a reduction of core symptoms of impulsivity, inattentiveness and hyperactivity, which results in a decreased likelihood of involvement in accidents27. Despite some common side effects such as nausea, headache and stomach ache, MPH has been shown to have the best safety profile among ADHD medications30; recent meta-analyses and systematic reviews also add support for the efficacy of pharmacological treatments for ADHD in reducing core symptoms of the disorder31,32. In addition, a recent study33 showed that MPH treatment had a positive effect on improving parent–child interactions and social cognition such as recognition of emotions and understanding of false belief among children with ADHD, through the oxytocin system. We therefore hypothesized that the use of pharmacological treatment for children with ADHD could lower the risk of physical abuse.
In view of the global increase in ADHD medication use25,26,34 and the lack of research on the effects of ADHD medication on child physical abuse, the aim of this study was to evaluate the effect of MPH on the risk of physical abuse using advanced pharmacoepidemiological approaches25,26.
Results
Following the self-controlled case series (SCCS) design (Fig. 1), we first identified 39,403 individuals aged 5–16 years with at least one MPH prescription, and finally included 1,064 patients with a first physical abuse event during the study period (Fig. 2), of which 818 (76.9%) were male and 246 (23.1%) were female. The overall incidence of physical abuse during MPH treatment was 3.53 per 1,000 patient-years. The mean (standard deviation) age at the start of the observation was 5.53 (1.57) years, and the mean duration of follow-up per participant was 8.48 (3.29) years. The mean MPH exposure was 2.59 (2.25) years per participant. Of the 1,064 patients with physical abuse, 867 (81.5%) had a recorded ADHD diagnosis. Broader psychiatric comorbidities for these patients are reported in Supplementary Table 1. Of the 1,064 first physical abuse events, 225 occurred during the MPH treatment and 839 occurred during the non-medicated period (Table 1). The median age of the index physical abuse event was 8.6 years (interquartile range, IQR, 7.0–10.7 years) (Extended Data Fig. 1). The crude incidences of physical abuse events in different risk windows are summarized in Table 2. There were three deaths during the study period.
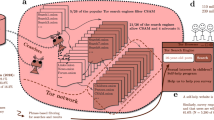
a, Outcome of incident physical abuse. b, First recurrent physical abuse. This is a hypothetical figure for an individual. *Incident case can occur at any time after or even before the observation start date. **New observation start date set as 1 January 2001, the child’s 5th birthday, day 7 after the incident abuse or the discharge date of the incident abuse hospitalization episode, whichever was later. ***Recurrent case can occur at any time during the newly defined observation period.
Risk of incident abuse
After adjusting for age, season and the COVID-19 stringency index, there was an increased risk of physical abuse during the 90 d period before MPH initiation (incidence rate ratio, IRR, 4.49; 95% confidence interval, CI, 3.76–5.36). The IRR was similar to baseline levels during the first 90 d of MPH treatment (IRR, 0.90; 95% CI, 0.63–1.29) and was lower than the baseline levels during prolonged MPH treatment (IRR, 0.63; 95% CI, 0.51–0.77) (Table 2). When directly compared with the pre-exposure period (Fig. 3), the risk of physical abuse was lowered by 80% during the first 90 d of MPH treatment (IRR, 0.20; 95% CI, 0.14–0.29) and by 86% in the subsequent MPH treatment period (IRR, 0.14; 95% CI, 0.11–0.18).
aICD-9-CM: 580–599. bICD-9-CM: 370, 373, 363.0–363.2, 372.0–372.3. This figure visually summarizes the IRRs and 95% CIs. *All estimates were adjusted for age in one-year age band and seasonal effect, and COVID-19 stringency index using conditional Poisson regression, with a significance level of 5% for a two-tailed test. The error bars represent the corresponding 95% CIs of the IRRs. #In 100 patient-years.
Risk of first recurrent physical abuse
A similar association was observed between MPH and recurrent physical abuse. We identified 219 children who had their first recurrent physical abuse events during the observation period, with 61 events occurring during the MPH treatment period (Table 2). Compared with the non-medicated period, we found an increased risk of recurrent physical abuse during the 90 d period before MPH initiation (IRR, 1.77; 95% CI, 1.08–2.90), slightly lower risk during the first 90 d of MPH treatment (IRR, 0.41; 95% CI, 0.16–1.03) and no differences during prolonged MPH treatment (IRR, 0.78; 95% CI, 0.51–1.20) (Table 2). The risk of recurrent physical abuse during the first 90 d of MPH treatment and during the subsequent MPH treatment period was lowered by 77% (IRR, 0.23; 95% CI, 0.09–0.61) and 56% (IRR, 0.44; 95% CI, 0.25–0.77), respectively, compared with the pre-exposure period (Fig. 3).
Sensitivity and negative control analyses
The sex-stratified results showed a similar pattern to the main analysis (Supplementary Table 2). No association was found in any risk window in the negative control analysis using diseases of the urinary system and eye infection as outcomes (Table 2, Fig. 3 and Supplementary Table 2). We also found a lower risk of physical abuse during the 90 d post-treatment period. After adjusting for additional time-varying factors, other psychiatric comorbidities and/or other psychotropic medication use, a decreased risk of physical abuse after treatment initiation compared with the short period before medication use remained (IRRs ranging from 0.14 to 0.20). For all types of child abuse and neglect (n = 1,123) the results were similar to the outcomes for the main analysis of physical abuse. Other sensitivity analyses showed similar results (Fig. 4 and Supplementary Table 3). The E-value analysis indicated that results were unlikely to be affected by unmeasured confounding factors (Supplementary Discussion).
Discussion
The incidence of physical abuse was 4.5 times higher during the 90 d period before the start of treatment with MPH, returned to a similar risk in the first 90 d of MPH treatment and decreased by around one-third during the subsequent treatment period compared with the other non-medicated period (reference period).
After initiation of MPH treatment, it is possible that the initial reduction in recorded child physical abuse is related to reduced contact with parents because of the disclosure or close monitoring by social care, education or healthcare professionals, rather than to the direct beneficial effects of MPH. However, we observed that the IRR of child physical abuse was lower with a longer duration of use (>90 d) beyond the initial separation period. Therefore, it is unlikely that our results are fully explained by the increased monitoring associated with the initiation of MPH.
To further examine the sensitivity of our results to any changes in surveillance of child physical abuse, we conducted an analysis to study the risk of first recurrent physical abuse events corresponding to the use of MPH. The results follow a similar pattern of risk to that observed in the main analysis. This subgroup analysis showed that, even in a group of children who were already under close surveillance due to previous history of abuse, there was still a higher risk of physical abuse directly before MPH initiation but not in other risk periods. Such findings further support the association between MPH treatment and lower risk of physical abuse beyond the potential effects of close surveillance by professionals.
Several factors may explain why the period before the initiation of MPH treatment coincides with higher incidence of physical abuse. The highest risk of physical abuse in children during the pretreatment period might be a trigger for screening, diagnosis and treatment engagement of ADHD. In clinical practice, the initiation of new medication often occurs when there are specific concerns about the child’s mental and physical health. The decision to start MPH treatment in these patients may be in response to changes in behavioural or related psychiatric problems associated with physical abuse events. In contrast, the negative control analysis using diseases of the urinary system and eye infection, which should not be associated with ADHD or MPH treatments, did not show the same risk patterns as in the primary or subgroup analyses. Furthermore, the robustness of the primary analyses was supported by the sensitivity analyses.
Previous studies have demonstrated that, when ADHD symptoms in children are reduced by medication, there is an associated reduction in parental stress, less negative parenting and improved parent–child relationships33,35,36, which can potentially reduce the risk of physical abuse. However, it is important to proactively address ways in which to bolster support for parents, for example, via parental training programmes37,38,39,40 to improve the quality of parenting and reduce parental stress levels, as well as incorporating resources via schools17,18 and community centres19.
The availability of psychosocial interventions is inconsistent and, if available, they are mostly focused on addressing children’s symptoms with a behavioural training approach21,41,42. It is widely acknowledged that there is a very limited availability of evidence-based behavioural parent training programmes in the publicly funded healthcare system in Hong Kong for parents of children with ADHD. Two previous research studies have shown that parenting stress ratings remained unchanged after attending a local parental training programme, ‘Multifamily Therapy for Children With ADHD’42,43.
Despite multiple studies on MPH incorporating real-world outcomes, there have been few examinations of potential effect on the risk of child physical abuse. Studies from Scandinavia and Hong Kong have reported that MPH not only improves ADHD symptoms31, but is also associated with lower risks of other more distal outcomes such as motor vehicle accidents44, traumatic brain injury45, substance use disorder46, criminality47 and more general functional outcomes48. A previous network meta-analysis31 has demonstrated that MPH can reduce ADHD core symptoms across different age groups. Therefore, it might be reasonable to assume that the effects of MPH on the risk of physical abuse could also be observed in other age groups.
It is worthwhile to note that, despite a well established safety profile, some children treated with MPH can experience side effects such as increased blood pressure, increased heart rate and poor appetite49,50. However, research has shown that these side effects are unlikely to be severe and can often be managed by changing the dose, timing of dose and/or formula of the medication; clinicians and parents should monitor if they continue to cause problems to the children and look at other options if necessary51,52.
Limitations
There are several limitations to our study. First, the data source—Clinical Data Analysis and Reporting System (CDARS)—used in this study only includes information from public hospitals or clinics, without cases seen by private medical practitioners. However, in Hong Kong, the public sector is the main provider of specialist care and there are only a few private child psychiatrists27,53,54. Therefore, the vast majority of patients receiving MPH should be included in this study. Another limitation is that our cohort included only clinically referred patients who had sufficiently severe ADHD symptoms to receive MPH treatment. Therefore, our cohort may have a higher baseline risk of physical abuse compared with non-medicated patients. However, since we applied the within-individual design—SCCS—the individual baseline risk should not affect our results and conclusion. Similarly, identifying child physical abuse cases using hospital records may result in an underestimation of numbers, as only severe cases would be hospitalized. Again, due to the nature of the SCCS design, this would only affect statistical power rather than the interpretation of the result. Nevertheless, our results may not be applicable to children with mild ADHD who do not require pharmacological treatment. Additionally, as we included a comparatively long follow-up period, time-varying confounding factors might influence study results. However, in addition to the adjustment of major time-varying confounders, age and seasons, we conducted sensitivity analyses by adjusting for various time-varying confounders including psychiatric comorbidities and medication use that did not yield any major changes in the results. Finally, the E values in our sensitivity analysis indicated that our estimates could only be explained by such confounding effects if they were associated with both treatment and outcome at a magnitude of 9.47–13.77-fold, respectively, in addition to the confounders already addressed. Any residual confounding is unlikely to exert such powerful effects on our study conclusions.
Conclusion
Results from the main analysis and sensitivity analyses are consistent with our hypothesis that the use of MPH for ADHD is associated with a lower risk of physical abuse. Medications could play an important role as part of the support package for families raising children with ADHD, creating a positive effect that could last during treatment and beyond.
Methods
Data source
This study used data from CDARS, the electronic health records database developed by the Hong Kong Hospital Authority, a statutory body that manages all public hospitals and their ambulatory clinics in Hong Kong. The Hospital Authority health services are available to all Hong Kong residents (over 7.4 million people) and cover about 80% of all hospital admissions in Hong Kong55. Data from CDARS have been validated and used in a variety of pharmacoepidemiological studies54,56,57. Patient-specific data in CDARS include diagnoses, hospital admissions/discharges and prescription/dispensing information58. The study protocol was approved by the institutional review board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (reference no. UW 12–136). This was a pharmacoepidemiology study without patient contact and therefore informed consent was exempted.
SCCS design
We used an SCCS design59,60 to investigate the association between MPH use and child physical abuse. We have previously used SCCS to investigate the effects of MPH on various conditions27,53,54,57, in which patients served as their own controls and comparisons were made within individuals who experienced both the outcome and the exposure of interest59. IRRs were derived by comparing the rate of events during medication exposure with the rate during non-medicated periods using conditional Poisson regression. The major advantage of SCCS design over conventional study designs (for example cohort design) is that it implicitly controls for measured and unmeasured time-invariant confounders that vary between individuals, such as genetic factors, socioeconomic status and underlying disease severity59. Furthermore, we adjusted for time-varying factors, including age, season and the COVID-19 stringency index in the main analysis as well as other mental disorders and other psychotropic medications in the sensitivity analyses, which potentially affect MPH prescribing34,61. As the COVID-19 pandemic has severely affected daily life, the COVID-19 stringency index62, an indicator that reflects the toughness of various regions in response to COVID-19, with a higher index representing a more stringent response measure, was further adjusted as another time-varying factor. Within-individual approaches such as the SCCS design have become a common methodology in ADHD medication research over the past decade63. Details of the SCCS assumptions relevant to the current study are available in Supplementary Note 1, Extended Data Fig. 2 and Supplementary Table 4.
Case identification
Children aged 5–16 years who had received at least one MPH prescription and experienced an incident physical abuse event during the study period (1 January 2001 to 31 December 2020) were identified from CDARS. The outcomes of physical abuse were identified using the ICD-9-CM diagnostic codes: E967 (perpetrator of child and adult abuse, external causes of injury and poisoning) and 995.54 (child physical abuse). Child physical abuse is strictly defined as any act of commission that endangers or impairs the physical health and development of a child64. While under the care of the Hong Kong Hospital Authority, for every case admitted for suspected child abuse, a multidisciplinary case conference is held within 10 working days after the case report to investigate the results and evidence from different parties within the context of the child and family to confirm case details and plan intervention64. The ICD-9-CM code of physical abuse will only be added after the decision is made by the conference as a statutory requirement, and therefore the recorded diagnosis extracted from CDARS has very high validity. We included all MPH users, regardless of whether they had a record of ADHD diagnosis, because MPH is almost exclusively used in children for the management of ADHD in Hong Kong. MPH is currently not licensed for narcolepsy in Hong Kong for children and the incidence of narcolepsy is between 25 and 50 per 100,000 people65. Hence MPH is very unlikely to be used for narcolepsy. Furthermore, the aim of this study was to evaluate the association between MPH use and risk of physical abuse, and such a definition for MPH exposure had been used in previous studies66,67. Atomoxetine was the only other licensed treatment for ADHD in Hong Kong and use was minimal during the study period34; thus observation periods were censored by atomoxetine treatment to avoid coprescribing situations that would affect the comparisons.
We commenced follow-up at 5 years of age as MPH is not recommended for children below this age68. Individual observation periods began on 1 January 2001 or on the child’s fifth birthday, whichever was later, and ended on 31 December 2020, on the child’s 17th birthday or on the registered date of death, whichever was earliest.
Exposures and outcomes
For each study subject, all MPH prescriptions and abuse events were identified. Exposure periods were defined as the time of receiving MPH, and the duration between prescription start and end dates was recorded in CDARS for each prescription as a time-varying variable. More than 99% of the prescriptions recorded start and end dates. Daily dosage and the quantity prescribed were used to determine the duration of treatment if the prescription end date was not available. Median values for the exposure duration were imputed when the above information was missing. We divided the patient-time into four discrete windows: (1) 90 d before the first MPH exposure (pre-exposure period), (2) first 90 d of MPH use, (3) subsequent MPH use (>90 d) and (4) baseline period (the patient-time that falls outside the three previously stated categories, including patient-time before pre-exposure and after completing MPH). The corresponding date of the abuse was identified as the event date. The study design and timeline for a single hypothetical participant are illustrated in Fig. 1a.
Statistical analysis
Risk of incident abuse
The association between MPH use and childhood physical abuse was calculated by comparing the rates of physical abuse during exposure and non-exposure periods. Adjusted IRRs and the corresponding 95% CIs were calculated and adjusted by age in 1-year bands, seasonal effects and COVID-19 stringency. A 90 d pre-exposure period was added to account for the possibility that a recent physical abuse event may affect the likelihood of MPH treatment, which in turn may introduce bias into the risk estimate during treatment. We separated the first 90 d of MPH use to allow detection of any temporary changes in the risk of physical abuse; we also compared the rate of physical abuse between the pre-exposure period and MPH-exposed periods. Stratified analyses were conducted to evaluate the effects by sex.
Risk of first recurrent physical abuse
To evaluate the risk of subsequent physical abuse during MPH treatment in those children who were already under vigilant surveillance after the physical abuse event, we further investigated the association between MPH and the risk of first recurrent physical abuse. Children with a history of physical abuse where the first recurrent physical abuse events were recorded during the individual’s observational period were included. The follow-up period began on 1 January 2001, the child’s fifth birthday, day 7 after the incident physical abuse or the discharge date of the incident physical abuse hospitalization episode, whichever was latest, and the IRR of the subsequent physical abuse was evaluated during the different exposure windows using the same definition and analysis as outlined above (Fig. 1b).
Sensitivity and negative control analyses
Sensitivity analyses were conducted to test the validity and robustness of the initial study results: (1) different drug non-adherence scenarios, (2) redefining the start of the observation as the latest of the first observed date of ADHD diagnosis/MPH treatment, (3) restriction to incident users of MPH, (4) >120 d of MPH exposure, (5) restricting the study period to 31 December 2019 to reduce the impact of COVID-19 on the results, (6) adding a 90 d post-exposure period, (7) adjusting for other comorbid psychiatric disorders, (8) adjusting for both other comorbid psychiatric disorders and other psychotropic medication use, (9) including all types of child abuse and neglect as the outcome, (10) two negative controls using diseases of the urinary system (ICD-9-CM: 580–599) and eye infection (ICD-9-CM: 370, 373, 363.0–363.2, 372.0–372.3) as alternative outcomes and (11) further assessment of the potential impact of any unmeasured confounders by computing the E value69. Detailed descriptions of these analyses are available in Supplementary Note 2.
A two-tailed P value of 0.05 was used in all statistical analyses. R4.0.3 was used for data manipulation and analyses. We have reported the results according to the Strengthening the Reporting of Observational Studies in Epidemiology Statement. According to the formula suggested by Musonda et al.70, our sample size of 1,064 is able to detect an IRR of 0.826 at 5% of significance and 80% power (detailed information on the formula and the exact calculation is shown in Supplementary Equation).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data cannot be shared as the data custodian—Hong Kong Hospital Authority—did not give permission due to patient confidentiality and privacy concerns. According to the conditions laid down by Hong Kong Hospital Authority, only local academic institutions, government departments or non-governmental organizations may apply for access to data through the Hospital Authority data sharing portal (https://www3.ha.org.hk/data).
Code availability
All relevant analysis codes are available online (https://github.com/legao513/child-abuse).
References
WHO Global Status Report on Violence Prevention 2014 (WHO Media Centre, 2014); https://www.who.int/publications/i/item/9789241564793
WHO Fact Sheet on Child Maltreatment (WHO Media Centre, 2022); https://www.who.int/news-room/fact-sheets/detail/child-maltreatment
Arango, C. et al. Risk and protective factors for mental disorders beyond genetics: an evidence-based atlas. World Psychiatry 20, 417–436 (2021).
Ip, P. et al. Child maltreatment hospitalisations in Hong Kong: incidence rate and seasonal pattern. Arch. Dis. Child. 101, 1107–1113 (2016).
Mandell, D. S., Walrath, C. M., Manteuffel, B., Sgro, G. & Pinto-Martin, J. A. The prevalence and correlates of abuse among children with autism served in comprehensive community-based mental health settings. Child Abus. Negl. 29, 1359–1372 (2005).
Hadianfard, H. Child abuse in group of children with attention deficit–hyperactivity disorder in comparison with normal children. Int. J. Community Based Nurs. Midwifery 2, 77–84 (2014).
Ford, J. D. et al. Child maltreatment, other trauma exposure, and posttraumatic symptomatology among children with oppositional defiant and attention deficit hyperactivity disorders. Child Maltreat. 5, 205–217 (2000).
Sari Gokten, E., Saday Duman, N., Soylu, N. & Uzun, M. E. Effects of attention-deficit/hyperactivity disorder on child abuse and neglect. Child Abus. Negl. 62, 1–9 (2016).
Schilling, S. & Christian, C. W. Child physical abuse and neglect. Child Adolesc. Psychiatr. Clin. N. Am. 23, 309–319 (2014).
Faraone, S. V. et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 128, 789–818 (2021).
Demontis, D. et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 51, 63–75 (2019).
Leitch, S. et al. Experience of stress in parents of children with ADHD: a qualitative study. Int. J. Qual. Stud. Health Well-being 14, 1690091 (2019).
Dykens, E. M. Family adjustment and interventions in neurodevelopmental disorders. Curr. Opin. Psychiatry 28, 121–126 (2015).
Brockington, I. et al. WPA guidance on the protection and promotion of mental health in children of persons with severe mental disorders. World Psychiatry 10, 93–102 (2011).
Coates, J., Taylor, J. A. & Sayal, K. Parenting interventions for ADHD: a systematic literature review and meta-analysis. J. Atten. Disord. 19, 831–843 (2015).
Lo, H. H. M. et al. The effects of family-based mindfulness intervention on ADHD symptomology in young children and their parents: a randomized control trial. J. Atten. Disord. 24, 667–680 (2020).
DuPaul, G. J., Gormley, M. J. & Laracy, S. D. School-based interventions for elementary school students with ADHD. Child Adolesc. Psychiatr. Clin. N. Am. 23, 687–697 (2014).
Power, T. J. et al. A family–school intervention for children with ADHD: results of a randomized clinical trial. J. Consult. Clin. Psychol. 80, 611–623 (2012).
Epstein, J. N. et al. Impact of a web-portal intervention on community ADHD care and outcomes. Pediatrics 138, e20154240 (2016).
Au, A. et al. The efficacy of a group Triple P (Positive Parenting Program) for Chinese parents with a child diagnosed with ADHD in Hong Kong: a pilot randomised controlled study. Aust. Psychol. 49, 151–162 (2014).
Wong, W. C. & Wong, I. Y. F. Burden and coping strategies of parents of children with attention deficit/ hyperactivity disorder in Hong Kong: a qualitative study. Nurs. Open 8, 3452–3460 (2021).
Crandell, J. L., Sandelowski, M., Leeman, J., Havill, N. L. & Knafl, K. Parenting behaviors and the well-being of children with a chronic physical condition. Fam. Syst. Health 36, 45–61 (2018).
Tamura, K., Morrison, J. & Pikhart, H. Children’s behavioural problems and its associations with socioeconomic position and early parenting environment: findings from the UK Millennium Cohort Study. Epidemiol. Psychiatr. Sci. 29, e155 (2020).
Pfiffner, L. J. & Haack, L. M. Behavior management for school-aged children with ADHD. Child Adolesc. Psychiatr. Clin. N. Am. 23, 731–746 (2014).
Cortese, S. Pharmacologic treatment of attention deficit–hyperactivity disorder. N. Engl. J. Med. 383, 1050–1056 (2020).
Cortese, S. et al. Starting ADHD medications during the COVID-19 pandemic: recommendations from the European ADHD Guidelines Group. Lancet Child Adolesc. Health 4, e15 (2020).
Man, K. K. et al. Methylphenidate and the risk of trauma. Pediatrics 135, 40–48 (2015).
Man, K. K. C. et al. Effectiveness of pharmacological treatment for attention-deficit/hyperactivity disorder on physical injuries: a systematic review and meta-analysis of observational studies. CNS Drugs 31, 1043–1055 (2017).
Ghirardi, L. et al. Use of medication for attention-deficit/hyperactivity disorder and risk of unintentional injuries in children and adolescents with co-occurring neurodevelopmental disorders. J. Child Psychol. Psychiatry 61, 140–147 (2020).
Solmi, M. et al. Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry 19, 214–232 (2020).
Cortese, S. et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 5, 727–738 (2018).
Correll, C. U. et al. Efficacy and acceptability of pharmacological, psychosocial, and brain stimulation interventions in children and adolescents with mental disorders: an umbrella review. World Psychiatry 20, 244–275 (2021).
Levi-Shachar, O. et al. The effect of methylphenidate on social cognition and oxytocin in children with attention deficit hyperactivity disorder. Neuropsychopharmacology 45, 367–373 (2020).
Raman, S. R. et al. Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. Lancet Psychiatry 5, 824–835 (2018).
Graziano, P. A., McNamara, J. P., Geffken, G. R. & Reid, A. Severity of children’s ADHD symptoms and parenting stress: a multiple mediation model of self-regulation. J. Abnorm. Child Psychol. 39, 1073–1083 (2011).
Theule, J., Wiener, J., Tannock, R. & Jenkins, J. M. Parenting stress in families of children with ADHD: a meta-analysis. J. Emot. Behav. Disord. 21, 3–17 (2013).
Ciesielski, H. A., Loren, R. E. A. & Tamm, L. Behavioral parent training for ADHD reduces situational severity of child noncompliance and related parental stress. J. Atten. Disord. 24, 758–767 (2020).
Larsen, L. B. et al. Effect of parent training on health-related quality of life in preschool children with attention-deficit/hyperactivity disorder: a secondary analysis of data from a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 60, 734–744 e733 (2021).
Mah, J. W. T., Murray, C., Locke, J. & Carbert, N. Mindfulness-enhanced behavioral parent training for clinic-referred families of children with ADHD: a randomized controlled trial. J. Atten. Disord. 25, 1765–1777 (2021).
Zwi, M., Jones, H., Thorgaard, C., York, A. & Dennis, J. A. Parent training interventions for Attention Deficit Hyperactivity Disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst. Rev. 12, CD003018 (2011).
Cheung, K. K. et al. Experiences of adolescents and young adults with ADHD in Hong Kong: treatment services and clinical management. BMC Psychiatry 15, 95 (2015).
Lai, K. Y. C., Ma, J. L. C. & Xia, L. L. L. Multifamily therapy for children with ADHD in Hong Kong: the different impacts on fathers and mothers. J. Atten. Disord. 25, 115–123 (2021).
Ma, J. L. C., Lai, K. Y. C. & Xia, L. L. L. Treatment efficacy of multiple family therapy for Chinese families of children with attention deficit hyperactivity disorder. Fam. Process 57, 399–414 (2018).
Lin, Y. C. et al. Stimulants associated with reduced risk of hospitalization for motor vehicle accident injury in patients with obstructive sleep apnea—a nationwide cohort study. BMC Pulm. Med. 20, 28 (2020).
Liao, Y. T. et al. Dosage of methylphenidate and traumatic brain injury in ADHD: a population-based study in Taiwan. Eur. Child Adolesc. Psychiatry 27, 279–288 (2018).
Chang, Z. et al. Stimulant ADHD medication and risk for substance abuse. J. Child Psychol. Psychiatry 55, 878–885 (2014).
Lichtenstein, P. et al. Medication for attention deficit—hyperactivity disorder and criminality. N. Engl. J. Med. 367, 2006–2014 (2012).
Boland, H. et al. A literature review and meta-analysis on the effects of ADHD medications on functional outcomes. J. Psychiatr. Res. 123, 21–30 (2020).
Buitelaar, J. K. et al. Long-term methylphenidate exposure and 24-hours blood pressure and left ventricular mass in adolescents and young adults with attention deficit hyperactivity disorder. Eur. Neuropsychopharmacol. 64, 63–71 (2022).
Hennissen, L. et al. Cardiovascular effects of stimulant and non-stimulant medication for children and adolescents with ADHD: a systematic review and meta-analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs 31, 199–215 (2017).
Carucci, S. et al. Long term methylphenidate exposure and growth in children and adolescents with ADHD. A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 120, 509–525 (2021).
Coghill, D. et al. The management of ADHD in children and adolescents: bringing evidence to the clinic: perspective from the European ADHD Guidelines Group (EAGG). Eur. Child Adolesc. Psychiatry https://doi.org/10.1007/s00787-021-01871-x (2021).
Man, K. K. C. et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl. Psychiatry 6, e956 (2016).
Man, K. K. C. et al. Association of risk of suicide attempts with methylphenidate treatment. JAMA Psychiatry 74, 1048–1055 (2017).
Leung, G. M. et al. The ecology of health care in Hong Kong. Soc. Sci. Med. 61, 577–590 (2005).
Lau, W. C. Y. et al. Association between treatment with apixaban, dabigatran, rivaroxaban, or warfarin and risk for osteoporotic fractures among patients with atrial fibrillation: a population-based cohort study. Ann. Intern. Med. 173, 1–9 (2020).
Man, K. K. C. et al. Association between methylphenidate treatment and risk of seizure: a population-based, self-controlled case-series study. Lancet Child Adolesc. Health 4, 435–443 (2020).
HAHO/ITD Clinical Data Analysis & Reporting System (CDARS) User’s Manual 3 (Hong Kong, 2003).
Whitaker, H. J., Farrington, C. P., Spiessens, B. & Musonda, P. Tutorial in biostatistics: the self-controlled case series method. Stat. Med. 25, 1768–1797 (2006).
Petersen, I., Douglas, I. & Whitaker, H. Self controlled case series methods: an alternative to standard epidemiological study designs. Br. Med. J. 354, i4515 (2016).
Suhail, K. & Cochrane, R. Seasonal variations in hospital admissions for affective disorders by gender and ethnicity. Soc. Psychiatry Psychiatr. Epidemiol. 33, 211–217 (1998).
Hale, T. et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 5, 529–538 (2021).
Chang, Z. et al. Risks and benefits of attention-deficit/hyperactivity disorder medication on behavioral and neuropsychiatric outcomes: a qualitative review of pharmacoepidemiology studies using linked prescription databases. Biol. Psychiatry 86, 335–343 (2019).
Protecting Children from Maltreatment—Procedural Guide for Multi-disciplinary Co-operation (Revised 2020) (Social Welfare Department of the Hong Kong Government, 2020); https://www.swd.gov.hk/en/index/site_pubsvc/page_family/sub_fcwprocedure/id_1447/
Longstreth, W. T. Jr., Koepsell, T. D., Ton, T. G., Hendrickson, A. F. & van Belle, G. The epidemiology of narcolepsy. Sleep 30, 13–26 (2007).
Lo, C. K. et al. Linking healthcare and social service databases to study the epidemiology of child maltreatment and associated health problems: Hong Kong’s experience. J. Pediatr. 202, 291–299.e1 (2018).
Lo, C. K. M. et al. Prevalence of child maltreatment and its association with parenting style: a population study in Hong Kong. Int. J. Environ. Res. Public Health 16, 1130 (2019).
National Guideline Centre (UK) Attention Deficit Hyperactivity Disorder: Diagnosis and Management (National Institute for Health and Care Excellence, 2018).
VanderWeele, T. J. & Ding, P. Sensitivity analysis in observational research: introducing the E-value. Ann. Intern. Med. 167, 268–274 (2017).
Musonda, P., Farrington, C. P. & Whitaker, H. J. Sample sizes for self-controlled case series studies. Stat. Med. 25, 2618–2631 (2006).
Acknowledgements
This study is funded by the Hong Kong Research Grant Council Collaborative Research Fund (grant number C7009-19GF). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We also thank L. Lam for proofreading the manuscript and acknowledge the support from European Commission Framework Horizon 2020 funding.
Author information
Authors and Affiliations
Contributions
K.K.C.M., L.G., P.I. and I.C.K.W. designed the study. K.K.C.M., L.G., W.C.Y.L. and M.F. extracted the data, conducted the statistical analyses and cross-checked the analyses. K.K.C.M. and L.G. wrote the first draft of the manuscript. D.C. and P.I. provided critical input to the interpretation of the analyses. P.I. and I.C.K.W. are the principal investigators, providing resources and supervising all aspects of the project. All authors contributed to the interpretation of the analyses and the review and editing of the manuscript, and approved the submission of the final version.
Corresponding authors
Ethics declarations
Competing interests
K.K.C.M. is the recipient of the CW Maplethorpe Fellowship, and reports grants from the National Institute for Health Research, UK, the European Commission Horizon 2020 Framework, EU, and the Research Grant Council, Hong Kong, and personal fees from IQVIA Ltd., unrelated to the submitted work. W.C.Y.L. reports a research grant from AIR@InnoHK administered by the Innovation and Technology Commission outside the submitted work. D.C. reports personal fees from Shire/Takeda, personal fees from Medice, personal fees from Servier and personal fees from Oxford University Press, outside the submitted work. E.W.C. reports grants from the Research Grants Council (RGC, Hong Kong), grants from the Narcotics Division of the Security Bureau of the Government of the Hong Kong SAR, grants from the Research Fund Secretariat of the Food and Health Bureau, grants from the National Natural Science Fund of China, grants from the National Health and Medical Research Council (NHMRC, Australia), grants from Wellcome Trust, grants from Bayer, grants from Bristol-Myers Squibb, grants from Pfizer, grants from Janssen, grants from Amgen, grants from Takeda and personal fees from Hospital Authority of Hong Kong, outside the submitted work. X.L. received grants from the Health and Medical Research Fund, Food and Health Bureau of the Government of Hong Kong, the Research Grants Council Early Career Scheme (RGC/ECS), Janssen, Pfizer, internal funding from the University of Hong Kong and a consultancy fee from Merck Sharp & Dohme and Pfizer, unrelated to this work. A.Y.L.C. reports a grant from the Innovation and Technology Commission of the Hong Kong Special Administration Region Government for salary at the University of Hong Kong. Y.K.W. reports grants from the Research Grant Council General Research Fund, grants from the Health and Medical Research Fund, personal fees from Eisai Inc., personal fees from Eisai Co., Ltd, and other fees from Lundbeck HK Limited, outside the submitted work. I.C.K.W. reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, the Hong Kong Health and Medical Research Fund, the National Institute for Health Research in England, the European Commission and the National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and Medice in the previous 3 years. All other authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Histogram of age at the incident physical abuse.
Extended Data Fig. 2 Time from the first physical abuse case to MPH initiation.
Abbreviation: MPH, methylphenidate.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Discussion, Notes 1 and 2 and equation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Man, K.K.C., Gao, L., Lau, W.C.Y. et al. Attention deficit hyperactivity disorder, physical abuse and methylphenidate treatment in children. Nat. Mental Health 1, 66–75 (2023). https://doi.org/10.1038/s44220-022-00008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-022-00008-6