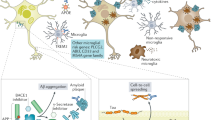
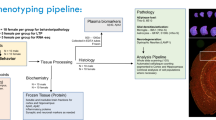
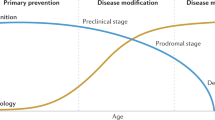
Abstract
Alzheimer’s disease (AD) and other, less prevalent dementias are complex age-related disorders that exhibit multiple etiologies. Over the past decades, animal models have provided pathomechanistic insight and evaluated countless therapeutics; however, their value is increasingly being questioned due to the long history of drug failures. In this Perspective, we dispute this criticism. First, the utility of the models is limited by their design, as neither the etiology of AD nor whether interventions should occur at a cellular or network level is fully understood. Second, we highlight unmet challenges shared between animals and humans, including impeded drug transport across the blood–brain barrier, limiting effective treatment development. Third, alternative human-derived models also suffer from the limitations mentioned above and can only act as complementary resources. Finally, age being the strongest AD risk factor should be better incorporated into the experimental design, with computational modeling expected to enhance the value of animal models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 16, 391–460 (2020).
Sengupta, U. & Kayed, R. Amyloid β, tau, and α-synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 214, 102270 (2022).
Jellinger, K. A. & Attems, J. Challenges of multimorbidity of the aging brain: a critical update. J. Neural Transm. 122, 505–521 (2015).
Tanne, J. H. Aduhelm: approval of Alzheimer’s drug was highly unorthodox, finds report. BMJ 380, 6 (2023).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Perry, R. J. & Hodges, J. R. Attention and executive deficits in Alzheimer’s disease. A critical review. Brain 122, 383–404 (1999).
Polanco, J. C. et al. Amyloid-β and tau complexity—towards improved biomarkers and targeted therapies. Nat. Rev. Neurol. 14, 22–39 (2018).
Götz, J. & Ittner, L. M. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat. Rev. Neurosci. 9, 532–544 (2008).
Dujardin, S., Colin, M. & Buee, L. Invited review: animal models of tauopathies and their implications for research/translation into the clinic. Neuropathol. Appl. Neurobiol. 41, 59–80 (2015).
Jankowsky, J. L. & Zheng, H. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener. 12, 89 (2017).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6, 916–919 (2000).
de la Torre, J. C. & Mussivand, T. Can disturbed brain microcirculation cause Alzheimer’s disease? Neurol. Res. 15, 146–153 (1993).
Grimm, A. & Eckert, A. Brain aging and neurodegeneration: from a mitochondrial point of view. J. Neurochem. 143, 418–431 (2017).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015).
Wirths, O. & Zampar, S. Neuron loss in Alzheimer’s disease: translation in transgenic mouse models. Int. J. Mol. Sci. 21, 8144 (2020).
Eimer, W. A. & Vassar, R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and caspase-3 activation. Mol. Neurodegener. 8, 2 (2013).
van Eersel, J. et al. Early-onset axonal pathology in a novel P301S-tau transgenic mouse model of frontotemporal lobar degeneration. Neuropathol. Appl. Neurobiol. 41, 906–925 (2015).
Hatch, R. J., Wei, Y., Xia, D. & Götz, J. Hyperphosphorylated tau causes reduced hippocampal CA1 excitability by relocating the axon initial segment. Acta Neuropathol. 133, 717–730 (2017).
Roy, D. S. et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531, 508–512 (2016).
Oh, H., Razlighi, Q. R. & Stern, Y. Multiple pathways of reserve simultaneously present in cognitively normal older adults. Neurology 90, e197–e205 (2018).
Stern, Y., Barnes, C. A., Grady, C., Jones, R. N. & Raz, N. Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol. Aging 83, 124–129 (2019).
Ammassari-Teule, M. Neural compensation in presymptomatic hAPP mouse models of Alzheimer’s disease. Learn. Mem. 27, 390–394 (2020).
Morrone, C. D., Lai, A. Y., Bishay, J., Hill, M. E. & McLaurin, J. Parvalbumin neuroplasticity compensates for somatostatin impairment, maintaining cognitive function in Alzheimer’s disease. Transl. Neurodegener. 11, 26 (2022).
Probst, A. et al. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 99, 469–481 (2000).
Allen, B. et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 22, 9340–9351 (2002).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
Götz, J., Bodea, L. G. & Goedert, M. Rodent models for Alzheimer disease. Nat. Rev. Neurosci. 19, 583–598 (2018).
Saito, T. et al. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 17, 661–663 (2014).
Sato, K. et al. A third-generation mouse model of Alzheimer’s disease shows early and increased cored plaque pathology composed of wild-type human amyloid β peptide. J. Biol. Chem. 297, 101004 (2021).
Xia, D., Gutmann, J. M. & Götz, J. Mobility and subcellular localization of endogenous, gene-edited tau differs from that of over-expressed human wild-type and P301L mutant tau. Sci. Rep. 6, 29074 (2016).
Bjorkhem, I. et al. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J. Lipid Res. 39, 1594–1600 (1998).
Huynh, T. V. et al. Lack of hepatic ApoE does not influence early Aβ deposition: observations from a new APOE knock-in model. Mol. Neurodegener. 14, 37 (2019).
Barisano, G. et al. A ‘multi-omics’ analysis of blood–brain barrier and synaptic dysfunction in APOE4 mice. J. Exp. Med. 219, e20221137 (2022).
Shi, Y. et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527 (2017).
Bales, K. R. et al. Human APOE isoform-dependent effects on brain β-amyloid levels in PDAPP transgenic mice. J. Neurosci. 29, 6771–6779 (2009).
Castellano, J. M. et al. Human ApoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3, 89ra57 (2011).
Hou, J., Chen, Y., Grajales-Reyes, G. & Colonna, M. TREM2 dependent and independent functions of microglia in Alzheimer’s disease. Mol. Neurodegener. 17, 84 (2022).
Fitz, N. F. et al. Trem2 deficiency differentially affects phenotype and transcriptome of human APOE3 and APOE4 mice. Mol. Neurodegener. 15, 41 (2020).
Humpel, C. Organotypic brain slice cultures: a review. Neuroscience 305, 86–98 (2015).
Fath, T., Ke, Y. D., Gunning, P., Götz, J. & Ittner, L. M. Primary support cultures of hippocampal and substantia nigra neurons. Nat. Protoc. 4, 78–85 (2009).
Pir, G. J., Choudhary, B. & Mandelkow, E. Caenorhabditis elegans models of tauopathy. FASEB J. 31, 5137–5148 (2017).
Griffin, E. F., Caldwell, K. A. & Caldwell, G. A. Genetic and pharmacological discovery for Alzheimer’s disease using Caenorhabditis elegans. ACS Chem. Neurosci. 8, 2596–2606 (2017).
Asadzadeh, J. et al. Retromer deficiency in tauopathy models enhances the truncation and toxicity of tau. Nat. Commun. 13, 5049 (2022).
Saleem, S. & Kannan, R. R. Zebrafish: an emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 4, 45 (2018).
Pang, K. et al. An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res. 32, 157–175 (2022).
Hurley, M. J. et al. Genome sequencing variations in the Octodon degus, an unconventional natural model of aging and Alzheimer’s disease. Front. Aging Neurosci. 14, 894994 (2022).
Reid, S. J. et al. Alzheimer’s disease markers in the aged sheep (Ovis aries). Neurobiol. Aging 58, 112–119 (2017).
Yan, S. et al. A huntingtin knockin pig model recapitulates features of selective neurodegeneration in Huntington’s disease. Cell 173, 989–1002 (2018).
Lee, S. E. et al. Production of transgenic pig as an Alzheimer’s disease model using a multi-cistronic vector system. PLoS ONE 12, e0177933 (2017).
Walker, L. C. & Jucker, M. The exceptional vulnerability of humans to Alzheimer’s disease. Trends Mol. Med. 23, 534–545 (2017).
Haque, R. U. & Levey, A. I. Alzheimer’s disease: a clinical perspective and future nonhuman primate research opportunities. Proc. Natl Acad. Sci. USA 116, 26224–26229 (2019).
Paspalas, C. D. et al. The aged rhesus macaque manifests Braak stage III/IV Alzheimer’s-like pathology. Alzheimers Dement. 14, 680–691 (2018).
Sasaguri, H. et al. Recent advances in the modeling of Alzheimer’s disease. Front. Neurosci. 16, 807473 (2022).
Yoshimatsu, S. et al. Multimodal analyses of a non-human primate model harboring mutant amyloid precursor protein transgenes driven by the human EF1α promoter. Neurosci. Res. 185, 49–61 (2022).
Seita, Y. et al. Generation of transgenic cynomolgus monkeys overexpressing the gene for amyloid-β precursor protein. J. Alzheimers Dis. 75, 45–60 (2020).
Tang, M. et al. Neurological manifestations of autosomal dominant familial Alzheimer’s disease: a comparison of the published literature with the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS). Lancet Neurol. 15, 1317–1325 (2016).
Lear, A. et al. Understanding them to understand ourselves: the importance of NHP research for translational neuroscience. Curr. Res. Neurobiol. 3, 100049 (2022).
Kennedy, M. E. et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl. Med. 8, 363ra150 (2016).
Amin, N. D. & Pasca, S. P. Building models of brain disorders with three-dimensional organoids. Neuron 100, 389–405 (2018).
Park, J. et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 21, 941–951 (2018).
Fair, S. R. et al. Electrophysiological maturation of cerebral organoids correlates with dynamic morphological and cellular development. Stem Cell Rep. 15, 855–868 (2020).
Sun, X. Y. et al. Generation of vascularized brain organoids to study neurovascular interactions. eLife 11, e76707 (2022).
Shin, N. et al. Vascularization of iNSC spheroid in a 3D spheroid-on-a-chip platform enhances neural maturation. Biotechnol. Bioeng. 119, 566–574 (2022).
Duque, A., Arellano, J. I. & Rakic, P. An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Mol. Psychiatry 27, 377–382 (2022).
Sloan, S. A. et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790 (2017).
Lopez-Otin, C. & Kroemer, G. Hallmarks of health. Cell 184, 33–63 (2021).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2022).
Turturro, A., Duffy, P., Hass, B., Kodell, R. & Hart, R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J. Gerontol. A Biol. Sci. Med. Sci. 57, B379–B389 (2002).
Blackmore, D. G. et al. Multimodal analysis of aged wild-type mice exposed to repeated scanning ultrasound treatments demonstrates long-term safety. Theranostics 8, 6233–6247 (2018).
van Praag, H., Shubert, T., Zhao, C. & Gage, F. H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685 (2005).
Blackmore, D. G. et al. Low-intensity ultrasound restores long-term potentiation and memory in senescent mice through pleiotropic mechanisms including NMDAR signaling. Mol. Psychiatry 26, 6975–6991 (2021).
Nisbet, R. M. et al. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain 140, 1220–1230 (2017).
Pandit, R., Leinenga, G. & Götz, J. Repeated ultrasound treatment of tau transgenic mice clears neuronal tau by autophagy and improves behavioral functions. Theranostics 9, 3754–3767 (2019).
Xu, G. et al. TAPPing into the potential of inducible tau/APP transgenic mice. Neuropathol. Appl. Neurobiol. 48, e12791 (2022).
Beckmann, N., Gerard, C., Abramowski, D., Cannet, C. & Staufenbiel, M. Noninvasive magnetic resonance imaging detection of cerebral amyloid angiopathy-related microvascular alterations using superparamagnetic iron oxide particles in APP transgenic mouse models of Alzheimer’s disease: application to passive Aβ immunotherapy. J. Neurosci. 31, 1023–1031 (2011).
Lewis, J. et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25, 402–405 (2000).
Coninx, E. et al. Hippocampal and cortical tissue-specific epigenetic clocks indicate an increased epigenetic age in a mouse model for Alzheimer’s disease. Aging 12, 20817–20834 (2020).
Gamache, J. et al. Factors other than hTau overexpression that contribute to tauopathy-like phenotype in rTg4510 mice. Nat. Commun. 10, 2479 (2019).
Polanco, J. C., Hand, G. R., Briner, A., Li, C. & Götz, J. Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol. 141, 235–256 (2021).
Thal, D. R. et al. Estimation of amyloid distribution by [18F]flutemetamol PET predicts the neuropathological phase of amyloid β-protein deposition. Acta Neuropathol. 136, 557–567 (2018).
Braak, H. & Braak, E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278 (1995).
Jucker, M. & Walker, L. C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 (2013).
de Calignon, A. et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–697 (2012).
Asai, H. et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18, 1584–1593 (2015).
Clavaguera, F. et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl Acad. Sci. USA 110, 9535–9540 (2013).
Ahmed, Z. et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 127, 667–683 (2014).
Ferris, S. H. et al. Positron emission tomography in the study of aging and senile dementia. Neurobiol. Aging 1, 127–131 (1980).
Palop, J. J. & Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17, 777–792 (2016).
Sheline, Y. I. et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 30, 17035–17040 (2010).
Palmqvist, S. et al. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 8, 1214 (2017).
Zott, B., Busche, M. A., Sperling, R. A. & Konnerth, A. What happens with the circuit in Alzheimer’s disease in mice and humans? Annu. Rev. Neurosci. 41, 277–297 (2018).
Lu, H. et al. Rat brains also have a default mode network. Proc. Natl Acad. Sci. USA 109, 3979–3984 (2012).
Whitesell, J. D. et al. Regional, layer, and cell-type-specific connectivity of the mouse default mode network. Neuron 109, 545–559 (2021).
Zhou, Y. et al. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer’s disease and mild cognitive impairment. Alzheimers Dement. 4, 265–270 (2008).
Quiroz, Y. T. et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann. Neurol. 68, 865–875 (2010).
O’Brien, J. L. et al. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74, 1969–1976 (2010).
Busche, M. A. et al. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 109, 8740–8745 (2012).
Palop, J. J. et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711 (2007).
Padmanabhan, P., Kneynsberg, A. & Götz, J. Super-resolution microscopy: a closer look at synaptic dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 22, 723–740 (2021).
Tzioras, M., McGeachan, R. I., Durrant, C. S. & Spires-Jones, T. L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 19, 19–38 (2023).
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood–brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78 (2019).
Pardridge, W. M. Tyrosine hydroxylase replacement in experimental Parkinson’s disease with transvascular gene therapy. NeuroRx 2, 129–138 (2005).
Neuwelt, E. et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 7, 84–96 (2008).
Golde, T. E. Open questions for Alzheimer’s disease immunotherapy. Alzheimers Res. Ther. 6, 3 (2014).
Budd Haeberlein, S. et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 9, 197–210 (2022).
Schneider, L. A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 19, 111–112 (2020).
Ayton, S. Ventricular enlargement caused by aducanumab. Nat. Rev. Neurol. 18, 383–384 (2022).
Leinenga, G., Koh, W. K. & Götz, J. A comparative study of the effects of aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer disease. Alzheimers Res. Ther. 13, 76 (2021).
Sun, T. et al. Focused ultrasound with anti-pGlu3 Aβ enhances efficacy in Alzheimer’s disease-like mice via recruitment of peripheral immune cells. J. Control. Release 336, 443–456 (2021).
Kovacs, Z. I. et al. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl Acad. Sci. USA 114, E75–E84 (2017).
McMahon, D. & Hynynen, K. Acute inflammatory response following increased blood–brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics 7, 3989–4000 (2017).
Clausznitzer, D. et al. Quantitative systems pharmacology model for Alzheimer disease indicates targeting sphingolipid dysregulation as potential treatment option. CPT Pharmacometrics Syst. Pharmacol. 7, 759–770 (2018).
Madrasi, K. et al. Systematic in silico analysis of clinically tested drugs for reducing amyloid-β plaque accumulation in Alzheimer’s disease. Alzheimers Dement. 17, 1487–1498 (2021).
Vogel, J. W. et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 27, 871–881 (2021).
Cornblath, E. J. et al. Computational modeling of tau pathology spread reveals patterns of regional vulnerability and the impact of a genetic risk factor. Sci. Adv. 7, eabg6677 (2021).
Meisl, G. et al. In vivo rate-determining steps of tau seed accumulation in Alzheimer’s disease. Sci. Adv. 7, eabh1448 (2021).
Kunze, T., Hunold, A., Haueisen, J., Jirsa, V. & Spiegler, A. Transcranial direct current stimulation changes resting state functional connectivity: a large-scale brain network modeling study. NeuroImage 140, 174–187 (2016).
Geerts, H. et al. A combined PBPK and QSP model for modeling amyloid aggregation in Alzheimer’s disease. CPT Pharmacometrics Syst. Pharmacol. https://doi.org/10.1002/psp4.12912 (2023).
Taubes, A. et al. Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer’s disease. Nat. Aging 1, 932–947 (2021).
Haeno, H. et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 148, 362–375 (2012).
Padmanabhan, P., Desikan, R. & Dixit, N. M. Modeling how antibody responses may determine the efficacy of COVID-19 vaccines. Nat. Comput. Sci. 2, 123–131 (2022).
Suberbielle, E. et al. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat. Neurosci. 16, 613–621 (2013).
Thadathil, N. et al. DNA double-strand break accumulation in Alzheimer’s disease: evidence from experimental models and postmortem human brains. Mol. Neurobiol. 58, 118–131 (2021).
Rolyan, H. et al. Telomere shortening reduces Alzheimer’s disease amyloid pathology in mice. Brain 134, 2044–2056 (2011).
Shu, L. et al. Genome-wide alteration of 5-hydroxymenthylcytosine in a mouse model of Alzheimer’s disease. BMC Genomics 17, 381 (2016).
Cadena-del-Castillo, C. et al. Age-dependent increment of hydroxymethylation in the brain cortex in the triple-transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 41, 845–854 (2014).
Trishina, E. et al. Defects in mitochondrial dynamics and metabolomic signatures of evolving energetic stress in mouse models of familial Alzheimer’s disease. PLoS ONE 7, e32737 (2012).
David, D. C. et al. Proteomic and functional analysis reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 280, 23802–23814 (2005).
Ittner, L. M. et al. Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc. Natl Acad. Sci. USA 105, 15997–16002 (2008).
Duboff, B., Götz, J. & Feany, M. B. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron 75, 618–632 (2012).
Cummins, N., Tweedie, A., Zuryn, S., Bertran-Gonzalez, J. & Götz, J. Disease-associated tau impairs mitophagy by inhibiting parkin translocation to mitochondria. EMBO J. 38, e99360 (2018).
Evans, H. T., Benetatos, J., van Roijen, M., Bodea, L. G. & Götz, J. Decreased synthesis of ribosomal proteins in tauopathy revealed by non-canonical amino acid labelling. EMBO J. 38, e101174 (2019).
Saito, T. & Saido, T. C. Neuroinflammation in mouse models of Alzheimer’s disease. Clin. Exp. Neuroimmunol. 9, 211–218 (2018).
Reinitz, F. et al. Inhibiting USP16 rescues stem cell aging and memory in an Alzheimer’s model. eLife 11, e66037 (2022).
Yu, W. H. et al. Macroautophagy—a novel β-amyloid peptide-generating pathway activated in Alzheimer’s disease. J. Cell Biol. 171, 87–98 (2005).
Sun, J. et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 9, 189 (2019).
Dodiya, H. B. et al. Gut microbiota-driven brain Aβ amyloidosis in mice requires microglia. J. Exp. Med. 219, e20200895 (2022).
Seo, D. O. et al. ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science 379, eadd1236 (2023).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Jiang, S. et al. Proteopathic tau primes and activates interleukin-1β via myeloid-cell-specific MyD88- and NLRP3–ASC–inflammasome pathway. Cell Rep. 36, 109720 (2021).
Lafay-Chebassier, C. et al. mTOR/p70S6k signalling alteration by Aβ exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J. Neurochem. 94, 215–225 (2005).
Caccamo, A., Majumder, S., Richardson, A., Strong, R. & Oddo, S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and tau: effects on cognitive impairments. J. Biol. Chem. 285, 13107–13120 (2010).
Dorigatti, A. O. et al. Brain cellular senescence in mouse models of Alzheimer’s disease. Geroscience 44, 1157–1168 (2022).
Zhang, P. et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 22, 719–728 (2019).
Acknowledgements
We thank R. Tweedale for critical reading of the manuscript. We acknowledge support by the estate of C. Jones, the state government of Queensland (Department of Science, Information Technology and Innovation), the National Health and Medical Research Council of Australia (GNT1176326) and the NHMRC-EU Joint Programme on Neurodegenerative Disease Research to J.G.
Author information
Authors and Affiliations
Contributions
Both authors discussed and wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Padmanabhan, P., Götz, J. Clinical relevance of animal models in aging-related dementia research. Nat Aging 3, 481–493 (2023). https://doi.org/10.1038/s43587-023-00402-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00402-4
This article is cited by
-
Scanning ultrasound-mediated memory and functional improvements do not require amyloid-β reduction
Molecular Psychiatry (2024)
-
Ultrasound and antibodies — a potentially powerful combination for Alzheimer disease therapy
Nature Reviews Neurology (2024)