Abstract
Pancreatic islets control glucose homeostasis by the balanced secretion of insulin and other hormones, and their abnormal function causes diabetes or hypoglycaemia. Here we uncover a conserved programme of alternative microexons included in mRNAs of islet cells, particularly in genes involved in vesicle transport and exocytosis. Islet microexons (IsletMICs) are regulated by the RNA binding protein SRRM3 and represent a subset of the larger neural programme that are particularly sensitive to SRRM3 levels. Both SRRM3 and IsletMICs are induced by elevated glucose levels, and depletion of SRRM3 in human and rat beta cell lines and mouse islets, or repression of particular IsletMICs using antisense oligonucleotides, leads to inappropriate insulin secretion. Consistently, mice harbouring mutations in Srrm3 display defects in islet cell identity and function, leading to hyperinsulinaemic hypoglycaemia. Importantly, human genetic variants that influence SRRM3 expression and IsletMIC inclusion in islets are associated with fasting glucose variation and type 2 diabetes risk. Taken together, our data identify a conserved microexon programme that regulates glucose homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA-seq data were submitted to the Gene Expression Omnibus under the project no. GSE198906. All other RNA-seq samples used in this study are publicly available and listed in Supplementary Table 1. Source data are provided with this paper.
Code availability
Code to quantify alternative splicing from RNA-seq data and identify tissue-specific exons has been published before and can be accessed in https://github.com/vastdb-pastdb/pastdb/, https://github.com/vastgroup/vastdb_framework_code_example/ and https://github.com/vastgroup/vast-tools/.
References
Arda, H. E. et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human beta cell function. Cell Metab. 23, 909–920 (2016).
Salinno, C. et al. β-Cell maturation and identity in health and disease. Int. J. Mol. Sci. 20, 5417 (2019).
Pasquali, L. et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet. 46, 136–143 (2014).
Miguel-Escalada, I. et al. Human pancreatic islet three-dimensional chromatin architecture provides insights into the genetics of type 2 diabetes. Nat. Genet. 51, 1137–1148 (2019).
Mitchell, S. M. & Frayling, T. M. The role of transcription factors in maturity-onset diabetes of the young. Mol. Genet. Metab. 77, 35–43 (2002).
Pan, Q., Shai, O., Lee, L. J., Frey, B. J. & Blencowe, B. J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40, 1413–1415 (2008).
Wang, E. T. et al. Alternative isoform regulation in human tissue transcriptomes. Nature 456, 470–476 (2008).
Tapial, J. et al. An atlas of alternative splicing profiles and functional associations reveals new regulatory programs and genes that simultaneously express multiple major isoforms. Genome Res. 27, 1759–1768 (2017).
Baralle, F. E. & Giudice, J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell Biol. 18, 437–451 (2017).
Villate, O. et al. Nova1 is a master regulator of alternative splicing in pancreatic beta cells. Nucleic Acids Res. 42, 11818–11830 (2014).
Juan-Mateu, J. et al. Neuron-enriched RNA-binding proteins regulate pancreatic beta cell function and survival. J. Biol. Chem. 292, 3466–3480 (2017).
Juan-Mateu, J. et al. SRp55 regulates a splicing network that controls human pancreatic beta-cell function and survival. Diabetes 67, 423–436 (2018).
Irimia, M. et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell 159, 1511–1523 (2014).
Li, Y. I., Sanchez-Pulido, L., Haerty, W. & Ponting, C. P. RBFOX and PTBP1 proteins regulate the alternative splicing of micro-exons in human brain transcripts. Genome Res. 25, 1–13 (2015).
Calarco, J. A. et al. Regulation of vertebrate nervous system alternative splicing and development by an SR-related protein. Cell 138, 898–910 (2009).
Quesnel-Vallieres, M., Irimia, M., Cordes, S. P. & Blencowe, B. J. Essential roles for the splicing regulator nSR100/SRRM4 during nervous system development. Genes Dev. 29, 746–759 (2015).
Quesnel-Vallieres, M. et al. Misregulation of an activity-dependent splicing network as a common mechanism underlying autism spectrum disorders. Mol. Cell 64, 1023–1034 (2016).
Nakano, Y., Wiechert, S. & Banfi, B. Overlapping activities of two neuronal splicing factors switch the GABA effect from excitatory to inhibitory by regulating REST. Cell Rep. 27, 860–871 (2019).
Torres-Mendez, A. et al. A novel protein domain in an ancestral splicing factor drove the evolution of neural microexons. Nat. Ecol. Evol. 3, 691–701 (2019).
Parras, A. et al. Autism-like phenotype and risk gene mRNA deadenylation by CPEB4 mis-splicing. Nature 560, 441–446 (2018).
Henquin, J. C. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in beta cells. Diabetes Res. Clin. Pract. 93, S27–S31 (2011).
Raj, B. et al. A global regulatory mechanism for activating an exon network required for neurogenesis. Mol. Cell 56, 90–103 (2014).
Imamura, H. et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl Acad. Sci. USA 106, 15651–15656 (2009).
Arous, C. & Halban, P. A. The skeleton in the closet: actin cytoskeletal remodeling in beta-cell function. Am. J. Physiol. Endocrinol. Metab. 309, E611–E620 (2015).
Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 50, 1505–1513 (2018).
Chen, J. et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 53, 840–860 (2021).
Gao, N. et al. Foxa2 controls vesicle docking and insulin secretion in mature beta cells. Cell Metab. 6, 267–279 (2007).
Fadista, J. et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc. Natl Acad. Sci. USA 111, 13924–13929 (2014).
Atla, G. et al. Genetic regulation of RNA splicing in human pancreatic islets. Genome Biol. 23, 196 (2022).
Wigger, L. et al. Multi-omics profiling of living human pancreatic islet donors reveals heterogeneous beta cell trajectories towards type 2 diabetes. Nat. Metab. 3, 1017–1031 (2021).
Wilhelmi, I., Neumann, A., Jahnert, M., Ouni, M. & Schurmann, A. Enriched alternative splicing in islets of diabetes-susceptible mice. Int. J. Mol. Sci. 22, 8597 (2021).
Gonatopoulos-Pournatzis, T. et al. Genome-wide CRISPR–Cas9 interrogation of splicing networks reveals a mechanism for recognition of autism-misregulated neuronal microexons. Mol. Cell 72, 510–524 (2018).
van Arensbergen, J. et al. Derepression of Polycomb targets during pancreatic organogenesis allows insulin-producing beta cells to adopt a neural gene activity program. Genome Res. 20, 722–732 (2010).
Arntfield, M. E. & van der Kooy, D. β-Cell evolution: how the pancreas borrowed from the brain: the shared toolbox of genes expressed by neural and pancreatic endocrine cells may reflect their evolutionary relationship. Bioessays 33, 582–587 (2011).
Perillo, M. et al. New neuronal subtypes with a ‘pre-pancreatic’ signature in the sea urchin Stongylocentrotus purpuratus. Front. Endocrinol. 9, 650 (2018).
El Marabti, E. & Abdel-Wahab, O. Therapeutic modulation of RNA splicing in malignant and non-malignant disease. Trends Mol. Med. 27, 643–659 (2021).
Martin, G., Marquez, Y., Mantica, F., Duque, P. & Irimia, M. Alternative splicing landscapes in Arabidopsis thaliana across tissues and stress conditions highlight major functional differences with animals. Genome Biol. 22, 35 (2021).
Kolberg, L., Raudvere, U., Kuzmin, I., Vilo, J. & Peterson, H. gprofiler2—an R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Res 9, ELIXIR–709 (2020).
Ciampi, L. et al. Specialization of the photoreceptor transcriptome by Srrm3-dependent microexons is required for outer segment maintenance and vision. Proc. Natl Acad. Sci. USA 119, e2117090119 (2022).
MacArthur, J. et al. The new NHGRI-EBI catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 45, D896–D901 (2017).
Xin, Y. et al. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. 24, 608–615 (2016).
Wang, Y. J. et al. Single-cell transcriptomics of the human endocrine pancreas. Diabetes 65, 3028–3038 (2016).
Segerstolpe, A. et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24, 593–607 (2016).
Lawlor, N. et al. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res. 27, 208–222 (2017).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Fang, Z. et al. Single-cell heterogeneity analysis and CRISPR screen identify key beta-cell-specific disease genes. Cell Rep. 26, 3132–3144 (2019).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Cortal, A., Martignetti, L., Six, E. & Rausell, A. Gene signature extraction and cell identity recognition at the single-cell level with Cell-ID. Nat. Biotechnol. 39, 1095–1102 (2021).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Zhu, A., Ibrahim, J. G. & Love, M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092 (2019).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Yu, G. Gene Ontology semantic similarity analysis using GOSemSim. Methods Mol. Biol. 2117, 207–215 (2020).
Ortis, F. et al. Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-kappaB activation. Mol. Endocrinol. 20, 1867–1879 (2006).
Ravassard, P. et al. A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. J. Clin. Invest. 121, 3589–3597 (2011).
Wang, X., Park, J., Susztak, K., Zhang, N. R. & Li, M. Bulk tissue cell type deconvolution with multi-subject single-cell expression reference. Nat. Commun. 10, 380 (2019).
Korotkevich, G. et al. Fast gene-set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2021).
Tsonkova, V. G. et al. The EndoC-βH1 cell line is a valid model of human beta cells and applicable for screenings to identify novel drug target candidates. Mol. Metab. 8, 144–157 (2018).
Beucher, A. et al. The HASTER lncRNA promoter is a cis-acting transcriptional stabilizer of HNF1A. Nat. Cell Biol. 24, 1528–1540 (2022).
Georgiadou, E. et al. Mitofusins Mfn1 and Mfn2 are required to preserve glucose- but not incretin-stimulated beta-cell connectivity and insulin secretion. Diabetes 71, 1472–1489 (2022).
Mousavy Gharavy, S. N. et al. Sexually dimorphic roles for the type 2 diabetes-associated C2cd4b gene in murine glucose homeostasis. Diabetologia 64, 850–864 (2021).
Acknowledgements
We thank B. Banfi (University of Iowa) for kindly sharing the Srrm3 gene-trapped mouse line with us; M. Ángel Maestro for excellent technical advice on multiple protocols related to the study of Srrm3 mutant mice; J. Permanyer and C. Rodriguez for help with mouse genotyping; D. Balboa, I. Miguel-Escalada and E. Bernardo, as well as members of the M.I. and J.V. groups for constant scientific discussion; A. Gohr for assistance on bioinformatic analyses; S. Taylor (University of Manchester) for kindly sharing the HeLa Flp-In T-Rex cell line with us; and CRG Genomics and Advanced Light Microscopy Units for the RNA-seq and microscopy services. The research has been funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ERC-StG-LS2-637591 and ERCCoG-LS2-101002275 to M.I., ERC-AdG-LS2-670146 to J.V., and ERC-AdG-LS4-789055 to J.F.), EU Horizon 2020 TDSystems (667191) to J.F., la Caixa Foundation (ID 100010434), under the agreement LCF/PR/HR20/52400008 to M.I., an EFSD award supported by EFSD/Lilly European Diabetes Research Programme, the Spanish Ministry of Science and Innovation (BFU-2017-89308-P to J.V., BFU-2017-89201-P to M.I. and RTI2018-095666-B-I00 to J.F.) and the ‘Centro de Excelencia Severo Ochoa’ (CEX2020-001049). G.A. was supported by the Marie Skłodowska-Curie project ZENCODE-ITN (No. 643062). S.B.-G. was supported by a Juan de la Cierva postdoctoral fellowship (MINECO; FJCI-2017-32090). J.J.-M. was supported by the Beatriu de Pinós Programme and the Ministry of Research and Universities of the Government of Catalonia, and a Marie Skłodowska-Curie Individual Fellowship from the European Union’s Horizon 2020 research and innovation programme (MSCA-IF-2019-841758; http://ec.europa.eu/).
Author information
Authors and Affiliations
Contributions
J.J.-M., J.V. and M.I. conceived the present study. S. Bajew discovered the presence of microexons in pancreatic islets. S. Bajew and L.P.I. conducted the computational analysis and code development. J.J.-M. performed the in vitro experiments using beta cell lines. J.J.-M., M.M.-C. and A.L.-P. conducted the mouse in vivo and ex vivo work. J.J.-M. performed the immunohistochemical analysis of mouse pancreata. S. Bonnal designed the splicing-switching ASO and conducted the experiments of SRRM3 overexpression in HeLa cells. G.A. and S.B.-G. performed the genetic and epigenetic analyses in human pancreatic islets. L.P.I. conducted the transcriptomic analyses of human single-cell and bulk pancreatic islets. J.F. contributed with material and reagents, helped with the interpretation of results and provided insights into pathophysiological mechanisms. J.J.-M., J.V. and M.I. wrote the manuscript with support of all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Anna Gloyn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 IsletLONGs display a different tissue inclusion pattern and conservation compared to IsletMICs.
(A) UMAP projections of single-cell RNA-seq (scRNA-seq) data from human islets and expression levels of pancreatic hormones according to cell type (n = 26288 cells examined over 28 non-diabetic donors). (B) Mean inclusion levels of IsletMICs and IsletLONGs in the different islet cell types from scRNA-seq. Data are shown as box-and-whisker plot, in which the lower and upper bounds of the box represent the upper and lower quartiles, the centre line represents the median, whiskers the 1.5x interquartile range, and points the outliers. (C) Predicted impact of IsletMICs and IsletLONGs on protein sequences. IsletMICs are largely predicted to generate new protein isoforms upon inclusion by preserving the open reading frame (ORF), while IsletLONGs have a higher proportion of events that disrupt ORFs (that is the exon leads to a frame shift and/or introduces a premature termination codon when included or excluded) or impact UTRs. (D) Heatmap showing the tissue inclusion levels (expressed as z-score of the PSIs) of human cassette exons ≥28 nt enriched in pancreatic islets (IsletLONGs). (E) Overlap between IsletLONGs and neuron-enriched cassette exons ≥28 nt, showing that 39% (in contrast to 3% of IsletMICs) of tissue-enriched exons are not shared between islet and neural tissues.
Extended Data Fig. 2 IsletMICs correspond to the subset of neuronal microexons with high sensitivity to SRRM3.
(A, B) mRNA expression of the neural microexons regulator Srrm4 and of its paralog Srrm3 in neural tissues, pancreatic islets, exocrine pancreas and other rat (A) and mouse (B) tissues (data from VastDB, n = 41 in rat and n = 30 in mouse, biologically independent samples). Data are shown as box-and-whisker plot, in which the lower and upper bounds of the box represent the upper and lower quartiles, the centre line represents the median, and whiskers the 10th and 90th percentile. (C–E) siRNA-mediated knockdown (KD) of Srrm3 in INS-1E rat beta cell line. (C) Srrm3 mRNA levels measured by qPCR following 48 h transfection with Srrm3 (siSrrm3) or control (siCTL) siRNAs, normalized to Gapdh (n = 5 independent experiments). Data is shown as mean ± s.e.m. P values was obtained from Student’s two-tailed unpaired t-test. (D) RT-PCR assays for selected microexons in control and Srrm3 KD cells. The positions of inclusion/skipping isoforms and the percentage of microexon inclusion are indicated for two biological replicates. (E) Global impact of Srrm3 KD on exon inclusion levels estimated by PSI values from RNA-seq data. Differentially included exons in Srrm3 KD vs control are shown in orange (microexons, length [le] ≤ 27 nt), light blue (short exons, 27 < le ≤ 51 nt) and dark blue (cassette exons, le > 51 nt). The pie chart shows the number of misregulated exons (|∆PSI| > 15) according to their size range. Lower panel shows ΔPSI cumulative proportions for microexons, short exons and alternative cassette exons. P value was obtained from two-sided Wilcoxon test comparing the distributions of microexons and cassette exons of length > 51 nt. PSI values are the mean of three independent experiments. (F) Overlap between Srrm3-regulated microexons in rat INS-1E cells and rat IsletMICs. Only microexons with sufficient read coverage in both comparisons are shown. (G) Overlap between SRRM3-regulated microexons in rat INS-1E (R) and human EndoC-βH1 (H). Microexons presenting no ortholog (‘no orth’) or with no sufficient read coverage (‘no cov’) in the other species are indicated. (H) SRRM3 overexpression in HeLa cells at three different levels reveals different sensitivities for IsletMICs and neuronal-only MICs (NeuralMICs). Exon inclusion levels were quantified from RNA-seq and differences in inclusion between control and SRRM3-overexpressing cells are shown for IsletMICs and NeuralMICs (n = 1 experiment). Data are shown as box-and-whisker plot, in which the lower and upper bounds of the box represent the upper and lower quartiles, the centre line represents the median, whiskers the 1.5x interquartile range, and points the outliers.
Extended Data Fig. 3 Srrm3 depletion in mouse islets causes IsletMIC downregulation and increased stimulated insulin release.
(A) Differences in inclusion levels (∆PSI) in islets from Srrm3 −/− vs wild type (WT) mice for all exons shorter than 300 nucleotides with sufficient read coverage. PSI values are the mean of three mice islet preparations (n = 3 animals). B) Number of misregulated alternative exons according to size range in Srrm3 −/− and Srrm3 +/− mouse islets compared to WT ones. Black and grey bars indicate exons with ΔPSI > 15 and ΔPSI < −15, respectively. (C) Differences in inclusion levels (∆PSI) in islets from Srrm3 +/− vs wild type (WT) mice for all exons shorter than 300 nucleotides with sufficient read coverage. PSI values are the mean of three mice islet preparations. (D) Density plots for ΔPSI distributions in Srrm3 +/ islets of IsletMICs, IsletLONGs and other alternative exons. P values were obtained from two-sided Wilcoxon test comparing the distributions of IsletMICs (orange) or IsletLONGs (red) against other alternative exons. (E) Total insulin protein content in mice islets measured by ELISA (n = 10 animals). (F) Insulin mRNA levels in mice islets measured by RNA-seq (n = 3 animals). (G) Total glucagon protein content in mice islets measured by ELISA (n = 4 animals). (H) Glucagon mRNA levels in mice islets measured by RNA-seq (n = 3 animals). (I) Overlap between differentially expressed genes in Srrm3 −/− islets and genes harbouring IsletMIC and IsletLONG alternative exons. (J) Distribution of log2 fold change values in genes containing either IsletMICs or IsletLONGs compared to background (genes expressed in mouse islets). Color code represents -log10 adjusted p values. Data in (E–H) are represented as mean ± s.e.m. P values were obtained from two-sided Wilcoxon test (D) or Student’s two-tailed unpaired t-test (E). Islets were isolated from 9–14 weeks old C57BL/6J male and female mice.
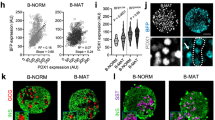
Extended Data Fig. 4 Srrm3 depletion disrupts islet cell composition and architecture.
(A) Representative immunofluorescence images of islets from WT and Srrm3 mutant neonatal mice stained for insulin (Ins; magenta) and glucagon (Gcg; green). (B, C) Quantification of the fraction of alpha cells (B) and insulin and glucagon double-positive cells (C) (n = average of 9–12 islets from 4 animals per genotype). (D) Percent of endocrine cell clusters in well-organized islet-like structures or displaying more dispersed organization. All data are shown as box-and-whisker plot, in which the lower and upper bounds of the box represent the upper and lower quartiles, the centre line represents the median, and whiskers minimum and maximum values. P values were obtained from Student’s two-tailed unpaired t-test. Pancreata were obtained from P1 C57BL/6J male and female mice.
Extended Data Fig. 5 Srrm3 mutant mice display hypoglycemic hyperinsulinemia.
Glycemia and plasma insulin and glucagon levels segregated by sex in Srrm3 mutant and wild type adult mice (9–14 weeks old). (A) Random blood glucose measurements in Srrm3 mutant and wild type mice fed ad libitum (n = 7 animals). (B) Blood glucose levels following a 4 h fast (n = 7 animals). (C) Blood glucose levels at 30 min postprandial (n = 7 animals). (D, E) Insulin plasma levels (D) and ratio between plasma insulin and blood glucose (E) at 30 min postprandial (n = 6 animals). (F) Plasma glucagon at 4 h fasting (n = 4 animals). All data is shown as box-and-whisker plot, in which the lower and upper bounds of the box represent the upper and lower quartiles, the centre line represents the median, and whiskers minimum and maximum values. P values were obtained from Student’s two-tailed unpaired t-test. All measurements were performed in 9–14 weeks old C57BL/6 J male and female mice.
Extended Data Fig. 6 The SRRM3 locus responds to glucose and harbors genetic variants associated with fasting glucose and type 2 diabetes risk.
(A) Regional plot of Fasting Glucose GWAS variants from the CMDKP database (hugeamp.org). (B) Allele frequency and predicted impact on a Foxa2 binding motif for the SNP rs67070387 associated with elevated Fasting glucose. (C) Luciferase assay in INS-1E cells following transfection of a control reporter vector or carrying the genomic sequence surrounding rs67070387 with one or the other allele (n = 7 independent experiments). (D) Quantile-quantile (QQ) plots showing distribution of p-values associated with type 2 diabetes from25 or fasting glucose from26 for variants located in 1Kb genomic regions containing microexons. (E) SRRM3 expression and IsletMIC inclusion in human islets from non-diabetic (ND) and type 2 diabetes (T2D) individuals from30 (n = 44 biologically independent samples). (F) Srrm3 expression and IsletMIC inclusion in islets from B6-ob/ob (OBOB) and New Zealand Obese (NZO) mice from31 (n = 5 animals). (G) SRRM3 expression and IsletMIC inclusion in human islets from non-diabetic (ND) and impaired glucose tolerant (IGT) individuals from28 (n = 48 biologically independent samples). (H) SRRM3 expression and IsletMIC inclusion in human islets from non-diabetic (ND) impaired glucose tolerant (IGT) individuals from30 (n = 50 biologically independent samples). (I) SRRM3 expression and IsletMIC inclusion in single beta cells from non-diabetic (ND) and type 2 diabetes (T2D) individuals from multiple studies (n = 8 biologically independent samples) (see methods). Data in (C, F–I) are shown as box-and-whisker plot, in which the lower and upper bounds of the box represent the upper and lower quartiles, the centre line represents the median and whiskers the 1.5x interquartile range. P values were obtained from Student’s two-tailed unpaired t-test (C) or two-sided Wilcoxon test (F).
Extended Data Fig. 7 Down-regulation of IsletMICs in T2D islets is not associated with changes in the expression of SRRM3 isoforms nor in co-regulatory RNA-binding proteins.
(A, B) Expression of SRRM3 alternative isoforms containing the enhancer of microexons domain (eMIC; ENST00000611745) and with a truncated eMIC (ENST00000612155) in islets from normo-glycemic (ND), and type 2 diabetic individuals (T2D) from the studies of Wigger et al. 2021 (A) (n = 44 biologically independent samples) and Fadista et al. 2014 (B) (n = 40 biologically independent samples). (C, D) RNA expression of RNA-binding proteins reported to co-regulate microexon splicing in neurons in islets from normo-glycemic (ND), glucose intolerant (IGT) and type 2 diabetic individuals (T2D) from Wigger et al. 2021 (C) (n = 78 biologically independent samples) and Fadista et al. 2014 (D) (n = 54 biologically independent samples) studies. (E) RT-PCR analysis of microexon inclusion/exclusion after transfection with control or SRSF6 siRNAs in EndoC-βH1 beta cells. Data in (A–D) are shown as box-and-whisker plot, in which the lower and upper bounds of the box represent the upper and lower quartiles, the centre line represents the median and whiskers the 1.5x interquartile range. P values were obtained from two-sided Wilcoxon test.
Supplementary information
Supplementary Table 1
List of RNA-seq data used or generated in this study.
Supplementary Table 2
List of islet-enriched alternative exons.
Supplementary Table 3
List of regulated Islet microexons by SRRM3.
Supplementary Table 4
Enriched GO terms for differentially expressed genes in Srrm3−/− islets.
Supplementary Table 5
Config files for the definition of islet-enriched alternative exons.
Supplementary Table 6
Overlap of islet-enriched alternative exons with GWAS genes.
Supplementary Table 7
Oligonucleotides sequences used for cloning, and gene expression and splicing analyses.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data and unprocessed gels.
Source Data Fig. 3
Statistical source data and unprocessed gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data and unprocessed gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Juan-Mateu, J., Bajew, S., Miret-Cuesta, M. et al. Pancreatic microexons regulate islet function and glucose homeostasis. Nat Metab 5, 219–236 (2023). https://doi.org/10.1038/s42255-022-00734-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00734-2
This article is cited by
-
FOXA1 and FOXA2: the regulatory mechanisms and therapeutic implications in cancer
Cell Death Discovery (2024)
-
Unique transcriptomes of sensory and non-sensory neurons: insights from Splicing Regulatory States
Molecular Systems Biology (2024)