Abstract
Here, a comprehensive study was designed to estimate the human risk assessment attributed to exposure of polycyclic aromatic hydrocarbons (PAHs)in sediment and fish in most polluted shore area in north of Persian Gulf. To this end, a total of 20 sediment and inhabitual Fish, as one of most commercial fish, samples were randomly collected from 20 different stations along Bushehr Province coastline. The 16 different components of PAHs were extracted from sediment and edible parts of inhabitual fish and measured with high-performance liquid chromatography (HPLC) and gas chromatography (GC), respectively. In addition, dietary daily intake (DDI) values of PAHs via ingestion Indian halibut and the incremental lifetime cancer risk (ILCR) attributed to human exposure to sediments PAHs via (a) inhalation, (b) ingestion, and (c) dermal contact for two groups of ages: children (1–11 years) and adults (18–70 years) were estimated. The results indicated that all individual PAHs except for Benzo(b)flouranthene (BbF) and Benzo(ghi) perylene (BgP) were detected in different sediment sample throughout the study area with average concentration between 2.275 ± 4.993 mg.kg–1 dw. Furthermore, Naphthalene (Nap) with highest average concentration of 3.906 ± 3.039 mg.kg–1 dw was measured at the Indian halibut. In addition, the human risk analysis indicated that excess cancer risk (ECR) attributed to PAHs in sediment and fish in Asaluyeh with high industrial activities on oil and derivatives were higher the value recommended by USEPA (10−6). Therefore, a comprehensive analysis on spatial distribution and human risk assessment of PAHs in sediment and fish can improve the awareness on environmental threat in order to aid authorities and decision maker to find a sustainable solution.
Similar content being viewed by others
Introduction
The human and environmental concerns attributed to emission of toxic, persistent and non-biodegradable elements into biotic and abiotic matrices are of outstanding importance for sustainable and health future1. These elements can accumulate in organisms and microorganisms and even food chain and increase the levels of potential toxicity2. Polycyclic aromatic hydrocarbons (PAHs) with characteristics including semi-volatile, carcinogenicity, mutagenicity, and teratogenicity, bioaccumulation and long distance migration are a class of concerning contaminants, causing human and ecological risk3. PAHs with one or more benzene rings in their chemical structure are difficult to break for microorganisms; these persistent elements have long existed in different media of environments including air, water, soil and living organisms4,5. The PAHs are produced via both natural factors and anthropogenic activities4. The biodegradation, leaking, forest fires, eruptions from volcanoes and microbial production of subterranean fossil fuels are the major natural sources of PAHs6. In addition, the most important anthropogenic source of PAHs are industrial activities, combustion of fossil fuels and, automobile exhausts7. Given the thermodynamic characteristics, PAHs are generally divided into three categories: (1) pyrogenic (emitted from incomplete combustion and pyrolysis of organic material), (2) petrogenic (comes from crude oil and oil spills), (3) biogenic/diagenetic (released from slow transformation of organic matter in sediments)1. It is believed that rivers and sediments are the main host for accumulation of PAHs, especially, petrogenic ones8. The organic and inorganic pollutants enter the rivers and sediments via polluted wastewater, stormwater and effluent discharge. The PAHs are adsorbed on particles in sediments; these can be resuspended and rereleased into water sources9. In addition, toxic contaminants such as PAHs is bioacumulated in aquatic species such as fish and mussels10. As fish are one main meal of people and serve as food, it can contribute to significant health risks for humans11. As a consequence of rapid industrialization and urbanization, a huge amount of PAHs may be discharged into aquatic environments; they can deposit into environment, accumulate in biotic organism used for food chain and consequently damage the human nerves, respiratory, circulatory system8,12. Therefore, given the possible dangers imposed via entrance of PAHs and other toxic contaminant into sediment, water bodies and biotic organisms, a comprehensive data collection on sediment quality and bioaccumulation of contaminants in fish can aid deeper understanding of ecological and human risk assessment in the area13,14. Furthermore, a comprehensive and intense monitoring and assessment of spatial distribution of PAHs in sediments and fish can aid the decision maker to find a sustainable solution15. In this regards, many studies have focused on ecological and human risk of PAHs in sediments and foods1,9,16,17,18. For instance, Balram Ambade and et al.16 investigated the toxicity of PAHs in sediment in Damodar River Basin, India. The results indicated that concentration of 4-ring PAHs such as Fluoranthene (Flur), Pyrene (Pye), Benzo(a)Anthracene (BaA), and Chrysene (Chry) in the sediments were 43 ± 41 ng.g–1, 32 ± 29 ng.g–1, 52 ± 50 ng.g–1, and 83 ± 105 ng.g–1, respectively16. In addition, Xiaoqin Lin and et al.18 surveyed the human risk assessment of marine organisms in Shenzhen coastal waters in China. The authors reported that the individual lifetime cancer risk levels associated with seafood consumption in this area were ranged from 1.10 × 10–5 to 1.52 × 10–5, indicating the potential cancer risk for human which need special attention 18. Therefore, the present study was developed to comprehensively estimate the human risk assessment attributed to exposure to sediment and fish in one of most polluted area in Iran and even the world, where the industrial activities and oil refinery discharge is a serious threat for the environment.
Methods and materials
Study area description and sampling
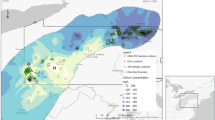
A comprehensive study on the sediment and Indian halibut samples was performed in order to determine the PAHs pollution in Bushehr province. To this end, five cities and island with high severity in oil pollution and industrial activities (Petrochemical and gas industries) throughout Busher Province, located at southeast of Iran were selected. Generally, a total of 20 samples were randomly collected from 20 different stations along Bushehr Province coastline including Asaluyeh, Kangan, Khark, Emam Hasan, and Bushehr from January 2019 to February 2019 (Fig. 1). The geographical location of selected stations is shown in Fig. 1. The sediment samples were withdrawn based on five-point sampling approach with a length of 10 m. Briefly, at each station, five sediment samples at four corners and center with a depth of 5 cm were collected. In general, 3 kg sediment samples were immediately placed in a pre-cleaned glass container with polytetrafluoroethylene screw caps and immediately transported to the laboratory.
Case study location ((a) Assaluyeh, (b) Kangan, (c) Khark, (d) Emam Hasan, and (e) Bushehr) (Figure was prepared by Arc GIS version 10.3 (https://www.esri.com/en-us/home).
In case of fish samples, 20 inhabitual Fish samples as one of most commercial fish along the Persian Gulf coastline were withdrawn by a trawl net in different cities and island mentioned earlier. The fish samples were immediately kept at cool box and transferred to laboratory for further analysis. Then, the fish samples were washed with distilled water and prepared to extract PAHs from their edible parts. Then, the edible parts of fish samples were put in the oven at 80 °C for 18 h. The dried samples were powdered for PAHs extraction and further analysis19.
PAHs extraction from sediment and fish samples
Extraction of PAHs from sediment samples was carried out according to the following procedure: 250 mL dichloromethane-n-hexane (1:1) was added to 10 g of each freeze-dried sediment and then the mixture was placed in soxhlet for 8 h. The residual was condensed to 15 mL by a rotary evaporator. Active copper (2–3 g) was added to the residual for eliminating sulfur, and so the mixture was filtered to 24 h. The residual was passed from a column comprising 10 g silica, 10 g active alumina, and 2 g anhydrous sodium sulfate. The mixture was concentrated to 5 ml again and was placed in scaled vials. Once the solvent was evaporated completely, 1 mL acetonitrile was added to the sample for high-performance liquid chromatography (HPLC) injection20. In case of PAHs in fish samples, 5 g dried edible part of Indian halibut was carefully mixed with 5 mL KOH (50%) and 75 mL methanol. The mixture was placed in soxhlet for 4 h. After transferring the liquid phase to the separator funnel, 100 mL n-hexane was added and vigorously shaken for 3 min. The methanol- KOH solution was evacuated and then hexane phase was also washed with 50 mL methanol–water solution (8:1) and 50 ml water. The samples were passed from the column containing 15 g silica, 10 g active alumina, and 1 g anhydrous sodium sulfate. 1 mL n-hexane was added to each sample to prepare for further analysis and Gas Chromatography (GC) analysis.
Analytical analysis
The measurement of different components of PAHs in the samples were performed by a gas chromatography–mass spectrometry (GC 6890, AGILENT, MS 5973N, Mode EI, detector:MS).
Generally, the concentrations 16 different aromatic compounds: Acenaphthylene, Naphthalene, Acenaphthene, Phenanthrene, Anthracene, Fluoranthene, Fluorene, Pyrene, Chrysene, Benzo(b)fluoranthene, Benzo(k)fluoranthene, Benzo(a)anthracene, Benzo[a]pyrene, Indeno(1,2,3,cd)pyrene, Benzo(g.h.i)perylene, Dibenz[a,h]anthracene were analyzed according to EPA method 3500C21 and National standard of Iran (19,238)22.
Risk assessment
The dietary daily intake (DDI) values of PAHs via ingestion Indian halibut were estimated by assumption an average weight of 70 kg for adults using Eq. (1)11,
where Ci is PAHs concentration as mg/kg and IFR denotes ingestion rate (kg/day). The toxicity and carcinogenity of the high molecular weight PAHs compared to BaP were determined by toxic equivalent quotient (TEQ). This quotient is related to the valence of any congener for create modification in DNA of human. The BaP carcinogenic equivalent (TEQ) for the individual PAHs in sediment and fish samples was calculated by Eq. (2)19.
where Ci = concentration of the individual PAHs in the sampling sediments and Indian halibuts (mg.kg–1 dw), and TEFn is toxic equivalence factor for individual PAHs.
The incremental lifetime cancer risk (ILCR) evaluates exposure health risk from PAHs according to USEPA standard. The major pathways for human exposure to sediments PAHs include (a) inhalation, (b) ingestion, and (c) dermal contact, with the age categories of children (1–11 years) and adults (18–70 years). The following Eqs. (3), (4), (5) were applied to evaluate the incremental lifetime cancer risk (ILCR) from PAHs in the sediments23.
In addition, the excess cancer risk of PAHs due to ingestion Indian halibut was estimated using Eq. (6)11.
Human risk model parameters in the sediment and Indian halibut samples are presented in Tables1 and 2, respectively.
Data analysis
The SPSS-23 software was applied to the statistical analyses of results. The results were presented as the average ± standard deviation (SD). The correlation between data was tested with Pearson’s correlation coefficient and analysis of variance (ANOVA). The level of P < 0.05 was significant.
Ethics declarations
We declare that this study was conducted in compliance with the ARRIVE (animal research: reporting of in vivo experiments) guidelines, adhering to its core principles and guidelines. We have followed the necessary steps outlined in the ARRIVE guidelines to minimize suffering and ensure appropriate care and welfare for the experimental animals. We have provided detailed descriptions of the methods and procedures of the animal experiments, and have reported relevant statistical analyses and data comprehensively. The method of this study has been approved by the Ethics Committee of Bushehr Branch, Islamic Azad University, Bushehr, Iran, and the animal experiments were approved by the Ethics Committee of Bushehr Branch, Islamic Azad University, Bushehr, Iran (16-11-8458, 1400/02/05).
Results and discussion
PAHs concentration in sediments
The mean concentration of different compounds of PAHs in different sediment samples drawn from five cities in Bushehr province is summarized in Table 3.
As summarized in Table 3, the individual PAHs except for Benzo(b)flouranthene (BbF) and Benzo(ghi) perylene (BgP) were detected in different sediment stations of study area. In addition, Acenaphthene was measured with highest average concentration of 2.275 ± 4.993 mg.kg–1 dw at the sediment. The lowest mean concentration of PAHs in the sediment belonged to Benzo(a) pyrene (BaP) 0.004 ± 0.012 mg.kg–1 dw. The average PAHs concentrations in the sediment samples of Asaluyeh, Kangan, Khark, Emam Hasan, and Bushehr stations are illustrated in Fig. 2.
As illustrated in Fig. 2, the average total PAHs concentration in Asaluyeh, Kangan, Khark, and Emam Hasan stations were 14.267, 2.777, 13.562, 3.865 mg.kg–1 and ND, respectively. Of interesting, no PAH components were detected in Bushehr station. The most possible reason for differences in PAHs concentration in different sampling stations are attributed to type and amounts of pollutants derived from industrial activities. In addition, factors such as organic carbon content, structure, diameter of sediment particles, and water solubility are effective in the distribution and abundance of PAHs in sediments24. Ali Ranjbar Jafarabadi and et al.25 surveyed the PAHs concentration in three islands (Qeshm, Hengam, Hormoz) with dense human activities; the authors reported that PAHs in reef surface sediments were in range of 274 to 1098 ng. g–1 dw25. In addition, Gui Wang and et al.4 surveyed the PAHs concentrations in surface sediments from Dingzi Bay, China. The authors reported that PAHs in sediment samples were ranged from 71.38 to 163.28 ng⋅g–14.
PAHs concentration in Indian halibut fish
Table 3 summarizes the mean concentration of different individual PAHs in edible parts of Indian halibut samples drawn from different stations. As summarized in Table 3, the individual PAHs were detected in different edible parts of Indian halibut samples. In addition, Naphthalene (Nap) was measured with highest average concentration of 3.906 ± 3.039 mg.kg–1 dw at the Indian halibut. The lowest mean concentration of PAHs in the Indian halibut belonged to Benzo(a) pyrene (BaP) 0.040 ± 0.055 mg.kg dw. The average PAHs concentrations in the Indian halibut samples of Asaluyeh, Kangan, Khark, Emam Hasan, and Bushehr stations are illustrated in Fig. 2. As illustrated in Fig. 2, the average total PAHs concentration in Asaluyeh, Kangan, Khark, Emam Hasan, and Bushehr stations were 22.999, 13.400, 20.977, 12.400 and 4.260 mg.kg–1 dw, respectively. The ƩPAH concentration was highest at Asaluyeh area, but was not significantly difference (p > 0.05) among the samples of PAHs. Furthermore, PAHs concentrations in the edible part of fishes were higher than those in the sediments, suggesting the ability of PAHs accumulation by the fishes. Due to the presence of fat tissues of living organisms, the pollutants are absorbed effectively in their tissues. On the other hand, hydrocarbon pollutants present in the sediments are decomposed in various ways such as optical photosynthesis and microbial processes26,27. Hongliang Zhang and et al.28 surveyed the PAHs in four common wild marine fish in Zhoushan Archipelago, East China Sea. The authors reported that PAHs concentration in edible muscles of fishes were 34.7–108 ng.g–1 wet weight and Four-ring and six-ring PAH congeners constitute the most and least percentages of the total PAHs, respectively28. Xiaoqin Lin and et al. surveyed the PAHs concentration in marine organisms in Shenzhen coastal waters of China and the results indicated that benzo[a]pyrene with concentration in range of non-detectable to 11.21 ng. g–1 dw was detected in most species18.
Human risk assessment
Human risk assessment attributed to exposure to sediments containing PAHs
Table 4 summarizes the ILCR attributed to exposure to PAHs in sediments via ingestion, inhalation and dermal. ILCR for two groups of ages including child and adults were calculated in the present study (See Table 4). As summarized in Table 4, the average cancer risk for adult living in Asaluyeh, Kangan, Khark and Emam Hasan were 1.00 × 10–5, 1.93 × 10–7, 1.10 × 10–5 and 1.82 × 10–7, respectively. In addition, the average cancer risk for child living in Asaluyeh, Kangan, Khark and Emam Hasan were 1.04 × 10–5, 1.11 × 10–7, 1.14 × 10–5, and 1.89 × 10–7, respectively. Generally, the order of average cancer risk in four sampling stations for adults were as follows: Khark > Asaluyeh > Kangan > Emam Hasan. In addition, the order of average cancer risk in four sampling stations for child were as follows: Khark > Asaluyeh > Emam Hasan > Kangan. It is important to note that and as earlier mentioned, no PAH components were detected in Bushehr station. Therefore, ILCR was not reported in Table 4.
Ruqayah Ali Grmasha and et al. surveyed the human risk assessment attributed to exposure to PAHs in sediment in along Euphrates River. The results indicated that the calculated incremental lifetime cancer risk (ILCR) for adult and children were in the 10–2–10–3 range, which are higher than the values calculated in this study1. In addition, Yanan Chen and et al. reported that the ILCR for children and adult exposed to PAHs in farmland soil of Yinma River Basin, China were 1 × 10–6–1.36 × 10–6 and 1.40 × 10–6–4.22 × 10–6, respectively, which are comparable with the results presented in the current study29.
Human risk assessment attributed to ingestion inhabitual fish containing PAHs
Table 5 summarizes concentration (Ci), Dietary daily intake (DDI), Carcinogenic potencies (B(A)Pteq), and excess cancer risk (ECR) of different PAHs in Indian halibut samples withdrawn from five sampling stations in Bushehr province. As summarized in Table 5, no PAHs were detected in Indian halibut samples withdrawn from Bushehr; there is no human risk for ingestion the Indian halibut in this area. However, almost all different components of PAHs were detected in Indian halibut samples withdrawn from Asaluyeh, Kangan and Khark. While some PAHs component were not detected in Emam Hasan (See Table 5). In addition, as summarized in Table 5, the excess cancer risk (ECR) for Asaluyeh, Kangan, Khark and Emam Hasan for different components of PAHs in Indian halibut samples were 1.76 × 10–9–6.2 × 10–6, 5.28 × 10–9–5.96 × 10–5, 9.78 × 10–10–1.74 × 10–5, and 2.34 × 10–9–5.61 × 10–6, respectively. Therefore, the highest ECR were calculated in Kangan and Asaluyeh with high industrial activities on oil and derivatives. In the present research, the ECR values for every individual PAH except for DbA were less than threshold value of USEPA (10−6) (Table 5), but the collective values of ECR in Indian halibut samples in all studied areas exceeded the USEPA acceptable level30. Thus, high consumption of Indian halibut is the main and most probable reason for high potential cancer risk in the study area. Xiaoqin Lin and et al. surveyed the human health risk assessment attributed to consumption of marine organisms containing PAHs from Shenzhen coastal. The authors reported that the individual lifetime cancer risk associated with seafood consumption ranged from 1.10 × 10–5 to 1.52 × 10–5, indicating a potential cancer risk needed special attention. These results are in line with the results presented in the current study18. Chuchu Zhang and et al. investigated the PAHs concentration and attributed human risk assessment due to consumption of marine organisms from two fishing grounds, South Yellow Sea, China. The authors reported that the ECR from PAH-contaminated seafood consumption was slightly higher than the guideline value (10–6)31. Overall, the high ECR attributed to consumption of fish contain PAHs in Bushehr province with high industrial activities and oil and gas refinery is a great risk for people living in this area. Therefore, preventative measurements are required for lower the human risk assessment; the authorities must make the industries to meet the standard for discharge of effluent into aquatic environments.
Conclusion
The present study was developed to comprehensively investigate the human exposure assessment of PAHs for people living in one of most polluted area in the world due to industrial activities and oil refinery. To this end, different components of PAHs in sediments and fish samples in the north area of Persian Gulf were measured and the corresponding human risk assessment following ingestion, inhalation and dermal pathway were estimated. Overall, the human risk analysis and ECR attributed to PAHs in sediment and fish in Asaluyeh, as well-known most polluted area, were much higher than the value recommended by USEPA (10−6). Furthermore, a comprehensive and intense monitoring and assessment on spatial distribution of PAHs in sediments and fish can aid the authorities and decision maker to find a sustainable solution.
Data availability
All data generated or analysed during this study are included in this published article.
References
Grmasha, R. A. et al. Polycyclic aromatic hydrocarbons in the surface water and sediment along Euphrates River system: Occurrence, sources, ecological and health risk assessment. Mar. Pollut. Bull. 187, 114568 (2023).
Agah, H., Shadi, R., Eslami, Z. & Raihanizadeh, A. Distribution pattern and ecological risk assessment of heavy metals and PAHs in sediments of the entrance of Musa estuary, Persian Gulf to establish desalination plant. Reg. Stud. Mar. Sci. 57, 102725 (2023).
Mitra, S. et al. Characterization, source identification and risk associated with polyaromatic and chlorinated organic contaminants (PAHs, PCBs, PCBzs and OCPs) in the surface sediments of Hooghly estuary, India. Chemosphere 221, 154–165 (2019).
Wang, G. et al. Distribution, source, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments from Dingzi Bay, China. J. Sea Res. 193, 102387 (2023).
Awe, A. A. et al. Occurrence and probabilistic risk assessment of PAHs in water and sediment samples of the Diep River, South Africa. Heliyon 6, e04306 (2020).
Benson, N. U. et al. Occurrence, depth distribution and risk assessment of PAHs and PCBs in sediment cores of Lagos lagoon, Nigeria. Reg. Stud. Mar. Sci. 37, 101335 (2020).
Lin, F. et al. Distribution characteristics, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in sediments from the Qinhuangdao coastal wetland, China. Mar. Pollut. Bull. 127, 788–793 (2018).
Wang, Y. B., Liu, C. W., Kao, Y. H. & Jang, C. S. Characterization and risk assessment of PAH-contaminated river sediment by using advanced multivariate methods. Sci. Total Environ. 524–525, 63–73 (2015).
Han, B., Zheng, L. & Lin, F. Risk assessment and source apportionment of PAHs in surface sediments from Caofeidian Long Island, China. Mar. Pollut. Bull. 145, 42–46 (2019).
Lee, C.-C., Hsieh, C.-Y., Chen, C. S. & Tien, C.-J. Emergent contaminants in sediments and fishes from the Tamsui River (Taiwan): Their spatial-temporal distribution and risk to aquatic ecosystems and human health. Environ. Pollut. 258, 113733 (2020).
Tongo, I., Ogbeide, O. & Ezemonye, L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 4, 55–61 (2017).
Zhang, M. et al. Distribution, sources, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Subei Shoal, China. Mar. Pollut. Bull. 149, 110640 (2019).
Wang, X. et al. Heavy metal contamination in surface sediments: A comprehensive, large-scale evaluation for the Bohai Sea, China. Environ. Pollut. 260, 113986 (2020).
An, N. et al. Spatial distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in the reservoir sediments after impoundment of Manwan Dam in the middle of Lancang River, China. Ecotoxicology 25, 1072–1081 (2016).
Hernandez-Ramirez, A. G. et al. Detection, provenance and associated environmental risks of water quality pollutants during anomaly events in River Atoyac, Central Mexico: A real-time monitoring approach. Sci. Total Environ. 669, 1019–1032 (2019).
Ambade, B., Sethi, S. S., Kurwadkar, S., Kumar, A. & Sankar, T. K. Toxicity and health risk assessment of polycyclic aromatic hydrocarbons in surface water, sediments and groundwater vulnerability in Damodar River Basin. Groundw. Sustain. Dev. 13, 100553 (2021).
Yu, Z. et al. Bioaccumulation of polycyclic aromatic hydrocarbons (PAHs)in wild marine fish from the coastal waters of the northern South China Sea: Risk assessment for human health. Ecotoxicol. Environ. Saf. 180, 742–748 (2019).
Lin, X., Lin, L., Liao, Z., Wu, P. & Yang, C. Occurrence and distribution of polycyclic aromatic hydrocarbons (PAHs) in marine organisms from Shenzhen coastal waters and human health risk assessment. Mar. Pollut. Bull. 195, 115498 (2023).
Nahar, A. et al. Dissemination and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in water and sediment of Buriganga and Dhaleswari rivers of Dhaka, Bangladesh. Heliyon 9, e18465 (2023).
Raeisi, A. et al. Polycyclic aromatic hydrocarbons (PAHs) in coastal sediments from urban and industrial areas of Asaluyeh Harbor, Iran: Distribution, potential source and ecological risk assessment. Water Sci. Technol. 74, 957–973 (2016).
Lee, H. K. & Chung, D. S. Extraction and sample preparation. J. Chromatogr. A 1300, 1 (2013).
National Standard of Iran. PAH-19238. https://standard.inso.gov.ir/ (2020).
Pongpiachan, S. Incremental lifetime cancer risk of PM2.5 bound polycyclic aromatic hydrocarbons (PAHs) before and after the wildland fire episode. Aerosol Air Qual. Res. 16, 2907–2919 (2016).
Mahmoodi, M., Safahieh, A., Nikpour, Y. & Ghanemi, K. Distribution and sources of polycyclic aromatic hydrocarbons in the sediment of Bushehr coastal zone-Iran, Iran. J. Energy Environ. https://doi.org/10.5829/idosi.ijee.2012.03.02.0311 (2012).
Ranjbar Jafarabadi, A., Riyahi Bakhtiari, A. & Shadmehri Toosi, A. Comprehensive and comparative ecotoxicological and human risk assessment of polycyclic aromatic hydrocarbons (PAHs) in reef surface sediments and coastal seawaters of Iranian Coral Islands, Persian Gulf. Ecotoxicol. Environ. Saf. 145, 640–652 (2017).
Ingrid, L., Sahraoui, A.L.-H., Frédéric, L., Yolande, D. & Joël, F. Arbuscular mycorrhizal wheat inoculation promotes alkane and polycyclic aromatic hydrocarbon biodegradation: Microcosm experiment on aged-contaminated soil. Environ. Pollut. 213, 549–560 (2016).
Partila, A. M. Biodegradation of polycyclic aromatic hydrocarbons in petroleum oil contaminating the environment (2013).
Zhang, H. et al. PAH residue and consumption risk assessment in four commonly consumed wild marine fishes from Zhoushan Archipelago, East China Sea. Mar. Pollut. Bull. 170, 112670 (2021).
Chen, Y., Zhang, J., Zhang, F., Liu, X. & Zhou, M. Contamination and health risk assessment of PAHs in farmland soils of the Yinma River Basin, China. Ecotoxicol. Environ. Saf. 156, 383–390 (2018).
Liao, C.-M. & Chiang, K.-C. Probabilistic risk assessment for personal exposure to carcinogenic polycyclic aromatic hydrocarbons in Taiwanese temples. Chemosphere 63, 1610–1619 (2006).
Zhang, C. et al. Polycyclic aromatic hydrocarbons (PAHs) in marine organisms from two fishing grounds, South Yellow Sea, China: Bioaccumulation and human health risk assessment. Mar. Pollut. Bull. 153, 110995 (2020).
Funding
The funding was supported by Islamic Azad University, Bushehr, Iran.
Author information
Authors and Affiliations
Contributions
Ghafour Nourian: Writing, Methodology, Investigation. Neamat Jaafarzadeh Haghighi Fard: Conceptualization, Supervision. Abdul Rahim Pazira: Methodology. Esmaeil Kohgardi: Investigation, Analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nourian, G., Jaafarzadeh Haghighi Fard, N., Pazira, A.R. et al. An extensive investigation on human risk associated with PAHs in fish and sediment in Bushehr, Northern of Persian Gulf. Sci Rep 14, 10585 (2024). https://doi.org/10.1038/s41598-024-61197-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61197-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.