Abstract
To elucidate the neurological features of Hansen disease. The medical records of patients with confirmed Hansen disease transferred from the neurology department were reviewed, and all medical and neurological manifestations of Hansen disease were assessed. Eleven patients with confirmed Hansen disease, 10 with newly detected Hansen disease and 1 with relapsed Hansen disease, who visited neurology departments were enrolled. The newly detected patients with Hansen disease were classified as having lepromatous leprosy (LL, n = 1), borderline lepromatous leprosy (BL, n = 2), borderline leprosy (BB, n = 2), borderline tuberculoid leprosy (BT, n = 1), tuberculoid leprosy (TT, n = 2), or pure neural leprosy (PNL, n = 2). All of the patients with confirmed Hansen were diagnosed with peripheral neuropathy (100.00%, 11/11). The symptoms and signs presented were mainly limb numbness (100.00%, 11/11), sensory and motor dysfunction (100.00%, 11/11), decreased muscle strength (90.90%, 10/11), and skin lesions (81.81%, 9/11). Nerve morphological features in nerve ultrasonography (US) included peripheral nerve asymmetry and segmental thickening (100.00%, 9/9). For neuro-electrophysiology feature, the frequency of no response of sensory nerves was significantly higher than those of motor nerves [(51.21% 42/82) vs (24.70%, 21/85)(P = 0.0183*)] by electrodiagnostic (EDX) studies. Nerve histological features in nerve biopsy analysis included demyelination (100.00%, 5/5) and axonal damage (60.00%, 3/5). In addition to confirmed diagnoses by acid-fast bacteria (AFB) staining (54.54%, 6/11) and skin pathology analysis (100.00%, 8/8), serology and molecular technology were positive in 36.36% (4/11) and 100.00% (11/11) of confirmed patients of Hansen disease, respectively. It is not uncommon for patients of Hansen disease to visit neurology departments due to peripheral neuropathy. The main pathological features of affected nerves are demyelination and axonal damage. The combination of nerve US, EDX studies, nerve biopsy, and serological and molecular tests can improve the diagnosis of Hansen disease.
Similar content being viewed by others
Introduction
Leprosy, also known as Hansen's disease, is caused by Mycobacterium leprae infection and affects mainly the skin and peripheral nervous system. Neuropathy is an integral symptom of Hansen disease1. Hansen disease is associated with neuropathy, which results in nerve function impairment and causes disabilities2. Neuropathy and its related disabilities are the major medical consequences of Hansen disease and remain a global medical concern3.
Hansen disease is one of the most common treatable peripheral neuropathies in the world4. Mycobacterium leprae (and M. lepromatosis) is the only pathogenic bacteria able to infect peripheral nerves5. Antimicrobial therapy is effective3, and early treatment is associated with good outcomes4; however, neuropathy remains a problem, especially if diagnosis and treatment are delayed3. Neural impairment results in a set of sensory, motor and autonomic disturbances5. Despite major advances in understanding the mechanisms of M. leprae entry into peripheral nerves, most aspects of the pathogenesis of Hansen disease neuropathy are poorly understood3.
According to the distinct clinical manifestations and immunological spectra of the disease, Ridley and Jopling classified Hansen disease into five polar forms: tuberculoid (TT), borderline tuberculoid (BT), borderline (BB), borderline lepromatous (BL), and lepromatous (LL)6. For treatment purposes, the World Health Organization (WHO) currently classifies Hansen disease cases into two groups according to the number of skin lesions: multibacillary (MB) and paucibacillary (PB)7. Pure neural leprosy (PNL) is a rare clinical form of Hansen disease in which patients do not present with classic skin lesions but have a high burden of disability associated with the disease8. Dermatologic clinical manifestations of Hansen disease are highly variable and are described as “great imitators”9, while the clinical neurological manifestations of Hansen disease are still unclear.
In this study, we retrospectively described the neurological clinical data of patients with confirmed Hansen disease transferred from the neurology department. We hope that this work will provide further insight into the clinical characteristics of Hansen disease in neurology.
Results
Clinical characteristics of patients with Hansen disease who visited the neurology department
A total of 11 patients (6 males, 5 females; mean age 43.55 ± 13.13 years) with confirmed Hansen disease who initially visited and/or were referred to the neurology department during the study period were evaluated (Table 1). The patients with Hansen disease were of Han nationality and were from 9 provinces in different endemic regions [Northeast: Heilongjiang and Jilin; North: Hebei; East: Anhui; Southwest: Hubei, Hunan, Sichuan, and Yunnan; and Northwest: Shaanxi and Xinjiang] in China. The main complaint patients mainly presented with was limb numbness (63.63%, 7/11), inability to walk (18.18%, 2/11), and inability to lift their finger (18.18%, 2/11). Through physical examination, obvious skin lesions were found in 81.81% (9/11) of the patients, and a skin numbness area was found in 18.18% (2/11) of the patients. Through consultation, only one patient (9.09%, 1/11) was diagnosed with newly detected Hansen disease, while 90.90% (10/11) of the patients denied a history of contact with an individual with Hansen disease.
Neurologic evaluation
Sensory and motor nerve dysfunction was evaluated by neurologists at the general hospital (Table 2). Shallow sensation deficits occurred in all patients (100.00%, 11/11). Muscle weakness caused by peripheral neuropathy occurred in 81.82% (9/11) of the patients. Froment's sign was positive in 45.45% (5/11) of the patients. Steppage gait was observed in 36.36% (4/11) patients.
Neurosonography findings
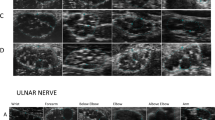
The peripheral nerves of nine patients were evaluated via ultrasonography (Table 3). Nerve cross-sectional area (CSA) was bilaterally measured via ultrasonography. There was a significant increase in the CSA of the affected nerves. The peripheral nerve form features revealed by ultrasonography also included asymmetrical and segmental thickening in all of the patients (100.00%, 9/9); swelling in 4 patients (44.44%, 4/9); decreased echo intensity in nerve bundles in 4 patients (44.44%, 4/9); and increased echo intensity in the epineurium in 2 patients (22.22%, 2/9). Good continuity of the nerve was observed in 66.67% (6/9) of the patients, and blood flow, as measured by color Doppler blood flow imaging (CDFI), was increased in 4 patients (44.44%, 4/9).
Electrodiagnostic findings
The nerve conduction velocity (NCV), F-wave, H reflex, skin sympathetic response (SSR) and needle electromyography (EMG) were performed for 10 patients (Table 4). The ulnar (20/20 & 20/20), median (20/20 & 20/20), radial (5/20 & 5/20), common peroneal (20/20 & 19/20), and tibial (20/20 & 18/20) peripheral nerves were tested for motor and sensory NCV, respectively.
The main electrodiagnostic (EDX) findings were as follows: (1) both motor and sensory fibers of peripheral nerves were affected in 100.00% of the patients (10/10); (2) asymmetrical nerve damage was present in not only motor but also sensory fibers in 100.00% of the patients (10/10); (3) motor fibers were most frequently affected in the common peroneal (90.00%, 18/20), tibial (55%, 11/20), median (40.00%, 8/20), ulnar (35%, 7/20), and radial (20.00%, 1/5) nerves; (4) sensory fibers were most frequently affected in the common peroneal (63.15%, 12/19), tibial (61.11%, 11/18), ulnar (60.00%, 12/20), median (50.00%, 10/20), and radial (40.00%, 2/5) nerves; (5) sensory fibers (57.31%, 47/82) and motor fibers (52.94%, 45/85) were similarly affected (P = 0.6997); and (6) sensory [62.16% ((23/37)) vs 53.33% (24/45)](P = 0.7162) and motor [72.50% (29/40) vs 35.55% (16/45)] fibers of the lower limbs and upper limbs were similarly affected (P = 0.0669).
The NCV findings were as follows (Table 5): (1) “No response” was the main feature for both sensory and motor fibers and occurred in more sensory fibers (51.21%, 42/82) than motor fibers (24.70%, 21/85) (P = 0.0183*). (2) “Decreased amplitude” was the secondary feature [24.70% (21/85) vs. 12.19% (10/82)] (P = 0.1160), followed by “decreased conduction velocity” [9.41% (8/85) vs. 8.53% (7/82)] (P > 0.9999), and “prolonged distal latency” [5.88% (5/85) vs. 0.00% (0/82)] (P = 0.0602) for both motor and sensory fibers. Moreover, there were no significant differences between motor and sensory fibers.
In addition, F-wave abnormalities were found in 3 patients; no response was observed in 1 patient, prolonged latency was observed in 2 patients, and a decreased frequency was observed in 1 patient.The H-reflex was normal in 7 patients. However, SSRs were not detected in 2 patients. EMG demonstrated neurogenic damage in 10 patients (Table 4).
Nerve and skin biopsy findings
Nerve biopsy was performed for 6 patients (Table 6). In hematoxylin–eosin (HE) staining, the myelin sheath and axon were damaged or absent in 66.66% (4/6) of the patients, while Schwann cells were hyperplastic in 50.00% (3/6) of the patients but were absent in 16.67% (1/6) of the patients. Inflammation was absent in 50.00% (3/6) of patients and was present in 50.00% (3/6) of patients. Tissue cell infiltration was observed in 33.33% (2/6) patients. Fibrous tissue of the nerve perineurium presented hyperplasia in 16.67% (1/6) of patients. Edema and mucus denaturation presenting as hyperplasia were observed in 16.67% (1/6) of patients.
In terms of special staining of nerve biopsy, samples from 2 patients underwent AFB staining, with negative results for both (0.00%, 0/2). Samples from two patients underwent weak acid-fast staining; a positive result was found for 1 patient (50.00%, 1/2), while a negative result was found for the other patient.
In terms of immunohistochemistry (ICH), the following positive results were obtained: (1) nerve-related indicators: myelin basic protein (MBP) (75%, 3/4); neurofilament (NF) (66.67%, 2/3); SRY-related HMG-box 10 (SOX-10) (100.00%, 3/3); Luxol fast blue (LFB) (33.33%, 1/3); and S-100-beta (100.00%, 2/2). (2) Cell-related indicators: macrophage (CD68) (100.00%, 6/6); T cells (CD3, CD4, CD5, and CD8) (50.00%, 3/6); B cells (Bcl-2, Bcl-6, CD10, CD20, CD21, and CD23) (50.00%, 3/6); and plasma cells (CD38 and CD138) (16.67%, 1/6).
Skin pathology analysis was performed for 8 patients (Table 7). The positive results were as follows: AFB, 75.00% (6/8); noninfiltration zone, 25.00% (2/8); lymphocytes, 87.50% (7/8); histiocytes, 62.50% (5/8); foam cells, 37.50% (3/8); and granulomas, 50.00% (4/8). All the skin pathology results supported the diagnosis of Hansen disease.
Indexes for differential diagnosis
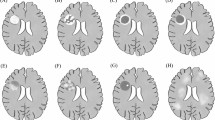
Other indexes, including indices of metabolism, rheumatism, immunity, nutrition, drugs, toxicity, tumors, infection, physical compression, the blood system, and the nervous system, were screened for the differential diagnosis of Hansen disease from neuropathy (Table 8). The indicators of Rheumatism & Immunity were positive in 37.50% (3/8) patients, involved positive results of Proliferating Cell Nuclear Antigen (PCNA) in case 4, Anti streptolysin O (ASO) in case 10, and Antinuclear antibody (ANA) in case 11, respectively. As an indicator of CNS demyelination, MBP IgG was also increased in Patient 4, and demyelination in the periventricular white matter of the lateral ventricle was detected by magnetic resonance imaging (MRI) in Patient 11. All the other results were negative.
Hansen disease symptom monitoring criteria
All patients with Hansen disease were evaluated with the Hansen disease Symptom Monitoring criteria (Supplementary Table S1), and those meeting the criteria of suspected Hansen disease were transferred from the neurology departments to the Hansen disease prevention facility.
Physical examination by Hansen disease prevention specialists
Skin lesions, nerve lesions, and disability grades were evaluated by Hansen disease prevention specialists (Table 9). Skin lesions occurred in 81.82% (9/11) of the patients. For 2 patients without obvious skin lesions, skin numbness was found in 1 patient, and a deceased prickle sensation was found in the other patient.
Nerve lesions occurred in all patients (100.00%, 11/11) and presented as more than 2 nerve lesions (Table 9). All of the patients (100.00%, 11/11) were evaluated for nerve thickening and nerve tenderness by nerve palpation, and the percentage of patients with nerve tenderness was 33.12% (51/154), which was greater than that with nerve thickening (12.34%, 19/154). (P < 0.05) (Table 5). The nerves most affected by tenderness were the tibial (54.54%, 10/22), ulnar (40.90%, 9/22), common peroneal (31.81%, 7/22), radial (22.72%, 5/22), medial (22.72%, 5/22), and supraorbital (9.09%, 2/22) nerves, but no tenderness of the greater auricular nerve was detected (0.00%, 0/22). The nerves most affected by thickening were the tibial (31.81%, 7/22), ulnar (31.81%, 7/22), supraorbital (9.09%, 2/22), greater auricular (9.09%, 2/22), and radial (4.54%, 1/22) nerves; however, no median (0.00%, 0/22) or common peroneal (0.00%, 0/22) nerve thickening was detected.
Twenty-one nerves were tested by both nerve ultrasonography and nerve palpation: [33.33% (7/21) vs 9.52% (2/21), P = 0.1595], [52.38% (11/21) vs 4.76% (1/21), P = 0.0171*] and [14.29% (3/21) vs 9.52% (2/21), P > 0.9999] of the nerves showed bilateral, unilateral, and a lack of nerve thickening according to nerve ultrasonography and nerve palpation, respectively. The positive finding of unilateral nerve thickness by nerve ultrasound were significantly higher than those by nerve palpation.
Disability occurred in all of the patients (100.00%, 11/11) (Table 9). Grade 2 disability (G2D) occurred in 81.81% (9/11) of patients, while grade 1 disability (G1D) occurred in 18.18% (2/11) of patients. Insensitivity occurred in all of the patients (100.00%, 11/11), claw-hand motion occurred in 36.36% (4/11) of the patients, foot droppage occurred in 36.36% (4/11) of the patients, and muscle atrophy occurred in 27.27% (3/11) of the patients. In addition, 9.09% (1/11) of the patients presented with lagophthalmos, 9.09% (1/11) presented with equinus, and 9.09% (1/11) presented with complex plantar ulcers, amputation, and wrist drop.
Auxiliary laboratory Hansen disease tests
For auxiliary laboratory Hansen disease tests, AFB staining and molecular tests were performed in all 11 patients: AFB staining was positive in 54.54% (6/11) patients, while molecular test [Nested PCR for Repetitive element (RLEP); dapsone resistance—associated target (folP1); rifampicin resistance-associated target (rpoB); quinolone resistance—associated target (gyrA), confirmed as M.leprae by sequencing and NGS] results were positive in 100.00% (11/11) patients. Skin pathology analysis, the “gold standard” for diagnosis of Hansen disease, was performed for 8 of the 11 (63.63%) patients, and the diagnosis of Hansen disease was confirmed for all the patients (8/8, 100.00%). Among the serological tests, the NDO-LID rapid test was performed for 11 patients, and results were positive for 36.36% (3/11) of the patients. In addition, ELISPOT was performed in only 1 patient (9.09%, 1/11), and a positive result was achieved (1/1, 100.00%) (Table 9).
Confirmed diagnosis of Hansen disease
Hansen disease was confirmed in eleven patients, including 10 patients with newly detected and 1 patient with relapse, according to the diagnostic criteria for Hansen disease in WHO10. The details are shown in Table 9.
Deteriorated disability due to delayed diagnosis
In this study, delayed diagnosis and G2D occurred in 90.90% (10/11) and 81.81% (9/11) of the patients, respectively. Multiple medical records of Hansen disease patients revealed worsening disability (100.00%, 3/3) (Table 10), decreased muscle strength (100.00%, 3/3) (Table 11), worsening nerve conduction (66.66%, 3/3) (Table 12) and worsening nerve ultrasonography findings (100.00%, 1/1) (Table 13) due to delayed diagnosis.
Discussion
Hansen disease is a chronic infection caused by Mycobacterium leprae that is associated with peripheral neuropathy. Early Hansen disease detection and treatment with multidrug therapy are the most important steps in preventing deformity and disability11.
The gold standard for Hansen disease diagnosis is dermatological and neurological clinical examination, bacilloscopy/AFB staining of the SSS and skin biopsy sample analysis12,13. To confirm the diagnosis, laboratory tests, AFB staining, skin and nerve biopsy, and serological and molecular tests were performed for all of the patients. In this study, the diagnoses of 11 patients with Hansen disease were confirmed: as 1 LL, 2 BL, 2 BB, 1 BT, 2 TT, 2 PNL, and 1 relapsed. The diagnoses of six (1 LL, 2 BL, 2 BB and 1 relapsed) patients were confirmed by AFB staining, and the diagnoses of 8 (1 LL, 2 BL, 2 BB, 1 BT, 1 TT, and 1 relapsed) patients were confirmed by skin biopsy. AFB staining results were negative in 1 TT and 2 PNL patients, and skin biopsy was not suitable for some TT or PNL patients, which implies the limited diagnostic value of traditional laboratory tests for Hansen disease. Notably, the nerve biopsy sample of 1 (BB) patient was positive for AFB staining, which provided morphological evidence of Mycobacterium leprae infection in the in situ nerve tissue.
Since the 1980s, components isolated from M. leprae have been identified14,15; an increasing number of experimental trials have used these components to detect antibodies in Hansen disease patients. These detection methods are mainly used for diagnosing MB of Hansen disease, monitoring treatment response, and predicting leprosy reactions and as active search strategies to identify new cases in high-risk populations16,17,18,19.
In the past three decades, definitive identification of M. leprae has been possible through the development of methods for the extraction, amplification, and identification of M. leprae DNA in clinical specimens via PCR. PCR has been ascertained to be especially valuable in diagnosing difficult cases, such as those with PNL, PB, and atypical clinical presentations and histopathological features compatible with Hansen disease20. These methods were also used in this study, and the positive results supported the diagnosis of Hansen disease, especially for TT and PNL patients.
In this study, serological (NDO-LID rapid test and ELISPOT) and molecular [multitarget genes, PCR, Sanger sequencing, and next-generation sequencing (NGS) in multiple specimens] methods were used for the detection of M. leprae. These methods successfully detected M. leprae and supported the diagnosis of Hansen disease, including in patients with tuberculoid forms and PNL. NDO-LID rapid test results were positive in 3 lepromatous form (1 LL, 1 BL, and 1 BB) patients, which implied that the method has limited value for the auxiliary diagnosis of tuberculoid form and/or PNL. However, the ELISPOT showed greater diagnostic value for tuberculoid form of Hansen disease, as it was performed in 1 patient with suspected Hansen disease without skin lesions, and the positive result supported the diagnosis of PNL. Notably, positive molecular signals were detected via nerve biopsy in 2 patients with Hansen disease (1 patient with PNL as determined by Sanger sequencing and 1 patient with a relapse as determined by NGS), which provided etiological evidence of Mycobacterium leprae infection of nerve tissue.
To confirm the diagnosis of Hansen disease, an endemic region, contact history, skin and nerve examination, AFB staining, and skin pathology analysis were performed for all patients. Six, three and two patients from high-, middle-, and low-endemic regions, respectively, were diagnosed with Hansen disease in China. One patient had a contact history of Hansen disease, and the other patient, a clinically cured patient, had neurologic symptoms and signs of Hansen disease onset after multidrug therapy (MDT) was completed. The remaining patients had no contact history of Hansen disease. Notably, (1) Two patients with Hansen disease, as the floating population, moved from a high-endemic region to a low-endemic region of Hansen disease in China (from Yunnan to Hebei, and from Sichuan to Xinjiang, respectively). This was in consistent with another study in China21, which implied Hansen disease patients in the floating population overflowed from high-endemic areas to low-endemic areas. (2) All 11 patients with confirmed Hansen disease were from provinces other than Beijing, the capital of China, and most of them experienced multiple hospital visits, delayed diagnosis and permanent disability and deformity. This implies that Hansen disease patients with difficult cases were transferred to areas with medical advantages according to their medical needs.
The clinical characteristics of patients with Hansen disease in neurology departments have mostly been described in case reports, with diagnoses based on a single technology, and overall knowledge of the neurological features of Hansen disease is still limited. To elucidate the neurological characteristics of Hansen disease, nerve ultrasonography, EDX, and nerve biopsy findings were thoroughly evaluated.
Nerve ultrasonography has been performed to assess peripheral nerves in patients with Hansen disease in multiple studies. A significant increase in the CSA of the ulnar, median, radial, common peroneal, and tibial nerves of Hansen disease patients has been mostly reported22,23,24,25,26,27,28,29. The sonographic findings of Hansen disease patients are characterized by an increased CSA and a pattern of asymmetry and focality of this thickening, with high sensitivity and specificity for early diagnosis24. In our previous study, we also observed a combination of an enlarged CSA of nerves in the upper limbs and atrophy of lower limb nerves in clinically cured Hansen disease patients30. In addition to an abnormal CSA, a loss of fascicles, hypoechogenicity and increased neural vascularity have also been reported in patients with Hansen disease22. In this study, asymmetrical and segmental thickening, swelling, and abnormal signs of echo intensity and blood flow were detected via nerve ultrasonography. The US results in our study are consistent with those of previous reports.
The risk factors for nerve enlargement in patients with Hansen disease were MB leprosy25, leprosy reaction26, neuritis27, and impairment of function28. Nerve ultrasonography for Hansen disease can be applied in the early diagnosis of Hansen disease in household contacts (HHCs)29 and for monitoring the therapeutic effects of MDT27. Compared with healthy volunteers, individuals with at least two thickened nerves assessed in the active search campaign had a 23.1 greater chance of having Hansen disease than healthy individuals24. Nerve ultrasonography showed greater sensitivity than clinical examination for detecting peripheral nerve thickening in Hansen disease patients, which may therefore improve the sensitivity of the diagnostic criterion of peripheral nerve enlargement in the diagnosis and classification of Hansen disease28. In this study, we also demonstrated that, when compared with the results of nerve ultrasonography, nerve palpation lacks precision in detecting nerve thickness. Due to the very limited accuracy of nerve palpation, the systematic and comprehensive development of nerve ultrasonography for both Hansen disease patients and close contacts is needed.
Abnormal nerve conduction in patients with Hansen disease was characterized by reduced conduction velocities in addition to changes in prolonged distal latency and decreased amplitude in the affected nerves31,32,33. Sensory latency and amplitude changes were more severe than motor latency and amplitude changes in patients presenting with muscle palsies32,33. Patients with the TT type of Hansen disease were the most affected33,34. Electrophysiological testing showed both axonal and demyelinating nerve involvement35,36.
The number of nerve abnormalities detected by electrophysiological testing is significantly greater than that detected clinically36. In Hansen disease patients, motor weakness, sonographic thickening, and motor conduction abnormalities are positively correlated37. An approach combining nerve ultrasonography and electrophysiological testing can improve Hansen disease diagnosis38.
Nerve histology is studied less often than cutaneous histology. Depending upon the host immune response, a spectrum of pathological changes in the skin are reflected in nerves. At the tuberculoid end of the spectrum, epithelioid granulomas with little or no AFB staining are observed, while at the lepromatous end, abundant AFB staining of Schwann cells, macrophages and plasma cell infiltrates are observed39. Other nerve changes include mild perineural edema, partial involvement of fascicles, a loss of fiber density and areas of demyelination40. Demyelination can occur primarily at the site of acute neuritis or secondary to chronic axonopathy. In severe cases, there is destruction of the nerve parenchyma with caseous necrosis with epithelioid infiltrates and segmental necrotizing granulomatous neuritis41. During spontaneous or posttreatment regression of nerve lesions, residual signs of chronic inflammation with lymphocytic infiltration and evidence of regeneration and fibrosis are observed42. The results of nerve biopsy in this study were consistent with those of previous studies.
Along with peripheral nerves, the central nervous system (CNS), spinal root ganglion and brachial plexus are involved in Hansen disease43,44. MBP, an indispensable protein of myelinated axons, is abundant in CNS myelin and has long been studied as a factor in the pathogenesis of neurodegenerative diseases, such as multiple sclerosis (MS)45 and reported in Hansen disease46. In this study, demyelination in the periventricular white matter of the lateral ventricle was detected by MRI in one patient, and MBP IgG was elevated in another patient. This finding provides evidence of demyelination in the CNS and peripheral nervous system in patients with Hansen disease.
Conclusion
Hansen disease patients may visit the neurology department due to neurological manifestations. Patients with peripheral neuropathy with or without skin lesions, after excluding other causes, were considered suspected to have Hansen disease.
The ultrasonographic features of Hansen disease included asymmetrical and segmental patterns of increased CSA. The electrophysiological features included no response, reduced conduction velocities, prolonged latency and decreased amplitude in the affected nerves. Nerve pathological features included AFB staining; axon and myelin sheath destruction; Schwann cell hyperplasia or an absence of macrophage, plasma cell, and/or lymphocyte infiltration; fibrosis; and edema.
The combination of ultrasonographic and electrophysiological testing with serological and molecular testing may improve the diagnosis of Hansen disease. Nerve biopsy can be chosen for difficult cases of PNL when the diagnosis is unclear and to provide in situ evidence for the pathogen in nerve tissue.
Methods
Ethics statement
This study was approved by the Medical Ethics Committee of Beijing Friendship Hospital, Capital Medical University, Beijing, P. R. China. Written informed consent was obtained from all adult participants. All the procedures that involved human participant selection were performed in accordance with the ethical standards of the institutional and/or national research committee and Declaration of Helsinki, 1964, and its later amendments or comparable ethical standards.
Hansen disease patients
This retrospective analysis was carried out at multiple centers. All the patients with suspected Hansen disease were transferred by neurologists from the neurology departments of general hospitals to the Hansen disease prevention facility. Patients with suspected Hansen disease for whom Hansen disease diagnosis was confirmed between January 1, 2018, and September 1, 2020, were included in this study. The diagnosis of Hansen disease was based on the Leprosy Diagnosis Criteria of WHO10.
Data collection
The medical records of all patients were retrospectively reviewed as follows:
-
(1)
The patients’ clinical data, including sex, age, ethnic group, domicile, place of residence, main complaint, disease duration, clinical symptoms, time of appearance of specific symptoms, Hansen disease contact history, physical examination (skin, nerve lesions, and grade of disability), and ancillary test results (AFB staining and skin pathology analysis), were collected from the Leprosy Management Information System in China (LEPMIS).
-
(2)
The patients’ neurologic examination results were retrieved from the medical records of the neurology departments of the transfer hospitals. Neurologic examinations were performed by neurologists. Skin lesions were assessed, as were the muscle strength, movement, and sensory impairment of limbs.
-
(3)
The features of the ulnar, median, radial, brachial plexus, common peroneal, tibial, and sciatic peripheral nerves were evaluated by nerve ultrasonography. The CSA, swelling, echo intensity of nerve bundles and the epineurium, and the continuity and CDFI of the bilateral nerves were measured via ultrasonography47,48.
-
(4)
The electrophysiologic features of the peripheral nerves, specifically the ulnar, median, radial, common peroneal, and tibial tract nerves, were analyzed. Motor and sensory NCV, F-wave, H reflex, SSR and EMG were conducted based on standard procedures.
-
(5)
The pathological features of the nerves were detected via HE staining, special staining, and ICH in the nerve biopsy.
-
(6)
The indexes for the differential diagnosis of Hansen disease from neuropathy were tested, and the results were collected49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,3,67,68,69,70,71,72,73,74,75.
Laboratory tests
Clinical specimens, including skin slit smear (SSS), nasal swab, blood, and biopsy specimens, were collected from patients with suspected Hansen disease. AFB staining and histological analyses of biopsy specimens were performed. Procedures for serological and molecular tests, which mainly included the NDO-LID rapid test, Nested-PCR, and the ELISPOT, were described in previous studies76,77,78,79,80. The sanger sequencing and next generation sequencing (NGS) were performed by Sangon Biotech (Shanghai) Co., Ltd. and Guangzhou Oumengweiyi Medical Laboratory Co., Ltd., respectively.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 software. The demographic data are reported using descriptive statistics, including percentages, means, and standard deviations. The clinical features of male and female patients were compared using the chi-square test. P < 0.05 indicated statistical significance.
Ethics approval and consent to participate
This study involving human participants was reviewed and approved by the Medical Ethics Committee of Beijing Friendship Hospital, Capital Medical University, Beijing, P.R. Chin. Written informed consent to participate in this study was provided by the participants.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Khadilkar, S. V., Patil, S. B. & Shetty, V. P. Neuropathies of leprosy. J. Neurol. Sci. 420, 117288 (2021).
Britton, W. J. & Lockwood, D. N. Leprosy. Lancet 363, 1209–1219 (2004).
Ebenezer, G. J. & Scollard, D. M. Treatment and evaluation advances in leprosy neuropathy. Neurotherapeutics 18, 2337–2350 (2021).
Wilder-Smith, E. P. & Van Brakel, W. H. Nerve damage in leprosy and its management. Nat. Clin. Pract. Neurol. 4, 656–663 (2008).
Barreto, J. G. & Salgado, C. G. Clinic-epidemiological evaluation of ulcers in patients with leprosy sequelae and the effect of low level laser therapy on wound healing: A randomized clinical trial. BMC Infect. Dis. 10, 237 (2010).
Tarique, M. et al. Fate of T cells and their secretory proteins during the progression of leprosy. Curr. Protein Pept. Sci. 19, 889–899 (2018).
World Health Organization (WHO). Classification of leprosy. https://www.who.int/lep/classification/en/ (2020).
Pitta, I. J. R. et al. Follow-up assessment of patients with pure neural leprosy in a reference center in Rio de Janeiro-Brazil. PLoS Negl. Trop. Dis. 16, e0010070 (2022).
Kundakci, N. & Erdem, C. Leprosy: A great imitator. Clin. Dermatol. 37, 200–212 (2019).
World Health Organization. Global Leprosy Strategy 2016–2020: Accelerating Towards a Leprosy-Free World: Monitoring and Evaluation Guide (World Health Organization, 2019).
Nascimento, O. J. Leprosy neuropathy: Clinical presentations. Arq. Neuropsiquiatr. 71, 661–666 (2013).
Dos Santos, D. F. et al. Peripheral nerve biopsy: A tool still needed in the early diagnosis of neural leprosy?. Trans. R. Soc. Trop. Med. Hyg. 114, 792–797 (2020).
El-Darouti, M. A. et al. Histopathological study of apparently normal skin of patients with leprosy. Int. J. Dermatol. 45, 292–296 (2006).
Hunter, S. W. & Brennan, P. J. Further specific extracellular phenolic glycolipid antigens and a related diacylphthiocerol from Mycobacterium leprae. J. Biol. Chem. 258, 7556–7562 (1983).
Hunter, S. W., Gaylord, H. & Brennan, P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 261, 12345–12351 (1986).
Duthie, M. S. et al. A rapid ELISA for the diagnosis of MB leprosy based on complementary detection of antibodies against a novel protein-glycolipid conjugate. Diagn. Microbiol. Infect. Dis. 79, 233–239 (2014).
Duthie, M. S. et al. Utility and limitations of serodiagnostic tests in monitoring the response to treatment of leprosy patients. Diagn. Microbiol. Infect. Dis. 96, 114984 (2020).
Hungria, E. M. et al. Leprosy reactions: The predictive value of Mycobacterium leprae-specific serology evaluated in a Brazilian cohort of leprosy patients (U-MDT/CT-BR). PLoS Negl. Trop. Dis. 11, e0005396 (2017).
Filho, F. B. et al. Active search strategies, clinicoimmunobiological determinants and training for implementation research confirm hidden endemic leprosy in inner São Paulo, Brazil. PLoS Negl. Trop. Dis. 15, e0009495 (2021).
Martinez, A. N., Talhari, C., Moraes, M. O. & Talhari, S. PCR-based techniques for leprosy diagnosis: From the laboratory to the clinic. PLoS Negl. Trop. Dis. 8, e2655 (2014).
Wu, L. et al. Temporal-spatial distribution characteristics of leprosy: A new challenge for leprosy prevention and control in Zhejiang, China. PLoS Negl. Trop. Dis. 15, e0008956 (2021).
Jain, S. et al. High-resolution sonography: A new technique to detect nerve damage in leprosy. PLoS Negl. Trop. Dis. 3, e498 (2009).
Goedee, H. S. et al. High resolution sonography in the evaluation of the peripheral nervous system in polyneuropathy–a review of the literature. Eur. J. Neurol. 20, 1342–1351 (2013).
Voltan, G. et al. Point-of-care ultrasound of peripheral nerves in the diagnosis of Hansen’s disease neuropathy. Front. Med. (Lausanne) 9, 985252 (2022).
Lugão, H. B., Nogueira-Barbosa, M. H., Marques, W. Jr., Foss, N. T. & Frade, M. A. Asymmetric nerve enlargement: A characteristic of leprosy neuropathy demonstrated by ultrasonography. PLoS Negl. Trop. Dis. 9, e0004276 (2015).
Nogueira-Barbosa, M. H. et al. Ultrasound elastography assessment of the median nerve in leprosy patients. Muscle Nerve 56, 393–398 (2017).
Lugão, H. B., Frade, M. A., Marques, W. J., Foss, N. T. & Nogueira-Barbosa, M. H. Ultrasonography of leprosy neuropathy: A longitudinal prospective study. PLoS Negl. Trop. Dis. 10, e0005111 (2016).
Sreejith, K. et al. High-resolution ultrasound in the assessment of peripheral nerves in leprosy: A comparative cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 87, 199–206 (2021).
Luppi, A. M. et al. High-resolution ultrasonography for early diagnosis of neural impairment in seropositive leprosy household contacts. PLoS One 18, e0285450 (2023).
Chen, X. et al. Coexistence of nerve enlargement and neuratrophy detected by ultrasonography in leprosy patients. Sci. Rep. 8, 7812 (2018).
Vashisht, D., Das, A. L., Vaishampayan, S. S., Vashisht, S. & Joshi, R. Nerve conduction studies in early tuberculoid leprosy. Indian Dermatol. Online J. 5, S71–S75 (2014).
Kar, S., Krishnan, A., Singh, N., Singh, R. & Pawar, S. Nerve damage in leprosy: An electrophysiological evaluation of ulnar and median nerves in patients with clinical neural deficits: A pilot study. Indian Dermatol. Online J. 4, 97–101 (2013).
Husain, S. & Malaviya, G. N. Early nerve damage in leprosy: An electrophysiological study of ulnar and median nerves in patients with and without clinical neural deficits. Neurol. India 55, 22–26 (2007).
Gupta, B. K. & Kochar, D. K. Study of nerve conduction velocity, somatosensory-evoked potential and late responses (H-reflex and F-wave) of posterior tibial nerve in leprosy. Int. J. Lepr. Other Mycobact. Dis. 62, 586–593 (1994).
Jardim, M. R. et al. Leprosy neuropathy evaluated by NCS is independent of the patient’s infectious state. Clin. Neurol. Neurosurg. 131, 5–10 (2015).
Vijayan, B. V., Dominic, M. R. & Nair, V. C. P. Leprous neuropathy: Observational study highlighting the role of electrophysiology in early diagnosis. J. Neurosci. Rural Pract. 12, 530–534 (2021).
Bathala, L., Kumar, K., Pathapati, R., Jain, S. & Visser, L. H. Ulnar neuropathy in hansen disease: Clinical, high-resolution ultrasound and electrophysiologic correlations. J. Clin. Neurophysiol. 29, 190–193 (2012).
Akita, J. et al. Comparison between nerve conduction study and high-resolution ultrasonography with color doppler in type 1 and type 2 leprosy reactions. Clin. Neurophysiol. Pract. 6, 97–102 (2021).
Antia, N. H. & Mistry, N. F. Plasma cells in caseous necrosis of nerves in leprosy. Lepr. Rev. 56, 331–335 (1985).
Shetty, V. P., Mehta, L. N., Antia, N. H. & Irani, P. F. Teased fibre study of early nerve lesions in leprosy and in contacts, with electrophysiological correlates. J. Neurol. Neurosurg. Psychiatry 40, 708–711 (1977).
Chandi, S. M., Chacko, C. J., Fritschi, E. P. & Job, C. K. Segmental necrotizing granulomatous neuritis of leprosy. Int. J. Lepr. Other. Mycobact. Dis. 48, 41–47 (1980).
Shetty, V. P., Suchitra, K., Uplekar, M. W. & Antia, N. H. Persistence of Mycobacterium leprae in the peripheral nerve as compared to the skin of multidrug-treated leprosy patients. Lepr. Rev. 63, 329–336 (1992).
Verma, S. et al. Central nervous system, spinal root ganglion and brachial plexus involvement in leprosy: A prospective study. J. Cent. Nerv. Syst. Dis. 14, 11795735221135476 (2022).
Polavarapu, K. et al. Brain and spinal cord lesions in leprosy: A magnetic resonance imaging-based study. Am. J. Trop. Med. Hyg. 100, 921–931 (2019).
Martinsen, V. & Kursula, P. Multiple sclerosis and myelin basic protein: Insights into protein disorder and disease. Amino Acids 54, 99–109 (2022).
Córsico, B., Croce, M. V., Mukherjee, R. & Segal-Eiras, A. Identification of myelin basic proteins in circulating immune complexes associated with lepromatous leprosy. Clin. Immunol. Immunopathol. 71, 38–43 (1994).
Mingsheng, L. & Liying, C. The utility of nerve ultrasound in diagnosis of peripheral neuropathy. Chin. J. Neurol. 53, 861–864 (2020).
Niu, J. et al. Cross-sectional area reference values for sonography of nerves in the upper extremities. Muscle Nerve 61, 338–346 (2020).
Sloan, G., Selvarajah, D. & Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 17, 400–420 (2021).
Misiunas, A., Niepomniszcze, H., Ravera, B., Faraj, G. & Faure, E. Peripheral neuropathy in subclinical hypothyroidism. Thyroid 5, 283–286 (1995).
Said, G. Uremic neuropathy. Handb. Clin. Neurol. 115, 607–612 (2013).
Kaeley, N., Ahmad, S., Pathania, M. & Kakkar, R. Prevalence and patterns of peripheral neuropathy in patients of rheumatoid arthritis. J. Family Med. Prim. Care 8, 22–26 (2019).
Shaban, A. & Leira, E. C. Neurological complications in patients with systemic lupus erythematosus. Curr. Neurol. Neurosci. Rep. 19, 97 (2019).
Martinez, A. R. M. et al. Sensory neuronopathy is a specific and disabling neurological manifestation of autoimmune hepatitis. Eur. J. Neurol. 27, 2072–2078 (2020).
Dalmau, J. & Graus, F. Diagnostic criteria for autoimmune encephalitis: Utility and pitfalls for antibody-negative disease. Lancet Neurol. 22, 529–540 (2023).
Koike, H. et al. ANCA-associated vasculitic neuropathies: A review. Neurol. Ther. 11, 21–38 (2022).
Rodrigues, C. E., Carvalho, J. F. & Shoenfeld, Y. Neurological manifestations of antiphospholipid syndrome. Eur. J. Clin. Invest. 40, 350–359 (2010).
Gwathmey, K. G. & Grogan, J. Nutritional neuropathies. Muscle Nerve 62, 13–29 (2020).
Fustes, O. & Rodriguez, C. A. Toxic neuropathy comment on organophosphate poisoning. Nurse Pract. 47, 1–2 (2022).
Ratnaike, R. N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 79(933), 391–396. https://doi.org/10.1136/pmj.79.933.391.PMID:12897217;PMCID:PMC1742758 (2003).
León-Ruiz, M., Jiménez-Jiménez, F. J. & Benito-León, J. Cadmium polyneuropathy: A rare, but not less important, cause of peripheral neuropathy. Rev. Neurol. 74, 403–407 (2022).
Honnorat, J. & Antoine, J. C. Paraneoplastic neurological syndromes. Orphanet J. Rare Dis. 2, 22 (2007).
Gabbai, A. A., Castelo, A. & Oliveira, A. S. HIV peripheral neuropathy. Handb. Clin. Neurol. 115, 515–529 (2013).
Corrêa, D. G. et al. Imaging features of neurosyphilis. J. Neuroradiol. 50, 241–252 (2023).
Kutlu, G. et al. Brucella: A cause of peripheral neuropathy. Eur. Neurol. 61, 33–38 (2009).
Hansen, K., Crone, C. & Kristoferitsch, W. Lyme neuroborreliosis. Handb. Clin. Neurol. 115, 559–575 (2013).
Sindic, C. J. Infectious neuropathies. Curr. Opin. Neurol. 26, 510–515 (2013).
Taso, M. et al. A randomised controlled trial comparing the effectiveness of surgical and nonsurgical treatment for cervical radiculopathy. BMC Musculoskelet. Disord. 21, 171 (2020).
Chaudhry, H. M., Mauermann, M. L. & Rajkumar, S. V. Monoclonal gammopathy—Associated peripheral neuropathy: Diagnosis and management. Mayo Clin. Proc. 92, 838–850 (2017).
Goodfellow, J. A. & Willison, H. J. Gangliosides and autoimmune peripheral nerve diseases. Prog. Mol. Biol. Transl. Sci. 156, 355–382 (2018).
Eshed-Eisenbach, Y., Brophy, P. J. & Peles, E. Nodes of Ranvier in health and disease. J. Peripher. Nerv. Syst. 28(Suppl 3), S3–S11 (2023).
Querol, L., Delmont, E. & Lleixà, C. The autoimmune vulnerability of the node of Ranvier. J. Peripher. Nerv. Syst. 28(Suppl 3), S12–S22 (2023).
Devaux, J. J. New insights on the organization of the nodes of Ranvier. Rev. Neurol. (Paris) 170, 819–824 (2014).
Wu, Y., Zhong, L. & Geng, J. Neuromyelitis optica spectrum disorder: Pathogenesis, treatment, and experimental models. Mult. Scler. Relat. Disord. 27, 412–418 (2019).
Derfuss, T. & Meinl, E. Identifying autoantigens in demyelinating diseases: Valuable clues to diagnosis and treatment?. Curr. Opin. Neurol. 25, 231–238 (2012).
Chen, X. et al. Evaluation of antigen-specific immune responses for leprosy diagnosis in a hyperendemic area in China. PLoS Negl. Trop. Dis. 12, e0006777 (2018).
Chen, X., You, Y. G., Yuan, Y. H., Yuan, L. C. & Wen, Y. Host immune responses induced by specific Mycobacterium leprae antigens in an overnight whole-blood assay correlate with the diagnosis of paucibacillary leprosy patients in China. PLoS Negl. Trop. Dis. 13, e0007318 (2019).
Chen, X. et al. Develop and field evolution of single tube nested PCR, SYBRGreen PCR methods, for the diagnosis of leprosy in paraffin-embedded formalin fixed tissues in Yunnan Province, a hyper endemic area of leprosy in China. PLoS Negl. Trop. Dis. 13, e0007731 (2019).
Chen, X. et al. Nested PCR and the taqman SNP genotyping assay enhanced the sensitivity of drug resistance testing of Mycobacterium leprae using clinical specimens of leprosy patients. PLoS Negl. Trop. Dis. 13, e0007946 (2019).
Jiang, H. et al. Utility of multi-target nested PCR and ELISPOT assays for the detection of paucibacillary leprosy: A possible conclusion of clinical laboratory misdiagnosis. Front. Cell Infect. Microbiol. 12, 814413 (2022).
Acknowledgements
We are grateful to Dr. Hongsheng Wang and his colleagues for their help in verifying difficult cases of leprosy disease. These patients were obtained from the Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, China; the National Center for Sexually Transmitted Disease and Leprosy Control, China Centers for Disease Control and Prevention, Nanjing, China; and the Centre for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China. We thank Dr. Yuwei Da and Dr. Hai Chen, who are from the Department of Neurology, Beijing Xuanwu Hospital, Capital Medical University, Beijing, China, for their great help in the frontier knowledge field of peripheral neuropathy in leprosy. We thank all the dermatologists, neurologists and disease control personnel in Beijing for providing care for leprosy prevention and control.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, X., Di, L., Qian, M. et al. Neurological features of Hansen disease: a retrospective, multicenter cohort study. Sci Rep 14, 10374 (2024). https://doi.org/10.1038/s41598-024-60457-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60457-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.