Abstract
Glyphosate is the active ingredient of glyphosate-based herbicides and the most commonly used pesticide in the world. The goal of the present study was to verify whether low doses of glyphosate (equivalent to the environmental exposure) evoke changes in galanin expression in intramural neurons in the small intestine in pigs and to quantitatively determine changes in the level of galanin receptor encoding mRNA (GALR1, GALR2, GALR3) in the small intestine wall. The experiment was conducted on 15 sexually immature gilts divided into three study groups: control (C)—animals receiving empty gelatin capsules; experimental 1 (G1)—animals receiving a low dose of glyphosate (0.05 mg/kg b.w./day); experimental 2 (G2)—animals receiving a higher dose of glyphosate (0.5 mg/kg b.w./day) orally in gelatine capsules for 28 days. Glyphosate ingestion led to an increase in the number of GAL-like immunoreactive intramural neurons in the porcine small intestine. The results of RT-PCR showed a significant increase in the expression of mRNA, which encodes the GAL-receptors in the ileum, a decreased expression in the duodenum and no significant changes in the jejunum. Additionally, intoxication with glyphosate increased the expression of SOD2-encoding mRNA in the duodenum and decreased it in the jejunum and ileum, but it did not affect SOD1 expression. The results suggest that it may be a consequence of the cytotoxic and/or neurotoxic properties of glyphosate and/or its ability to induce oxidative stress.
Similar content being viewed by others
Introduction
Along with the GAL-like peptide, alarin and GAL-message-associated peptide, galanin (GAL) is a neuropeptide of the GAL family1. GAL is an important neurotransmitter in the central and peripheral nervous systems, including the neurons that supply the gastrointestinal (GI) tract. The presence of GAL has been confirmed in the neurons of the dorsal motor vagal nucleus (DMX)2, the coeliac-superior mesenteric ganglion complex (CSMG)3, dorsal root ganglia (DRG)4 as well as in intramural ganglia—both myenteric and submucous in various sections of the GI tract) of many animal species and humans5,6,7. GAL plays a significant role in the control of the GI tract function; e.g. it regulates the intestine peristalsis, gastric juice secretion, and intestinal absorption and modulates inflammatory and neoplastic processes1,5,6. It performs its function owing to interactions with one of the three types of galanin receptors (GALRs): GALR1, GALR2 and GALR3, which are members of the G-protein-coupled receptor family1. The presence of GALRs has been demonstrated both in the central nervous system and in many peripheral tissues, including the GI tract8,9. It has been shown in many studies that GAL is an important neuroprotective factor, participating in the neural response to various pathological stimuli within the GI tract. GAL and GALRs expression has been shown to change in the course of gastric ulcer10, colitis11,12, injured axons in the intestine12, intoxication with acrylamide7, bisphenol A13 or systemic diseases, such as type I diabetes14.
The agricultural production intensification observed in recent years is linked to the widespread use of pesticides. These include glyphosate (N-(phosphonomethyl)-glycine)—the active ingredient of glyphosate-based herbicides (GBH) and, at the same time, the most commonly used pesticide in the world15. The widespread presence of glyphosate in groundwater, in precipitations and in soil results in its high levels in food products, such as beer, eggs, mineral water, oily seeds and vegetable oils, breakfast cereals, crackers and other cereal products and—in consequence—continuous exposure of humans and animals to this substance16. The herbicidal action of glyphosate is effected by the inhibition of 5-enolpyruvylshikimate-3-phosphate synthase and, in consequence, the biosynthesis of aromatic amino acids in plants15. Since the shikimic acid metabolic pathway does not occur in vertebrates, it was assumed that glyphosate would not have negative effects on animals or people16. However, many toxicology studies conducted during the past decade have confirmed the neurotoxic, teratogenic and carcinogenic effects of glyphosate16,17,18. Exposure to glyphosate leads to liver and kidney function disorders, endocrinal disorders and metabolic changes17. Studies using the rat as a research model showed that glyphosate administrated in low doses (approved for use in agriculture) induced endocrine effects and altered reproductive developmental parameters in both males and females19, impairs male offspring reproductive development by disrupting gonadotropin expression20 and alters gut microbial composition21. Glyphosate also has a negative impact on amphibians, causing a teratogenic effect on Xenopus laevis embryos incubated with 1/5000 dilutions of commercial glyphosate-based herbicides (GBH)22. Further, the neurotoxicity of glyphosate (administered in increasing doses) has been confirmed in studies using rodents23, while the genotoxicity was recorded in erythrocytes of broad-snouted caiman (Caiman latirostris) after in ovo exposure to high doses of glyphosate24. One of the main mechanisms responsible for glyphosate cytotoxicity is the ability to generate oxidative stress, i.e. excessive and unregulated production of reactive oxygen species25. The ability to induce oxidative stress has been confirmed both in aquatic animals and in mammals25,26,27,28. A low glyphosate concentration in water increased the level of reduced glutathione (GSH) in fish25 and rat livers26. Further studies of rats showed sublethal doses of glyphosate to increase malondialdehyde (MDA) levels in the liver27, whereas its long-term administration in low doses results in a higher level of MDA in the brain, plasma, kidneys and liver28. In effect, glyphosate intoxication leads to the activation of an antioxidative response expressed as an increase in the activity of antioxidative enzymes.
The International Agency for Research on Cancer classified glyphosate as being a probable human carcinogen (group 2A) in 201418. There is an ongoing debate between scientists and regulatory bodies concerning a ban on glyphosate use in agriculture and safe doses of the substance for humans16. The European Food Safety Authority (EFSA) established that the acceptable daily intake (ADI) of glyphosate is 0.5 mg/kg b.w. per day29. Although glyphosate is poorly absorbed from the GI tract, it was shown that approx. 34% of glyphosate is in the small intestine of the rat, where a high level persists even seven days after ingestion30. Despite significant interest in glyphosate, its impact on the GI tract, including the enteric nervous system (ENS), has not been sufficiently identified. Having a large autonomy in controlling the GI tract function, the ENS is indisputably involved in the adaptation and defence processes in response to GI tract function disorders, referred to as neural plasticity31. Given the above, the objective of this study was to verify whether low doses of glyphosate (equivalent to the theoretical maximum daily intake (TMDI) and the acceptable daily intake (ADI) in Europe) bring about changes in galanin expression in intramural neurons in the small intestine in pigs and to quantitatively determine changes in the level of galanin receptor encoding mRNA (GALR1, GALR2, GALR3) in the small intestine wall.
Results
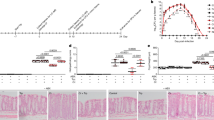
This study has shown that neurons in the ENS changed their neurochemical profile in response to glyphosate ingestion, showing an increase in galanin expression. Change intensity depended on the intestine section and the plexus type under examination (Table 1, Figs. 1, 2, 3). Regarding the myenteric plexus (MP), statistically significant changes in the number of GAL-like immunoreactive (LI) neurons in the duodenum and jejunum were found only in the group receiving the larger dose of glyphosate (increase from 3.65 ± 0.46% to 7.86 ± 0.39% and from 6.88 ± 0.41 to 16.51 ± 1.39%, respectively) (Fig. 1C,F). In the ileum, GAL-LI neurons accounted for 5.06 ± 0.56% of HuC/D-positive neurons in the control group (Fig. 1G) and glyphosate supplementation led to an increase in the number of GAL-positive neurons in both experimental groups: to 9.23 ± 0.40% in G1 (Fig. 1H) and to 15.88 ± 1.01% in G2 (Fig. 1I).
Distribution pattern of enteric nervous system neurons immunoreactive to HuC/D—used as a panneuronal marker and galanin (GAL) in the myenteric plexuses in the porcine small intestine in the control group (A, D, G), after lower (B, E, H) and higher doses (C, F, I) of glyphosate administration. Photographs (A–C) showing myenteric plexuses in the duodenum, (D–F) myenteric plexuses in the jejunum and (G–I) myenteric plexuses in the ileum. All photographs have been created by digital superimposition of two colour channels (green for HuC/D and red for GAL). Neurons immunoreactive to GAL are indicated with arrows.
Distribution pattern of enteric nervous system neurons immunoreactive to HuC/D—used as a panneuronal marker and galanin (GAL) in the outer submucous plexuses in the porcine small intestine the control group (A, D, G), after lower (B, E, H) and higher doses (C, F, I) of glyphosate administration. Photographs (A–C) showing outer submucous plexuses in the duodenum, (D–F) outer submucous plexuses in the jejunum and (G–I) outer submucous plexuses in the ileum. All photographs have been created by digital superimposition of two colour channels (green for HuC/D and red for GAL). Neurons immunoreactive to GAL are indicated with arrows.
Distribution pattern of enteric nervous system neurons immunoreactive to HuC/D—used as a panneuronal marker and galanin (GAL) in the inner submucous plexuses in the porcine small intestine the control group (A, D, G), after lower (B, E, H) and higher doses (C, F, I) of glyphosate administration. Photographs (A–C) showing inner submucous plexuses in the duodenum, (D–F) inner submucous plexuses in the jejunum and (G–I) inner submucous plexuses in the ileum. All photographs have been created by digital superimposition of two colour channels (green for HuC/D and red for GAL). Neurons immunoreactive to GAL are indicated with arrows.
In the outer submucous plexus (OSP), only supplementation of the larger dose brought about significant changes in GAL expression in the duodenum: an increase in the number of GAL-positive neurons from 35.68 ± 1.35 to 47.71 ± 0.87% (Fig. 2A,C). In turn, significant differences in the jejunum were observed in the gilts receiving both glyphosate doses: increase from 36.56 ± 1.62% in control to 43.57 ± 2.13% in G1 and to 45.20 ± 1.06% in G2 (Fig. 2D–F). Similarly, in the ileum, an increase in the GAL-LI neuron population was observed in the gilts receiving both glyphosate doses: from 38.46 ± 1.36% in the control group to 43.33 ± 1.38% in G1 and 53.54 ± 1.18% in G2 (Fig. 2G–I).
A similar trend was observed in the inner submucous plexus. Both glyphosate doses brought about significant changes in the duodenum: from 41.48 ± 1.59% in the control to 47.87 ± 1.53% in G1 and to 64.81 ± 1.58% in G2 (Fig. 3A–C). The increase in the jejunum was significant only in G2: from 48.56 ± 1.52% to 61.06 ± 1.04% (Fig. 3D,F). In the ileum, an increase in GAL-immunoreactivity was significant in both experimental groups: from 48.14 ± 1.77% in control to 55.09 ± 0.80% in G1 and to 67.41 ± 1.09% in G2 (Fig. 3G–I).
The results of RT-PCR showed a significant increase in the expression of mRNA which encodes the GALR1 receptor (in G1 and G2) (Fig. 4A), GALR2 receptor (in G1 and G2) (Fig. 4B) and GALR3 receptor (in G2) (Fig. 4C) in the ileum. The mRNA receptor expression in the duodenum was found to decrease: GALR1 in G2 (Fig. 4A), GALR2 receptor in G1 (Fig. 4B) and GALR3 in G1 (Fig. 4C). The expression of galanin receptors in the jejunum did not change significantly (Fig. 4A–C). Intoxication with glyphosate also increased the expression of SOD2-encoding mRNA in animals receiving higher dose of glyphosate in the duodenum (Fig. 5B) and decreased it in the G1 group in both the jejunum and ileum (Fig. 5B). However, glyphosate in both doses did not affect SOD1 expression in all fragments of intestines investigated (Fig. 5A).
Expression of mRNA encoding GALR1, GALR2, GALR3 in the small intestines tissue. Expression of GALR1 (A), GALR2 (B) and GALR3 (C) mRNA in the duodenum, jejunum and ileum collected from the control (grey bars), G1 (hatched bars) and G2 (black bars) groups. Levels of GALR1, GALR2 and GALR3 mRNA were measured by Real-Time PCR. The data obtained from each sample were normalized to GAPDH. Relative quantities (RQ) of mRNA were analysed using the comparative Ct method. Each cDNA sample was amplified in triplicate and all data are expressed as the mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
Expression of mRNA encoding SOD1 and SOD2 in the small intestines tissue. Expression of SOD1 (A) and SOD2 (B) mRNA in the duodenum, jejunum and ileum collected from the control (grey bars), G1 (hatched bars) and G2 (black bars) groups. Levels of SOD1 and SOD2 mRNA were measured by Real-Time PCR. The data obtained from each sample were normalized to GAPDH. Relative quantities (RQ) of mRNA were analysed using the comparative Ct method. Each cDNA sample was amplified in triplicate and all data are expressed as the mean ± SEM, **p < 0.01, ***p < 0.001.
Discussion
This study was the first to demonstrate that intoxication with low doses of glyphosate changed GAL expression in intramural neurons and galanin receptors in the small intestine wall of the pig. Due to comparable physiological processes, intestinal flora, and anatomy similar to humans, the pig is a useful animal model for studying intestinal pathology32, and thus, the obtained results can be referred to human medicine. Studies conducted to date have shown that GAL participates in the regulation of the GI tract function in mammals and in humans, not only in the physiological state but also in the regulation of pathological processes. Inflammatory processes in the intestines, such as proliferative enteropathy33, Brachyspira hyodysenterieae infection11, experimentally and naturally induced colitis12, or gastric ulcers10, had a considerable impact on the GAL level and increased its expression. Likewise, long-term NSAID (aspirin, naproxen and indometacin) supplementation increased GAL expression in all intestinal ganglia in pig duodenum and jejunum34,35. The same effect was observed as a result of pig intoxication with bisphenol A13 and acrylamide7. The increased density of GAL-containing fibres was also observed in the duodenum of people with alcoholic disease36. Further, diabetes, which often involves intestinal peristalsis disorders, has led to an increase in the number of intestinal neurons in the pig small intestine37, whereas it decreased the GAL concentration in the gastrointestinal tract in mice38. An increase in the population of GAL-positive neurons following glyphosate intoxication, as observed in this experiment, and earlier studies of the role of GAL in pathological processes in the GI tract, suggest that GAL may be an important factor involved in adaptive or neuroprotective processes of intestinal neurons, known for their neuroplastic properties expressed as a change of their neurochemical nature.
Earlier studies have shown that GALRs mRNA expression is high in the GI tract of many animal species and in humans. The presence of the GALR1 receptor has been reported in the muscularis externa, mucosal membrane, on ENS neurons in the GI tract of the mouse39, rat40, guinea pig41, pig10,11 and humans42. GAL decreases the peak pressure and contractions of longitudinal muscles, inhibiting intestinal peristalsis by GALR1 stimulation43. Moreover, GALR1 activation brings about the inhibition of voltage-dependent Ca2+ influx in a culture of rat muscularis externa neurons44. Further, the participation of the GALR1 receptor in motility and secretion control in the GI tract is largely associated with the regulatory impact on the secretion of other neurotransmitters45. Changes in GALR1 expression were observed in CNS neurons and in many peripheral tissues, including the GI tract, in many inflammatory and neoplastic processes10,39,42. Kiezun et al.42 suggest that GALR1 participates in the modulation of cancer cell proliferation in people with colon cancer. Increased GALR1 expression was observed in inflammatory processes in the GI tract, including experimentally induced antral ulcers in pigs10, dextran sulphate sodium-induced colitis in mice39 and Crohn’s disease in humans46. This confirms that GALR1 is a key element of communication between the immune and neuroendocrine systems of the intestine. In turn, the findings of the study conducted by Wąsowicz et al.11 were different. Namely, decreased GALR1 expression was observed in Brachyspira hyodysenteriae-induced colitis in pigs. The authors explain this discrepancy by extensive mechanical damage of tissues caused by inflammation or a different role of GAL in this part of the GI tract. The second hypothesis can also explain the findings of this experiment. Glyphosate supplementation decreased GALR1 expression in the group receiving higher doses in the duodenum and increased expression in both experimental groups in the ileum. This may result from the different functions of these intestine sections. The duodenum is the part of the GI tract where the food digestion process is the most intense and, at the same time, the concentration of glyphosate contained in the food is the highest. Whereas, ileum, with its concentration of lymphatic tissue, participates in the local inflammatory response and has thinner wall, with more narrow walls and fewer blood vessels. Food spends most of the time in it so it have the longest contact with glyphosate contained in food47.
GALR2 mRNA expression was also confirmed in all the GI tract sections, mainly in the muscularis externa, on myenteric neurons and sensory nerves8,9,10,11,48,49. The receptor’s location is linked to its function—regulation of the GI tract peristalsis, modulation of the sensory signal and response to pain48,49. Changes in the receptor expression in the GI tract occurred in inflammation and axotomy49. Given the increase in the receptor expression in the ileum wall and an increase in the GAL-LI neuron population in the myenteric plexus in both groups, it is suspected that peristalsis of this part of the small intestine increases in response to glyphosate supplementation. However, further research is necessary to confirm this hypothesis.
Although GALR3 distribution has been confirmed both in muscularis externa and on myenteric neurons, mRNA expression is considerably lower than the other galanin receptors8,9,10,11,50. Not much is also known about the function of this receptor in the GI tract. There have only been reports on GALR3 participation in the control of oedema formation and microcirculation regulation in dermatitis51. It may play a similar role in the regulation of local blood circulation and the inflammation process in the intestine, but this requires further research.
The mechanism of glyphosate’s toxic effect on mammalian tissues, including ENS, has not been elucidated. Studies of the harmful effects of glyphosate-based herbicides on the GI tract have shown it to cause histopathological changes, change the expression profile of genes involved in biotransformation and the level of pro-inflammatory cytokines in the chicken liver and small intestine52, damage the muscularis externa in gastric glands53. It also leads to hepatocyte degeneration in rats53, damages epithelial cells by the loss of the paracellular barrier in tight connections in the human intestinal epithelial cell line54, and causes injuries in the upper GI tract in humans55. Previous research demonstrated that oxidative stress is one of the main mechanisms responsible for glyphosate cytotoxicity25,26,27,28. In the present study, the antioxidant potential was evaluated through the expression of SOD mRNA in the intestinal wall. An increase in the expression of SOD2-encoding mRNA in the duodenum is consistent with earlier studies of the species, where an increase in SOD and catalase (CAT) activity was observed in the duodenum of piglets receiving glyphosate with fodder56. On the other hand, a decrease in SOD2 expression in the jejunum and ileum is consistent with the findings of a study on rats28. This suggests that glyphosate disrupts the antioxidant protection system in the intestines. However, further studies focusing on the activity of antioxidant enzymes and the production of free radicals are needed to confirm that oxidative stress is responsible for the changes observed in the present study. Moreover, oxidative stress is also the main mechanism of glyphosate neurotoxicity in the CNS and an important risk factor for neurodegenerative diseases23. Its harmful effect on the nervous system is well documented, especially for the CNS, where it inhibits enzyme activity, blocks cell proliferation, and disrupts the serotoninergic, noradrenergic and dopaminergic system function, leading to changes in neurotransmitter levels23,57,58. It has also been shown to lead to a change in the neurochemical profile of ENS neurons59. Taking into account the well-known neuroprotective function of GAL in the ENS5,12, we may speculate that a GAL expression increase and changes in its receptor expression, as observed in the current study, are associated with the neuroprotective function of the peptide, activated in response to glyphosate supplementation.
It cannot be ruled out that changes observed in the present study may be the result of intestinal microflora disorders caused by glyphosate. According to the findings of earlier studies, glyphosate considerably changes the composition of intestinal bacteria, leading to dysbiosis and neurobehavioral changes21,60. The impact of changes in intestinal microflora on the ENS has been documented earlier61.
It should be emphasized that further research is necessary to elucidate the mechanisms of the toxic effect of glyphosate on the GI tract and ENS neurons. Measuring the level of markers of neuronal damage may contribute to confirmation of the neurotoxic effect of glyphosate on ENS neurons. However, the determination of the level of antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase) or the level of lipid peroxidation, protein and/or DNA oxidation can clearly answer the question of whether the cytotoxic effect of glyphosate in the intestines is due to its induction of oxidative stress.
In summary, this study was the first to show that low doses (acceptable for intake) of glyphosate have an impact on the number of intestinal galaninergic neurons and the expression of galanin receptors in the pig small intestine wall. Although the mechanism of the toxic effect of glyphosate on the ENS has not been elucidated, the data supported by earlier reports suggest that it is a consequence of the cytotoxic and/or neurotoxic properties of glyphosate or its ability to induce oxidative stress. However, further research is needed to elucidate all aspects of glyphosate's impact on the ENS. Given that only low glyphosate doses have been applied in this study, there is a topical question of whether current legal regulations are sufficient to protect consumer health.
Materials and methods
Experimental procedures and tissue collection
All of the procedures on animals were conducted in compliance with the relevant Polish and EU regulations in the field of Animal Protection and Welfare (Leg. Decree 26/2014 implementing EU directive 2010/63/EU) and were approved by the Local Committee for Animal Experiments in Olsztyn (Approval No. 62/2020). The experiment was conducted on 15 sexually immature gilts (8 weeks, approx. 20 kg b.w.) divided into 3 study groups (5 animals in each): ((1) control (C)—animals receiving empty gelatin capsules, (2) experimental 1 (G1)—animals receiving a low dose of glyphosate (analytical standard, purity > 99.5%, Sigma-Aldrich, CAS-No.: 1071-83-6)—equivalent to the theoretical maximum daily intake (TMDI) in Europe—0.05 mg/kg b.w./day29, (3) experimental 2 (G2)—animals receiving a higher dose of glyphosate—equivalent to the acceptable daily intake (ADI)—0.5 mg/kg b.w./day29 orally in gelatine capsules for 28 days during the morning meal. The animals were weighed once a week during the experiment to establish the right glyphosate dose. After supplementation, all gilts were treated with azaperone (Stresnil, Jansen Pharmaceutica N.V., 4 mg/ kg of body weight, i.m.) and after 15 min euthanized using a lethal dose of sodium pentobarbital (Morbital, Biowet Puławy; 0.6 ml/kg of body weight, i.v.). Subsequently, sections of the small intestine (duodenum, jejunum, ileum) were collected for further examination. The intestine sections intended for immunofluorescence staining were fixed in a 4% buffered paraformaldehyde solution (pH 7.4) for one hour. The tissues were washed for three days in phosphate-buffered saline (PBS, pH 7.4) and ultimately immersed in 30% sucrose solution and kept at the temp. of 4 °C. The collected intestine sections (duodenum, jejunum, ileum) for Real-Time PCR were fixed in RNAlater® (Ambion, Austin, TX, USA) and kept overnight at − 4 °C, and subsequently frozen to − 80 °C and kept until the analysis.
Double immunofluorescent staining
The 12 µm-thick frozen duodenum, jejunum and ileum sections were doubly stained (as described previously by Palus et al.52 with a mixture of primary antibodies against nerve cell marker HuC/D (mouse polyclonal, Invitrogen, Waltham, MA, USA, Cat. No. A-21271, working dilution: 1:1000) and galanin (GAL, rabbit, cat. No. RIN7153, Peninsula, San Carlos, CA, USA, working dilution 1:3000) and the corresponding secondary antibodies (Alexa Fluor 488 nm donkey anti-mouse, ThermoFisher Scientific, Waltham, MA, USA; Cat. No. A21202; working dilatation: 1:1000 and Alexa Fluor 546 nm goat anti-rabbit, ThermoFisher Scientific, Waltham, MA, USA, Cat. No. A11010, working dilution: 1:1000). The specificity control for the antibodies used in the tests included a test of omission, replacement and pre-adsorption. Sections distanced by at least 100 µm were taken for staining to avoid analysing the same neurons. Neurons immunoreactive towards GAL were counted and photographed under an epifluorescence microscope (Olympus BX51). The population of GAL-positive neurons was counted in each of the enteric plexuses (myenteric plexus (MP), outer submucous plexus (OSP), inner submucous plexus (ISP)) in each small intestine section as a percent of Hu C/D-positive neurons. At least 500 HuC/D-positive neurons were counted for each plexus type.
Real-time PCR
Total RNA was isolated from 50 µg of tissue homogenate (perpendicular section covering all layers (serosa, muscularis propria, submucosa and mucous membranes) of the intestine) (GeneJET™ RNA, cleaning kit, Thermo Fisher Scientific, Waltham, MA) as per the manufacturer’s protocol. Subsequently, the samples were evaluated qualitatively and quantitatively (Spectrophotometer NanoVue®, Thermo Fisher Scientific, Waltham, MA) and reverse transcription of mRNA to cDNA was performed (Maxima First Strand cDNA Synthesis Kit for RT-qPCR, Thermo Fisher Scientific, Waltham, MA). The reverse transcription was performed in a Biometra Thermocycler (Biometra T Gradient Professional Basic) for 10 min at 25 °C, 30 min at 65 °C, 5 min at 85 °C, and subsequently kept at 4 °C for 10 min. All samples were frozen at − 80 °C.
Six genes were examined in each cDNA sample: GALR1, GALR2, GALR3, SOD 1, SOD 2 and porcine glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene in three replicates on a 96-well plate. The primers were designed with Primer-BLAST (http://ncbi.nlm.nih.gov), and their precise description can be found in an earlier publication11. Table 2 shows the primers used in the study. The PCR was performed in the 7500 fast real-time PCR system (Applied Biosystems, Waltham, MA, USA) with a thermal profile comprising: pre-denaturation 10 min at 95 °C, denaturation 15 s at 95 °C and hybridisation 1 min at 60 °C for 40 cycles.
Statistical analysis
The data from immunofluorescence staining and RT-PCR analysis were determined to be normally distributed using the D’Agostino and Person omnibus normality test using Statistica 13 software. Determinations of differences were made using a one-way analysis of variance (ANOVA) with the Dunnett test and presented as a mean ± SEM.
Ethical approval
The studies presented in the manuscript were carried out in accordance with the ARRIVE guidelines. All study procedures were approved by the Local Ethics Committee for Experiments on Animals (University of Warmia and Mazury in Olsztyn, Poland; Approval No. 62/2020). The guidelines in EU Directive 2010/63/EU for animal experiments were included.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Lang, R., Gundlach, A. L. & Kofler, B. The galanin peptide family: Receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol. Ther. 115, 177–207 (2007).
Gańko, M. & Całka, J. Prolonged acetylsalicylic-acid-supplementation-induced gastritis affects the chemical coding of the stomach innervating vagal efferent neurons in the porcine dorsal motor vagal nucleus (DMX). J. Mol. Neurosci. 54, 188–198 (2014).
Palus, K. & Całka, J. Alterations of neurochemical expression of the coeliac-superior mesenteric ganglion complex (CSMG) neurons supplying the prepyloric region of the porcine stomach following partial stomach resection. J. Chem. Neuroanat. 72, 25–33 (2016).
Zhang, X. et al. Regulation of expression of galanin and galanin receptors in dorsal root ganglia and spinal cord after axotomy and inflammation. Ann. N. Y. Acad. Sci. 863, 402–413 (1998).
Ekblad, E., Rökaeus, A., Håkanson, R. & Sundler, F. Galanin nerve fibers in the rat gut: Distribution, origin and projections. Neuroscience 16, 355–363 (1985).
Godlewski, J. & Kmiec, Z. Colorectal cancer invasion and atrophy of the enteric nervous system: Potential feedback and impact on cancer progression. Int. J. Mol. Sci. 21, 3391 (2020).
Palus, K., Makowska, K. & Całka, J. Alterations in galanin-like immunoreactivity in the enteric nervous system of the porcine stomach following acrylamide supplementation. Int. J. Mol. Sci. 20, 3345 (2019).
Anselmi, L. et al. Galanin receptors in the rat gastrointestinal tract. Neuropeptides 39, 349–352 (2005).
Arciszewski, M. B., Barabasz, S. & Całka, J. Immunohistochemical localization of galanin receptors (GAL-R1, GAL-R2, and GAL-R3) on myenteric neurons from the sheep and dog stomach. Ann. Anat. 190, 360–367 (2008).
Zalecki, M., Sienkiewicz, W., Franke-Radowiecka, A., Klimczuk, M. & Kaleczyc, J. The influence of gastric antral ulcerations on the expression of galanin and GalR1, GalR2, GalR3 receptors in the pylorus with regard to gastric intrinsic innervation of the pyloric sphincter. PLoS ONE 11, e0155658 (2016).
Wasowicz, K. et al. Changes in the expression of galanin and galanin receptors in the wall of the colon in pigs experimentally infected with Brachyspira hyodysenteriae. Bull. Vet. Inst. Pulawy 58, 23–28 (2014).
Gonkowski, S., Burliński, P., Skobowiat, C., Majewski, M. & Całka, J. Inflammation- and axotomy-induced changes in galanin-like immunoreactive (GAL-LI) nerve structures in the porcine descending colon. Acta Vet. Hung. 58, 91–103 (2010).
Szymanska, K. & Gonkowski, S. Neurochemical characterization of the enteric neurons within the porcine jejunum in physiological conditions and under the influence of bisphenol A (BPA). Neurogastroenterol. Motil. 31, e13580 (2019).
Bulc, M., Palus, K., Zielonka, Ł, Gajęcka, M. & Całka, J. Changes in expression of inhibitory substances in the intramural neurons of the stomach following streptozotocin- induced diabetes in the pig. World J. Gastroenterol. 23, 6088–6099 (2017).
Duke, S. O. The history and current status of glyphosate. Pest Manag. Sci. 74, 1027–1034 (2018).
Myers, J. P. et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health. 15, 19 (2016).
Bai, S. H. & Ogbourne, S. M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. Int. 23, 18988–19001 (2016).
Martins-Gomes, C., Silva, T. L., Andreani, T. & Silva, A. M. Glyphosate vs glyphosate-based herbicides exposure: A review on their toxicity. Xenobiot. 12, 21–40 (2022).
Manservisi, F. et al. The Ramazzini Institute 13-week pilot study glyphosate-based herbicides administered at human-equivalent dose to Sprague Dawley rats: Effects on development and endocrine system. Environ. Health. 18, 15 (2019).
Romano, M. A. et al. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch. Toxicol. 86, 663–673 (2012).
Tang, Q., Tang, J., Ren, X. & Li, C. Glyphosate exposure induces inflammatory responses in the small intestine and alters gut microbial composition in rats. Environ. Pollut. 261, 114129 (2020).
Paganelli, A., Gnazzo, V., Acosta, H., López, S. L. & Carrasco, A. E. Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling. Chem. Res. Toxicol. 23, 1586–1595 (2010).
Cattani, D. et al. Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: Involvement of glutamate excitotoxicity. Toxicology 320, 34–45 (2014).
Poletta, G. L., Larriera, A., Kleinsorge, E. & Mudry, M. D. Genotoxicity of the herbicide formulation Roundup (glyphosate) in broad-snouted caiman (Caiman latirostris) evidenced by the Comet assay and the Micronucleus test. Mutat. Res. 672, 95–102 (2009).
Slaninova, A., Smutna, M., Modra, H. & Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuro Endocrinol. Lett. 30(Suppl 1), 2–12 (2009).
Larsen, K., Najle, R., Lifschitz, A. & Virkel, G. Effects of sub-lethal exposure of rats to the herbicide glyphosate in drinking water: Glutathione transferase enzyme activities, levels of reduced glutathione and lipid peroxidation in liver, kidneys and small intestine. Environ. Toxicol. Pharmacol. 34, 811–818 (2012).
El-Shenawy, N. S. Oxidative stress responses of rats exposed to roundup and its active ingredient glyphosate. Environ. Toxicol. Pharmacol. 28, 379–385 (2009).
Astiz, M., de Alaniz, M. J. & Marra, C. A. Antioxidant defense system in rats simultaneously intoxicated with agrochemicals. Environ. Toxicol. Pharmacol. 28, 465–473 (2009).
EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 13, 4302 (2015).
Brewster, D. W., Warren, J. & Hopkins, W. E. Metabolism of glyphosate in Sprague-Dawley rats: Tissue distribution, identification, and quantitation of glyphosate-derived materials following a single oral dose. Fundam. Appl. Toxicol. 17, 43–51 (1991).
Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
Verma, N., Rettenmeier, A. W. & Schmitz-Spankem, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 11, 776–793 (2011).
Pidsudko, Z. et al. Distribution and chemical coding of intramural neurons in the porcine ileum during proliferative enteropathy. J. Comp. Pathol. 138, 23–31 (2008).
Czajkowska, M., Rychlik, A. & Całka, J. Long-term treatment with naproxen changes the chemical coding of the porcine intramural duodenum neurons. Ann. Anat. 227, 151425 (2020).
Brzozowska, M., Jana, B. & Całka, J. Effect of NSAIDs supplementation on the PACAP-, SP- and GAL-immunoreactive neurons in the porcine jejunum. Int. J. Mol. Sci. 22, 11689 (2021).
Hauge, T., Persson, J. & Sjölund, K. Neuropeptides in the duodenal mucosa of chronic alcoholic heavy drinkers. Alcohol Alcohol. 36, 213–218 (2001).
Bulc, M., Całka, J. & Palus, K. Effect of streptozotocin-inducted diabetes on the pathophysiology of enteric neurons in the small intestine based on the porcine diabetes model. Int. J. Mol. Sci. 21, 2047 (2020).
El-Salhy, M. Neuroendocrine peptides in stomach and colon of an animal model for human diabetes type I. J. Diabetes Compl. 13, 170–173 (1999).
Marrero, J. A., Matkowskyj, K. A., Yung, K., Hecht, G. & Benya, R. V. Dextran sulfate sodium-induced murine colitis activates NF–κB and increases galanin-1 receptor expression. Am. J. Physiol. Gastrointest. Liver Physiol. 278, 797–804 (2000).
Pham, T., Guerrini, S., Wong, H., Reeve, J. Jr. & Sternini, C. Distribution of galanin receptor 1 immunoreactivity in the rat stomach and small intestine. J. Comp. Neurol. 450, 292–302 (2002).
Anselmi, L. et al. Identification of galanin receptor 1 on excitatory motor neurons in the guinea pig ileum. Neurogastroenterol. Motil. 17, 273–280 (2005).
Kiezun, J. et al. Galanin receptors (GALR1, GALR2, and GALR3) immunoexpression in enteric plexuses of colorectal cancer patients: Correlation with the clinico-pathological parameters. Biomolecules 12, 1769 (2022).
Sternini, C. et al. Role of galanin receptor 1 in peristaltic activity in the guinea pig ileum. Neuroscience 125, 103–112 (2004).
Anselmi, L., Stella, S. L. Jr., Brecha, N. C. & Sternini, C. Galanin inhibition of voltage dependent Ca(2+) influx in rat cultured myenteric neurons is mediated by galanin receptor 1. J. Neurosci. Res. 87, 1107–1114 (2009).
Sarnelli, G., Vanden Berghe, P., Raeymaekers, P., Janssens, J. & Tack, J. Inhibitory effects of galanin on evoked [Ca2+] responses in cultured myenteric neurons. Am J Physiol. Gastrointest. Liver Physiol. 286, G1009–G1014 (2004).
Benya, R. V., Matkowskyj, K. A., Danilkovich, A. & Hecht, G. Galanin causes Cl– secretion in the human colon: Potential significance of inflammation–associated NF–kappa B activation on galanin-1 receptor expression and function. Ann. N Y. Acad. Sci. 863, 64–77 (1998).
Fish, E. M. & Burns, B. Physiology, Small Bowel (StatPearls Publishing, 2022).
Wang, S. et al. The GalR2 galanin receptor mediates galanin-induced jejunal contraction, but not feeding behavior, in the rat: Differentiation of central and peripheral effects of receptor subtype activation. FEBS Lett. 434, 277–282 (1998).
Sten Shi, T. J., Zhang, X., Holmberg, K., Xu, Z. Q. & Hokfelt, T. Expression and regulation of galanin–R2 receptors in rat primary sensory neurons: Effect of axotomy and inflammation. Neurosci. Lett. 237, 57–60 (1997).
Waters, S. M. & Krause, J. E. Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience 95, 265–271 (2000).
Schmidhuber, S. M., Rauch, I., Kofler, B. & Brain, S. D. Evidence that the modulatory effect of galanin on inflammatory edema formation is mediated by the galanin receptor 3 in the murine microvasculature. J. Mol. Neurosci. 37, 177–181 (2009).
Fathi, M. A. et al. Disruption of cytochrome P450 enzymes in the liver and small intestine in chicken embryos in ovo exposed to glyphosate. Environ. Sci Pollut. Res. Int. 27, 16865–16875 (2020).
Tizhe, E. V. et al. Influence of zinc supplementation on histopathological changes in the stomach, liver, kidney, brain, pancreas and spleen during subchronic exposure of Wistar rats to glyphosate. Comp. Clin. Path. 23, 1535–1543 (2014).
Vasiluk, L., Pinto, L. J. & Moore, M. M. Oral bioavailability of glyphosate: Studies using two intestinal cell lines. Environ. Toxicol. Chem. 24, 153–160 (2005).
Chang, C. Y. et al. Clinical impact of upper gastrointestinal tract injuries in glyphosate-surfactant oral intoxication. Hum. Exp. Toxicol. 18, 475–478 (1999).
Qiu, S. et al. Toxic effects of glyphosate on intestinal morphology, antioxidant capacity and barrier function in weaned piglets. Ecotoxicol. Environ. Saf. 187, 109846 (2020).
Bali, Y. A., Kaikai, N. E., Ba-M’hamed, S. & Bennis, M. Learning and memory impairments associated to acetylcholinesterase inhibition and oxidative stress following glyphosate based-herbicide exposure in mice. Toxicology 415, 18–25 (2019).
Martínez, M. A. et al. Neurotransmitter changes in rat brain regions following glyphosate exposure. Environ. Res. 161, 212–219 (2018).
Palus, K., Bulc, M. & Całka, J. Glyphosate affects the neurochemical phenotype of the intramural neurons in the duodenum in the pig. Neurogastroenterol. Motil. 35, e14507 (2023).
Aitbali, Y. et al. Glyphosate based- herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol. Teratol. 67, 44–49 (2018).
Zizmare, L. et al. Roux-En-Y gastric bypass (RYGB) surgery during high liquid sucrose diet leads to gut microbiota-related systematic alterations. Int. J. Mol. Sci. 23(3), 1126 (2022).
Acknowledgements
This study was supported by the National Science Centre [grant no. 2020/04/X/NZ7/00338]. Funded by the Minister of Science under the Regional Initiative of Excellence Program.
Author information
Authors and Affiliations
Contributions
K.P.: contributed to the conception and design of the study, performed surgical procedures, participated in the laboratory analyses, analysed and interpreted data, written draft, reviewed and edited the manuscript; M.Ch-K: participated in the laboratory analyses and edition of the manuscript; B.J.: participated in the analysis and interpretation of acquired data and reviewed the manuscript; J.C.: reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palus, K., Chmielewska-Krzesińska, M., Jana, B. et al. Glyphosate-induced changes in the expression of galanin and GALR1, GALR2 and GALR3 receptors in the porcine small intestine wall. Sci Rep 14, 8905 (2024). https://doi.org/10.1038/s41598-024-59581-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59581-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.