Abstract
To figure out how does SARS-CoV-2 affect sperm parameters and what influencing factors affect the recovery of sperm quality after infection? We conducted a prospective cohort study and initially included 122 men with SARS-CoV-2 infection. The longest time to track semen quality after infection is 112 days and 58 eligible patients were included in our study eventually. We subsequently exploited a linear mixed-effects model to statistically analyze their semen parameters at different time points before and after SARS-CoV-2 infection. Semen parameters were significantly reduced after SARS-CoV-2 infection, including total sperm count (211 [147; 347] to 167 [65.0; 258], P < 0.001), sperm concentration (69.0 [38.8; 97.0] to 51.0 [25.5; 71.5], P < 0.001), total sperm motility (57.5 [52.3; 65.0] to 51.0 [38.5; 56.8], P < 0.001), progressive motility (50.0 [46.2; 58.0] to 45.0 [31.5; 52.8], P < 0.001). The parameters displayed the greatest diminution within 30 days after SARS-CoV-2 infection, gradually recovered thereafter, and exhibited no significant difference after 90 days compared with prior to COVID-19 infection. In addition, the patients in the group with a low-grade fever showed a declining tendency in semen parameters, but not to a significant degree, whereas those men with a moderate or high fever produced a significant drop in the same parameters. Semen parameters were significantly reduced after SARS-CoV-2 infection, and fever severity during SARS-CoV-2 infection may constitute the main influencing factor in reducing semen parameters in patients after recovery, but the effect is reversible and the semen parameters gradually return to normal with the realization of a new spermatogenic cycle.
Similar content being viewed by others
Introduction
In the aftermath of the global pandemic engendered by the novel coronavirus pneumonia (COVID-19) and caused by SARS-CoV-2, medical scientists are now focusing on the mechanisms by which the SARS-CoV-2 virus infects cells through ACE2 and how it generates multi-organ damage in humans. SARS-CoV-2 enters cells through binding and membrane fusion with ACE2 on the cell membrane, mediated by the spike (S) protein receptor-binding domain, and the trans-membrane serine protease 2 (TMPRSS2) and furin protease are also involved in this process1. Due to the abundant expression of ACE2 in the lung, the virus is most likely to invade lung tissue, causing acute respiratory distress syndrome (ARDS) in severe cases. Uncontrolled inflammatory immune responses, high levels of cytokines, and multi-organ failure can then occur and produce high mortality rates. Thus, other organs such as liver, intestines, brain, eyes, heart, blood vessels, and testes can be severely damaged by SARS-CoV-22. ACE2 is also highly expressed in testicular tissue3,4, and the proportion of ACE2-positive cells in the testes is even significantly higher than that in the lungs. TMPRSS2 expression is also localized in the male reproductive system, indicating the testes as potential organs at high risk of SARS-CoV-2 infection5. Single-cell sequencing data from human testes revealed wide expression of ACE2 in Sertoli cells, Leydig cells, and germ cells at different developmental stages6, while in sperm, ACE2 was primarily detected in the flagellar mid-piece and the post-acrosomal region7. These results indicate that ACE2 may serve as a receptor mediating SARS-CoV-2 invasion into testicular cells and that this process causes injury to the testes and negatively affects spermatogenesis.
Previous investigators demonstrated that SARS-CoV-1 infection generated problematic complications within the reproductive system8,9, and that SARS-CoV-2 invaded human cell populations through the same receptor (ACE2) that was extensively expressed in multiple organs of the human body. In one study, two SARS-CoV-2-positive testicular samples were detected in five samples taken from COVID-19 patients10, and in another, the authors detected three SARS-CoV-2-positive testicular samples in 26 patients11, suggesting an ability of SARS-CoV-2 to invade and damage the testes. Research data indicated that viral orchitis is caused by SARS-CoV-2 and that it is manifested as a large number of degenerative germ cells in the seminiferous tubules, swelling and vacuolation in Sertoli cells, and infiltration of a large number of inflammatory cells (including T lymphocytes, B lymphocytes, and macrophages) in the testicular interstitium and seminiferous tubules; more severe cases even displayed features of Sertoli cell-only syndrome10,12. Researchers also uncovered pathological manifestations of autoimmune orchitis in COVID-19 patients13, with most of these signs accompanied by epididymitis. However, controversies remain as to whether testicular damage is caused by the direct actions of the virus or by virally induced autoimmune orchitis, and the specific molecular mechanisms underlying testicular disruption remain unclear.
Although injury to the testes due to SARS-CoV-2 has been observed, it is debatable whether the virus can be determined in semen. In one study of 38 semen samples, six cases of SARS-CoV-2-positivity were identified, two of which were demonstrated in semen from patients who recovered from COVID-1914. However, other studies revealed that viral mRNA was absent in semen10,15,16,17. Although changes in semen parameters (including reduced semen volume, sperm concentration, and sperm count) were determined in men with testicular damage and SARS-CoV-2 infection13,15, the impacts on sperm motility, viability, and morphology are still disputed. It is therefore currently unclear whether the decrease in semen parameters in infected individuals is due to the viral infection itself or the febrile symptoms. The results of meta-analysis additionally revealed a large heterogeneity in the literature, which may have affected the evaluation of the effects of SARS-CoV-2 on sperm motility18. Normal spermatogenesis requires approximately 3 months, and semen volume, concentration, and sperm motility fluctuate under physiological conditions during this timeframe. Therefore, larger sample sizes and longer time-scales are needed to analyze the effects of SARS-CoV-2 infection on spermatogenesis and sperm quality. In addition, SARS-CoV-2 activates cellular oxidative stress, causing sperm DNA fragmentation that is associated with poor embryonic development, reduced implantation rates, and higher miscarriage rates19,20,21. The observation of pregnancy outcomes is therefore also clinically relevant. However, there is a paucity of studies on the long-term effects, potential for sustained sperm quality, and pregnancy outcomes in recovered COVID-19 patients. Our study comprised 58 patients with SARS-CoV-2 infection at our hospital, and we statistically analyzed their clinical characteristics and semen parameters before and after SARS-CoV-2 infection. We collected semen samples several times from these men and analyzed them after the men showed infection with SARS-CoV-2; our latest detection time was 112 days after infection. We expected to clarify the actions of SARS-CoV-2 infection on semen parameters through the present study and further explored the recovery time and potential influencing factors on male sperm quality after SARS-CoV-2 infection (with the latter including fever), thus providing useful guidance in the clinical treatment and assisted reproductive outcomes of patients with SARS-CoV-2 infection.
Results
Current studies on the effects of SARS-CoV-2 infection on semen parameters
Previous studies have indicated that SARS-CoV-2 invades body cells through membrane binding and fusion mediated by ACE2 and TMPRSS21, subsequently causing orchitis and injury10,12 (Fig. 1). To clarify the actions of SARS-CoV-2 infection on sperm parameters and recovery after disease, we summarized recent studies on the impact of SARS-CoV-2 infection on sperm quality and found that these investigators reported relatively similar conclusions regarding the effects of SARS-CoV-2 infection on semen parameters15,22,23,24,25,26,27,28,29 (Table 1). Most studies revealed that semen parameters that included total sperm count, sperm concentration, and total motility were significantly reduced within 3 months after recovery, although no changes were found with respect to semen volume. There is, however, controversy regarding changes in progressive motility, as some studies indicate a decrease, while others suggest no significant changes after recovery. For example, no impact was observed on sperm survival or testosterone concentrations, except in one study that showed a decrease in blood testosterone after infection29. Furthermore, none of these studies indicated the presence of SARS-CoV-2 in semen, suggesting that the reduction in semen parameters caused by SARS-CoV-2 infection may not be due to a direct action of the virus on sperm. Considering the heterogeneity of these studies (such as most of the studies were non-self-controlled, and that there has been no long-term or systematic tracking of the recovery time of males after SARS-CoV-2 infection), additional systematic studies need to be conducted to evaluate these scientific questions. Therefore, our study aim was to improve upon these shortcomings. We included 58 patients with SARS-CoV-2 infection in our analysis of semen parameters and collected multiple semen samples before and after infection (the detailed screening process for our study patients is displayed in Fig. 2). Samples were collected at various time points, i.e., at 1, 2, and 3 months and longer after SARS-CoV-2 infection, with the longest collection time at 109 days after recovery. We aimed to clarify the effects of SARS-CoV-2 infection on sperm parameters and on the recovery of semen quality, providing guidance for clinical treatment and assisted reproduction practices of the patients who exhibited altered sperm quality.
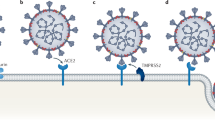
SARS-CoV-2 invades and damages the testes through the widely expressed receptors ACE2 on testicular cells. Various cell types, including leydig cells, sertoli cells, and germ cells with different developing stages widely express ACE2, which medicates the intrusion of SARS-CoV-2 through the blood–testis barrier together with TMPRSS2.
Analysis of basic clinical characteristics of the study patients
We conducted a comprehensive analysis of the basic clinical information of the 58 patients involved in this study, including age, BMI, timing of semen analysis before and after SARS-CoV-2 infection, and duration of infection. Additionally, following the criteria outlined in the 10th edition of the COVID-19 Diagnosis and Treatment Guidelines, we categorized the patients into mild and moderate cases, with no occurrences of severe or critical cases in our study. We meticulously recorded the temperature and duration of fever, classifying patients into low, moderate, and high fever groups. This classification was undertaken to investigate the impact of fever on sperm parameters and the post-infection recovery, as this factor remains a subject of considerable debate. Further details of the clinical features were presented in Table 2.
Changes in semen parameters of males with SARS-CoV-2 infection
Previous studies have depicted a SARS-CoV-2 infection as causing inflammation to the testes, but the specific effects of SARS-CoV-2 infection on semen parameters are still debated. We conducted a comparative analysis of indices in semen from 58 male patients before and after SARS-CoV-2 infection (as shown in Table 3) and noted no significant differences in the semen volume between the two groups, while the median total sperm count and concentration was reduced after infection. The proportions reflecting total sperm motility and progressive motility were significantly reduced after infection, while the proportion of immotile sperm was increased. The sperm survival rate and the normal morphology rate were also reduced, mainly manifested as increased head defect, however, showed no differences in the numbers of sperm with neck, mid-piece, or tail defects. In addition, we observed no differences in the number of round cells, anti-sperm antibodies, semen liquefaction time, or viscosity before vs. after SARS-CoV-2 infection. More detailed information is presented in Table 3.
Trends in semen quality before vs. after SARS-CoV-2 infection (using a linear mixed-effects model)
To further analyze the changes in and restoration of semen quality after SARS-CoV-2 infection, we constructed a linear mixed-effects model for the semen parameters that were significantly decreased after SARS-CoV-2 infection. Parameters included total sperm count, sperm concentration, percentage of normal sperm, proportions of sperm with head defects and those showing normal motility and progressive motility, the proportion of immobile sperm, and sperm survival rate. Time was designated as the fixed effect and participant ID as the random effect. We divided observations into different groups according to detection time: 1 represented pre-SARS-CoV-2 infection; 2, the observation time within 30 days after infection; 3, the observation time between 30 and 60 days after infection; 4, the observation time between 60 and 90 days after infection, and 5 represented the observation time of over 90 days. The numbers of observations at 1–5 time points were 58, 13, 32, 23, and 31, respectively, and Figs. 3 and 4 display the observations of total sperm counts and sperm concentrations at different time points. The changing trends in semen quality before vs. after infection are shown in Table 4. Figures 5 and 6 show the EMMs (estimated marginal means) with corresponding standard errors (SE) across time for sperm count and concentration. It is notable that the greatest diminution in sperm count and concentration occurred within 30 days after SARS-CoV-2 infection, followed by a gradual recovery after 30 days, and we noted no difference between before and after SARS-CoV-2 infection 90 days later. Intriguingly, the proportion of normal sperm fell and the proportion of sperm with head defects rose significantly between 30 and 60 days after infection and also showed a tendency to recover after 60 days (Table 4, Figs. S1 and S2). Moreover, assessments of sperm motility indicated that the greatest decrease in total motility occurred within 30 days after infection, followed by a gradual recovery and that motility returned to normal after 60 days (Table 4, Fig. S3). There was, however, a slight decrease in sperm motility after 90 days, which may have been attributable to the elevation in the proportion of immobile sperm after 90 days (Table 4, Fig. S4). The proportion of sperm exhibiting progressive motility significantly decreased within 30 days after infection and subsequently displayed a recovery between 30 and 60 days—with no significant difference between the two groups before vs. after 60 days of infection (Table 4, Fig. S5). Sperm survival rate decreased most significantly within 30 days and recovered after 30 days (Table 4, Fig. S6). Figures S7–S12 depict the distributions of observed values with respect to the proportions of normal sperm, sperm with head defects, motile sperm, progressively motile sperm, and immobile sperm, and sperm survival rate at different time points. These results indicated that the most significant diminutions in various semen parameters were observed within 30 days after infection, followed by a gradual recovery. Despite the variation in the recoveries of different indicators, all parameters basically returned to normal after 90 days.
Observations of total sperm count at different time points of the patients. 1 represents pre-SARS-CoV-2 infection; 2 represents the observation time was within 30 days after infection; 3 represents the observation time was between 31 and 60 days after infection; 4 represents the observation time was between 61 and 90 days after infection; 5 represents the observation time was more than 91 days.
Observations of sperm concentration at different time points of the patients. 1 represents pre-SARS-CoV-2 infection; 2 represents the observation time was within 30 days after infection; 3 represents the observation time was between 31 and 60 days after infection; 4 represents the observation time was between 61 and 90 days after infection; 5 represents the observation time was more than 91 days.
The estimated marginal means (EMMs) of all the observations of total sperm count at different time points of the patients. 1 represents pre-SARS-CoV-2 infection; 2 represents the observation time was within 30 days after infection; 3 represents the observation time was between 31 and 60 days after infection; 4 represents the observation time was between 61 and 90 days after infection; 5 represents the observation time was more than 91 days.
The estimated marginal means (EMMs) of all the observations of sperm concentration at different time points of the patients. 1 represents pre-SARS-CoV-2 infection; 2 represents the observation time was within 30 days after infection; 3 represents the observation time was between 31 and 60 days after infection; 4 represents the observation time was between 61 and 90 days after infection; 5 represents the observation time was more than 91 days.
Interestingly, compared to the control group, the malondialdehyde (MDA) concentration in patients' sperm significantly increased within 30 days post infection, but showed no significant difference after 90 days (Fig. S13C). However, the expression of COX-IV (the mitochondrial respiratory chain key enzyme) showed no significant difference from the control group either within 30 days or 90 days post infection (Fig. S13A,B). Moreover, immunofluorescence results indicated that the distribution of COX-IV in SARS-CoV-2 infected patients' sperm was more diffuse compared to the control group (Fig. S13A), unlike the well-localization in mitochondrial sheath in the control group, suggesting potential abnormalities in mitochondrial morphology in SARS-CoV-2 infected patients.
High core body temperature during SARS-CoV-2 infection contributes to the changes in semen parameters after recovery
To analyze the factors that influenced semen parameters after recovery from SARS-CoV-2 infection, we allotted the patients to different subgroups based on their clinical symptoms and subsequently analyzed their semen parameters before and after infection. When we allotted 58 patients to the two groups of those with low-grade fevers (19) and those with moderate-to-high fevers (39) according to fever severity, we uncovered no significant differences in age, BMI, timing of semen detection before vs. after infection, duration of disease, or other clinical symptoms except for fever (Table S1). Results from the 19 patients in the low-grade fever group showed no significant differences in semen parameters other than anti-sperm antibody levels before vs. after infection (Table 5). However, analysis of the semen parameters after infection in the 39 patients with moderate-to-high fever showed a significant reduction in total sperm count, sperm concentration, normal sperm proportion, total motility, progressive motility, and sperm survival rate. We also noted an elevation in the proportions of sperm with head defects and immotile sperm (Table 6). These results suggested that moderate-to-high fever may constitute a risk factor for the decline in semen parameters after recovery from SARS-CoV-2 infection, while low-grade fevers exerted a much smaller impact.
Discussion
How does viral infection affect sperm quality?
Spermatogenesis is a major physiological event that occurs in the seminiferous epithelium, and it encompasses four phases30: spermatogonial stem cells first self-renew and spermatogonia proliferate and differentiate into primary spermatocytes31; primary spermatocytes undergo meiosis to form round spermatids32; round spermatids transform to elongated spermatids and spermatozoa33; and finally spermatozoa are released into the seminiferous tubule lumen. This is the normal process that only takes place in the testis. However, congenital disorders of sexual development, hormonal imbalances, anatomical anomalies of the reproductive system, acquired traumas, drug-induced injury, radiation exposure, bacterial and viral infections, and genetic factors may affect the orderly process of sperm production in the seminiferous tubules, leading to male infertility34. More than 27 viruses are found in human semen35, and several viruses exert a negative effect on male reproduction and spermatogenesis36. Jorge Hallak37 and colleagues' review extensively elucidates the evidence of the presence of 12 viruses in the male reproductive tract and their adverse effects. The review clarifies the routes of infection, target organs and cells, the prevalence, and patterns of viral shedding in semen, as well as diagnostic/testing and treatment strategies. Acute bacterial infections generally affect the epididymis and accessory glands via the ascending urogenital tract, while viral infection predominantly perturbs the testes through blood circulation38. Mumps virus, human immunodeficiency virus (HIV), Zika virus, and coronaviruses can all cause orchitis. Meanwhile, hepatitis B virus (HBV), hepatitis C virus (HCV), herpes simplex virus (HSV), HIV, influenza virus, and Zika virus can lead to changes in sperm parameters. The most of the viruses primarily target the testes, with only a few, such as HIV, human papillomavirus (HPV), HSV, and Zika virus, directly affecting accessory gland organs like the epididymis, vas deferens, seminal vesicles, prostate, and penis. Detection of viruses in tissues suggests their potential to directly damage the male reproductive system and semen parameters. Acute viral infections have also been confirmed to induce systemic reactions, thereby impacting sperm quality systematically37. For example, there is evidence to indicate that influenza damages the integrity of sperm DNA39,40,41 and that impaired sperm quality can be detected from four to 11 weeks after fever. It is speculated that the potential mechanisms underlying the untoward effects on sperm may entail (I) fever that causes increased testicular temperature and damages germ cell lines, and (II) viruses that induce orchitis and impair the exocrine and endocrine functions of the testes. Therefore, the mechanisms by which different viral infections impair semen parameters and male reproductive function are highly heterogeneous and not well-defined. A better understanding of the infection pathways and target cells in the male reproductive tract is crucial for devising appropriate treatment and prevention strategies. Additionally, existing data indicate that Ebola virus, HBV, HCV, HSV, HIV, HPV, and Zika virus can be detected in semen, while there is no clear evidence for the presence of influenza virus, mumps virus, and coronaviruses in the semen of infected individuals. Research on this aspect of the novel coronavirus is not sufficiently deep and is controversial. Most studies have not provided data on the presence of SARS-CoV-2 in semen, with a few reporting no detection of the virus in the semen of SARS-CoV-2-infected patients. Although these viruses detectable in semen can be transmitted sexually (except for Zika virus), their presence does not necessarily indicate infectivity, as this requires a certain viral load and titer. Further in-depth research is needed to clarify these aspects and provide a better understanding of the etiology, infection pathways, and target tissues.
SARS-CoV-2 invades testes through ACE2 receptors and causes orchitis
The widespread expression of the receptor protein ACE2 that mediates the entrance of SARS-CoV-2 into Sertoli cells, Leydig cells, and germ cells at different developmental stages6, facilitates viral invasion and allows testicular tissue function to be compromised. The study conducted by Jorge Hallak et al.42 provides robust support for this conclusion. They observed the expression of ACE2 and TMPRSS2 in all cases, unaffected by age. These immunofluorescence staining were concurrently present in Leydig cells, Sertoli cells, spermatogonia, endothelial cells, and fibroblasts. Even in atrophic tubules, ACE2 and TMPRSS2 were co-expressed. SARS-CoV-2 enters cells through binding to ACE2 on the cell surface, mediated by the receptor-binding domain of the spike protein, and membrane fusion, with the cell transmembrane serine protease 2 (TMPRSS2) participating in this process1. This establishes the molecular basis for the invasion and damage of testicular tissue by SARS-CoV-2. The findings by Jorge Hallak et al.42 further confirm this, as electron microscopy results reveal the presence of viral particles in various cells, including supporting cells, interstitial cells, fibroblasts, endothelial cells, sperm cells, and reticular testicular epithelial cells. Studies have revealed that infection with SARS-CoV-2 generates viral orchitis, which is manifested as a large number of degraded germ cells and Sertoli cells, cellular swelling, vacuolization, increased apoptosis, and infiltration of inflammatory cells; the latter includes T lymphocytes, B lymphocytes, and macrophages in the testicular interstitium and seminiferous tubules10,12. Jorge Hallak42 and colleagues observed distinctive changes, including thickening of the basal membrane of seminiferous tubules and vascular alterations. Regions with thickened basal membranes exhibited fibroblasts simultaneously expressing SARS-CoV-2 N protein-positive immune markers and viral particles, indicating that infected fibroblasts might trigger extracellular matrix deposition in the seminiferous tubules. Furthermore, they provided the first description of vascular changes in testicular tissue of patients with COVID-19. All cases showed congestion and endothelial swelling, with five cases displaying fibrinoid thrombi and one case accompanied by venous thrombosis. Since viral particles and antigens were scarcely detected in endothelial cells, the authors suggested that virus-induced endothelial changes may not be the primary mechanism for thrombotic alterations. Testicular vascular changes were predominantly attributed to systemic alterations associated with COVID-19, such as refractory hypoxemia, multisystem thrombosis, and secondary infections. Ischemia of the testicles due to shock and thrombosis may lead to detachment of seminiferous tubule cells from the basal membrane, increased apoptosis, consequently impairing spermatogenesis. Considering that most investigators have not detected SARS-CoV-2 RNA in semen, we hypothesize that the reduction in sperm quality after infection may not be attributable to the virus’s direct effect on sperm, but that it is the orchitis and injury to the seminiferous epithelium caused by SARS-CoV-2’s actions on the testes that may be responsible for the reduction in semen parameters in male patients. In our study, 91.4% of the patients showed symptoms that were classified as mild, with 8.6% of patients manifesting moderate symptoms, and 93.1% of the men only felt mild symptoms. And none of the patients reported any testicular discomfort. These results cause us to believe that orchitis may not have been present in the patients enrolled in our study, and this will be confirmed in the future by testicular puncture and biopsy. Interestingly, consistent with Jorge Hallak43 et al.’s study, none of the 26 mild to moderate COVID-19 patients they investigated complained of testicular discomfort, and testicular ultrasound did not reveal the presence of orchitis. This suggests that orchitis may be present in more severe COVID-19 cases and is often accompanied by testicular pain or discomfort. Surprisingly, ultrasound indicated signs of epididymitis in 42.3% of males. Up to 80% of epididymitis cases are caused by bacterial infection44, while viral epididymitis is often challenging to detect and prone to misdiagnosis. In symptomatic patients, ultrasound patterns of viral epididymitis resemble those of bacterial epididymitis, but the former typically presents clinically with orchitis, where the testis is usually the first affected organ, followed by epididymal inflammation or abnormalities45. Isolated viral epididymitis is relatively uncommon. In this study, the discovery of radiological epididymitis reveals the potential for asymptomatic damage that may be overlooked in the clinical assessment of SARS-CoV-2-infected males. Even with a thorough physical examination by experienced surgeons, ultrasound assessment is irreplaceable for detecting potential subclinical epididymitis, and epididymal injury may have adverse effects on sperm parameters. Therefore, clinicians should be attentive to this condition.
In addition to testicular and epididymal inflammation, researchers found reduced testosterone levels in reproductive-age males with SARS-CoV-2 infection42,46,47. This aligns with the pathological features of interstitial cell damage found in orchitis, as testosterone is primarily produced in interstitial cells and regulates spermatogenesis. Another study on changes in testicular endocrine function in 119 reproductive-age males with SARS-CoV-2 infection indicated elevated LH levels and a decreased testosterone/LH ratio37,48,49. This highlights the complexity of male hormone level regulation. Interestingly, in another study by Jorge Hallak50 and colleagues, inoculation of the SARS-CoV-2 nucleocapsid protein into the testes, epididymis, prostate, and seminal vesicles of rats did not show significant histological changes. However, the treated group exhibited higher sperm counts and lower testosterone levels, while LH levels did not change significantly. This suggests that simple immunization does not affect male reproductive tract tissues, and the decrease in testosterone levels is not a direct result of viral invasion, possibly involving mechanisms mediated by the testis. Given the systemic and complex regulation of male hormone production, more relevant research may be needed to confirm the pathogenic mechanisms of SARS-CoV-2 antigens in the human testicular microenvironment, especially regarding the functional impact on interstitial cells of the testis. Further studies are required to elucidate the relationship between SARS-CoV-2 nucleocapsid protein, immune response, and regulation of testicular hormone production. Moreover, the decline in sperm parameters was significantly attenuated within 1 month after SARS-CoV-2 infection, indicating that the infection may directly affect spermatids during the developmental process, as normal spermatogenesis requires approximately 72 days51.
Is fever the main factor influencing sperm quality after SARS-CoV-2 infection?
Fever is a risk factor for the diminution in sperm parameters after SARS-CoV-2 infection. As a common symptom of viral infection, fever can temporarily disturb spermatogenesis52, and fever is also a common symptom of SARS-CoV-2 infection53,54, with more than 80% of patients experiencing fever55. As the testes require a temperature below that of core body temperature to maintain normal spermatogenesis, even mild heat stress will lead to germ cell death and impairment of spermatogenesis (albeit for a limited period)56. Authors have demonstrated that semen quality was affected by fever-related illnesses, as fever during meiotic or post-meiotic periods reduced sperm concentration by 32.6% and 35%, respectively52. One study involving 18 patients with SARS-CoV-2 infection revealed that patients who were febrile during their infection exhibited reduced sperm concentration, quantity, and motility after recovery relative to individuals with a normal body temperature54. Another research group also described lowered sperm count and progressive motility in the febrile vs. non-febrile group15. In our study, we assigned the 58 patients with SARS-CoV-2 infection to two groups: one with low-grade fever and the other with moderate-to-high fever, but noted no significant differences between the two groups in terms of age, BMI, time of semen examination before and after infection, duration of disease, or other clinical symptoms in addition to fever. The sperm parameters in the low-grade-fever group showed a decreasing tendency after infection that was not statistically significant, while those in the moderate-to-high fever group showed significant drops in sperm concentration; in the proportions of normal sperm, sperm showing normal motility and progressive motility; and in sperm survival rate compared with prior to SARS-CoV-2 infection. The proportions of spermatozoa with head defects and of immotile sperm were also significantly increased. These results indicate that fever may be a risk factor for reduced sperm parameters after SARS-CoV-2 infection. In addition, the mean maximal temperature and fever duration in the group with moderate-to-high fevers were significantly higher than the same indices in the low-grade fever group, and this may be a reason for the greater declines in the sperm parameters in this group. These findings suggest that controlling fever severity and reducing fever duration may be useful for the recovery of sperm parameters in patients with moderate-to-high fevers. As to the reasons for fever-induced decreases in sperm parameters, we hypothesize three distinct factors. First, prolonged high temperatures during the viral infection period can exaggerate inflammatory reactions, increase damage to the blood–testis barrier, and further exacerbate viral invasion and injury to the testes13. Second, fever and systemic inflammation caused by SARS-CoV-2 infection may affect luteinizing hormone and testosterone secretion, leading to changes in semen parameters57. Third, fever can also induce oxidative stress, thus increasing oxidative damage to sperm in the seminiferous epithelium. Therefore, in the clinical diagnosis and treatment of COVID-19 patients, attention should be given to the control of fever.
Energy metabolism and recovery after infection
In addition to the negative impacts of inflammation and fever on sperm parameters, our unpublished data suggest that ACE2 plays a critical role in the energy metabolism of sperm. After the deletion of ACE2, for example, we noted significantly reduced mitochondrial function and ATP levels in mouse sperm. Therefore, changes in ACE2 expression after SARS-CoV-2 infection may constitute a reason for the reduction in sperm motility, but this hypothesis requires further confirmation. From the perspective of time, our study indicated that within 30 days after infection with SARS-CoV-2, sperm parameters (including total sperm count, sperm concentration, total motility, and progressive motility) were significantly reduced, followed by recovery within 30–60 days. After 60 days, there were no differences in sperm motility, viability, or the proportion of normal sperm compared with levels before SARS-CoV-2 infection. However, the recoveries of sperm count and concentration remained slightly slower, with no statistical difference observed after 90 days. Considering that the complete spermatogenic cycle in normal humans is 72 days, we posit that the negative impacts of SARS-CoV-2 on sperm viability and motility gradually lessen and that sperm recover after one cycle of spermatogenesis as the damaged sperm will be replaced by developing healthy sperm. Due to the presence of damaged and dead germ cells at different developmental stages caused by inflammation, immune reactions, and heat stress after SARS-CoV-2 infection, we expect the lowered sperm count and concentration to continue until the numbers of spermatogonia are restored by mitosis (which requires a predictably longer period of time). In summary, our results indicate that the negative impacts of SARS-CoV-2 infection on male sperm parameters are reversible and that the impairment of spermatogenesis is gradually repaired during an extended recovery period. All sperm parameters then basically return to normal 90 days after infection, and this is consistent with previously published studies.
Current research and our new contributions
While there have been numerous studies on the impact of SARS-CoV-2 infection on male reproduction and sperm quality, including cohort studies, prospective investigations, retrospective analyses, and meta-analyses, the heterogeneity in research methods, complexity of included populations, limited sample sizes, and variations in the duration of studies have made it challenging to reach consistent conclusions. Therefore, alongside our current study, we provide a comprehensive review of the existing research landscape in this field, aiming to contribute a meaningful piece to the scientific puzzle. Based on the published studies, we tend to conclude that SARS-CoV-2 has a negative impact on sperm parameters in the short term. This is supported not only by the “material basis” of SARS-CoV-2 infecting testicular cells through TMPRSS2 and ACE258, but also by recent meta-analysis results18,59,60,61. Interestingly, there are discrepancies between case–control and self-controlled studies. Case–control studies show significant debates on the impact of SARS-CoV-2 infection on semen volume, sperm concentration, and sperm vitality, with half of the studies suggesting an impact and the other half indicating no effect. However, more studies suggest a decrease in total sperm count, with no significant effect on forward sperm movement18,59,60,61. In self-controlled studies, two meta-analyses59,60 suggest a significant decrease in various sperm parameters after SARS-CoV-2 infection, aligning more with our current findings. This may be attributed to the self-controlled design eliminating individual differences, resulting in higher study efficiency and consistency with the same sample size. Notably, in the four self-controlled studies included in the first subsection of our results, three showed no significant impact of SARS-CoV-2 infection on sperm parameters. However, this is not contradictory to our earlier conclusion, as the semen collection times in these three studies exceeded 3 months post-recovery, while the only study showing a significant impact had an average semen collection time of 51 days. Consistent with our current study's conclusion, the most substantial decrease in sperm parameters occurred within 30 days after SARS-CoV-2 infection, followed by a gradual recovery after 30 days, with no significant differences 90 days post-infection.
Building on the current research foundation, our study offers several new insights in this field. Firstly, the literature review in the first part of our study summarizes the current research status, combining it with our prospective investigation to provide readers with a panoramic view of the impact of SARS-CoV-2 on sperm parameters and male reproductive health. The self-controlled design of our study eliminates individual differences, achieving higher research efficiency and accuracy with a sample size of 58 cases and multiple samplings at different time points post-recovery. Additionally, the use of linear mixed-effects models for analyzing repeated measurements contributes to improved test efficiency, offering higher-quality research evidence. Secondly, while existing conclusions on the impact of COVID-19 on sperm parameters mostly focus on significant differences in semen volume, sperm concentration, and total sperm count, with seemingly no significant difference in forward sperm motility, our study supplements these findings by examining the influence on forward sperm motility. Furthermore, we compare changes in sperm morphology before and after infection, an aspect less explored in other studies. Thirdly, our study collects semen samples within 1 year before COVID-19 and at various time points (1 month, 2 months, 3 months, and up to 109 days) post-infection. This systematic analysis over the time axis provides a comprehensive understanding of the short-term and long-term effects of SARS-CoV-2 infection on sperm parameters and the recovery process post-infection. Fourthly, we meticulously control for confounding factors, excluding the impact of non-COVID febrile illnesses, sleep deprivation, drug use, smoking, alcohol consumption, steroid hormone medication, testicular diseases, and age on semen quality. This comprehensive approach enhances the reliability of our study results. Additionally, the detailed collection of clinical information from patients, including various symptoms, allows for subgroup analysis based on different symptom categories, especially high, moderate, and low fever groups, offering a more comprehensive exploration of factors influencing semen parameters amid confounding variables. When analyzing the impact of fever on sperm quality, we conduct comparisons across different age groups and BMI, excluding the influence of factors other than fever, thereby enhancing study accuracy—a consideration not explicitly mentioned in the cited studies. Fifthly, the impact of COVID-19 on male fertility remains unclear, with considerable variation in published research results possibly due to small sample sizes and population heterogeneity. Our study provides research evidence from a population in western China, contributing to a more complete understanding of the impact of SARS-CoV-2 on sperm parameters globally. Lastly, our study includes only mild to moderate infection cases, and all patients did not report testicular discomfort. This suggests that SARS-CoV-2 infection may not necessarily affect sperm parameters through severe orchitis. However, whether these patients have orchitis needs confirmation through ultrasound, and the specific mechanisms require further investigation.
Methods
Study participants
The patients included in this study were sourced from the Reproductive Medicine Center of Sichuan Provincial Maternity and Child Health Care Hospital. They underwent preconception medical examinations or were males with potential assisted reproductive needs. Among this population, some individuals required assisted reproductive treatment for their partners, potentially undergoing repeated semen analyses before and after enrollment. The patients were diagnosed with SARS-CoV-2 infection through positive nucleic acid or antigen detection. After infection, we notified patients to return for semen collection at different time points (including 1 month, 2 months, and 3 months). Semen was obtained through patient masturbation. We selected patients who had at least one semen analysis before and after infection for inclusion in the study. We extracted the basic clinical information of the patients from the medical records at our hospital. The corresponding self-perceived clinical symptoms were extracted using a pre-designed questionnaire. The patients were 18–65 years of age; we excluded individuals outside this range. Moreover, any other diseases or abnormalities that could reduce infertility were eliminated, including radio-chemotherapy, varicocele, inflammation of the testis and epididymis, mumps, congenital factors, and endocrine abnormalities. Patients diagnosed with other febrile diseases after SARS-CoV-2 infection or treated with antiviral medications such as ribavirin and ritonavir that could affect semen parameters were also excluded. Fifty-eight patients with semen evaluations performed before and after SARS-CoV-2 infection were ultimately included in our study. Routine semen analysis was performed by the Male Reproductive Medicine Laboratory of the Sichuan Provincial Maternity and Child Health Care Hospital. Sperm concentration and motility in the fresh semen samples were analyzed using a phase-contrast microscope (Olympus, BX43).
Methods for routine semen analysis
Chinese expert consensus on routine semen analysis
The semen analysis methods in our laboratory refer to the Chinese Expert Consensus on Routine Semen Analysis62. This consensus was compiled by the Reproductive Laboratory Subcommittee of the Chinese Association of Sexual Medicine based on references of the 5th edition (WHO5)63 and 6th edition (WHO6)64 of the “WHO Laboratory Manual for the Examination and Processing of Human Semen” and the ISO 23162:2021 “Basic Examination of Semen—Standardization and Testing Methods”65.
Semen volume
Semen volume was calculated using the weighing method. The weight of a labeled semen collection cup was measured in advance using an electronic balance, followed by another weighing after semen collection. The difference between the two weights represents the semen volume (assuming a semen density of 1 g/ml, the actual average density of semen is about 1.01 g/ml)66.
Semen liquefaction
The initial ejaculated semen was usually in a gel-like form and starts to liquefy and becomes thinner within a few minutes. As liquefaction continues, the semen became more and better uniform under 37 °C and in a slow-rotating semen collection cup. The liquefied semen samples were taken out from the 37 °C incubator every 15 min to mix and then observe for liquefaction. Record and note the status of liquefaction either when it occurred within 30 min or not even after 60 min.
Semen pH value
The semen pH value was immediately tested after liquefaction, with a drop of semen evenly applied to the pH test paper after the mixture. The color in the soaking area was compared with the colors of the standard strip within 30 s. The matched pH value was then read (the pH value of semen from a fertile men should be more than 7.2).
Semen viscosity
Semen was drawn into a disposable plastic pipette with a wide diameter (1.5 mm). The pipette was gently squeezed to allow the semen to drop by gravity, and the length of the thread was observed to evaluate semen viscosity. Normal semen forms discontinuous small drops, while the thread might exceed 2 cm in cases of abnormal, which was taken as the criterium to record semen viscosity.
Sperm morphology analysis
Sperm staining and morphology analysis were performed according to the 5th edition (WHO5) and 6th edition (WHO6) of the “WHO Laboratory Manual for the Examination and Processing of Human Semen.” Briefly speaking, after complete liquefaction of semen, the semen samples were thoroughly mixed. Using a pipette, 5–10 μl of semen was dropped onto one side of a clean glass slide, immediately contacting the semen drop with the non-sandblasted side of a second glass slide at a 45° angle. The second slide was then slowly dragged along the long axis of the first slide to create a smear. The dried semen smear was slowly immersed in 95% ethanol for fixation for 15 min. Subsequently, the fixed smear was sequentially immersed in the following solutions for different times: 80% ethanol (v/v) for 30 s, 50% ethanol (v/v) for 30 s, distilled water for 30 s, Harris' hematoxylin for 4 min, distilled water for 30 s, acid ethanol immersion 4–8 times (approximately 1 s per immersion), rinsed with running tap water for 5 min, 50% ethanol (v/v) for 30 s, 80% ethanol (v/v) for 30 s, 15 min at least in 95% ethanol (v/v), orange G6 for 1 min, 95% ethanol (v/v) for 30 s, 95% ethanol (v/v) for 30 s, 95% ethanol (v/v) for 30 s, EA-50 green staining for 1 min, 95% ethanol (v/v) for 30 s, 95% ethanol (v/v) for 30 s, 100% ethanol for 15 s, 100% ethanol for 15 s, xylene: ethanol, (1:2) for 1 min, 100% xylene for 1 min. Finally, a drop of sealing gel was added to the glass slide for subsequent sperm morphology analysis. The proportions of normal sperm, sperm with head defects, and sperm with neck and middle piece defects were calculated among 200 sperm. Orange G and hematoxylin dye liquors were obtained from Besso Biotechnology Co., Ltd (BA4035, BA4041, Zhuhai, China).
Teratozoospermia index (TZI) and Sperm deformity index (SDI)
Sperm with morphological abnormalities usually have multiple defects (head defects, middle piece or principal piece defects, or a combination of these defects). Detailed examination of the occurrence rate of various morphological abnormalities may be more useful than a single assessment of the percentage of normal morphology sperm, especially in studying the extent of sperm damage in humans67,68. By using a multiple-entry system to record each defect in the head, middle piece, and principal piece of the sperm, two indices can be obtained: teratozoospermia index (TZI)69,70 and sperm deformity index (SDI)71,72. Research has shown that TZI is associated with in vivo fertility67,70,73, while SDI is associated with in vitro fertilization71. TZI was calculated as the total number of defects divided by the total number of abnormal sperm, with a maximum count of 4 for each abnormal sperm defect, and additional counts of 1 for excess residual cytoplasm in the head, middle piece, and principal piece; SDI was calculated as the total number of defects divided by the total number of sperm (not just abnormal sperm), with the merging of several head defects counted as 1, while middle piece and principal piece defects were counted separately as 1.
Detection of sperm agglutination
In the direct immunobead experiment, the microbeads covalently conjugated with rabbit anti-human IgG or IgA immunoglobulins were directly mixed with washed sperm. The binding of microbeads with anti-human IgG or IgA indicated the presence of IgG or IgA antibodies on the surface of the sperm. The experiment was performed according to the standard experimental procedure of the Sperm Agglutination Test Kit for IgG (Boruide Biotechnology Co., Ltd., BRED-012, Shenzhen, China).
Sperm survival rate
The sperm survival rate was measured using eosin-nigrosine staining. The experiment was conducted according to the standard experimental procedure of the Sperm Vitality Staining Reagent Kit (Boruide Life Science Technology Co., Ltd., BRED-014, Shenzhen, China). Briefly speaking, 50 μl of semen was mixed with an equal volume of eosin- nigrosine suspension, and a sperm smear was prepared on a glass slide after 30 s. After drying, the stained sperm (dead sperm) and unstained sperm (live sperm) on the slide were counted using a laboratory counter under a microscope, with 200 sperm evaluated for each replicate sample.
Sperm concentration and motility analysis
Sperm concentration and motility analysis were performed manually as follows:
-
(1)
Sperm motility analysis: After thorough mixing of liquefied semen, 10 μl of semen was immediately placed on a clean glass slide and covered with a 22 mm × 22 mm × 0.4 mm cover glass (resulting in a thickness of approximately 20 μm). After 1 min of standing, a sperm motility assessment was performed, and the dilution factor of semen required for sperm count was determined63. Sperm motility was evaluated using an eyepiece with a grid, while the evaluation area was at least 5 mm away from the edge of the cover glass. Sperm with progressive motility were counted first, followed by non-progressive motility in the same grid, and immobile sperm were counted last. At least 5 different views were systematically observed for each sample, and the number of analyzed sperm was more than 200. The difference between the two analysis results within the 95% confidence interval was acceptable, or the sample would be thoroughly mixed again and re-checked.
-
(2)
Sperm concentration analysis and calculation of semen dilution factor: Based on the initial estimation of sperm concentration during sperm motility analysis, the semen dilution factor needed for sperm counting was determined63. If there was a large difference in the number of sperm in each field of view, it indicated insufficient mixing of the sample, and the sample would be thoroughly mixed again before further analysis. The preparation method of the semen dilution solution was as follows: 50 g of NaHCO3 and 10 ml of 35% formaldehyde solution were added to 1000 ml of distilled water. Optionally, 0.25 g of thymol blue or 5 ml of saturated methylene blue solution (> 4 mg/ml) was added to enhance the background and make the sperm heads clearer. The solution was stored at 4 °C, for at most 1 year using. The sperm concentration was calculated based on the volume of the counting chamber (depth × area) and the dilution factor.
Statistical analysis
Continuous data were determined to be normally distributed with the Shapiro–Wilk’s test. Normally distributed and non-normally distributed data are presented as mean (standard deviation, SD) or median (interquartile range, IQR), respectively, and analyzed using the Student’s t test or Kruskal–Wallis test to compare between/among groups. Categorical data are presented as frequency (percentage, %), and differences in rates were compared using the Chi-squared test. When the expected count was lower than 5, we adopted Fisher’s exact-probability test to compare the differences in rates. The Wilcoxon signed-rank test and McNamar’s test were used in dependent groups to compare semen parameters before and after SARS-CoV-2 infection. Because we repeated semen parameters over time, we assessed the differences in change from before SARS-CoV-2 infection to days ≤ 30, 31–60, 61–90, and > 90 after testing positive for COVID-19 using a linear mixed-effects model. In the linear mixed-effects model, we entered the time of the semen quality test as the fixed effect, and participant ID was entered as the random effect. We completed all analyses using R-4.2.0 (R Foundation for Statistical Computing), and analyses were two-sided, with P values < 0.05 indicating statistical significance.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Medical Ethics Committee of Sichuan Provincial Maternity and Child Health Care Hospital (20230626-188), and all patients provided signed informed consent.
Informed consent
All patients provided signed informed consent for their data for publication and we promise to strictly protect patient privacy.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request and all of them were presented in the submitted manuscript.
References
Jackson, C. B. et al. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 23(1), 3–20 (2022).
Wadman, M. et al. A rampage through the body. Science 368(6489), 356–360 (2020).
Douglas, G. C. et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 145(10), 4703–4711 (2004).
Barbagallo, F. et al. The testis in patients with COVID-19: Virus reservoir or immunization resource?. Transl. Androl. Urol. 9(5), 1897–1900 (2020).
Shen, Q. et al. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J. Cell. Mol. Med. 24(16), 9472–9477 (2020).
Wang, Z. & Xu, X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells 9(4), 920 (2020).
Ramal-Sanchez, M. et al. ACE2 receptor and its isoform short-ACE2 are expressed on human spermatozoa. Int. J. Mol. Sci. 23(7), 3694 (2022).
Zhao, J. M. et al. Clinical pathology and pathogenesis of severe acute respiratory syndrome. Chin. J. Exp. Clin. Virol. 17(3), 217–221 (2003).
Xu, J. et al. Orchitis: A complication of severe acute respiratory syndrome (SARS). Biol. Reprod. 74(2), 410–416 (2006).
Ma, X. et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell. Mol. Immunol. 18(2), 487–489 (2021).
Yao, X. H. et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 31(8), 836–846 (2021).
Yang, M. et al. Pathological findings in the testes of COVID-19 patients: Clinical implications. Eur. Urol. Focus 6(5), 1124–1129 (2020).
Li, H. et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 28, 100604 (2020).
Li, D. et al. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw. Open 3(5), e208292 (2020).
Holtmann, N. et al. Assessment of SARS-CoV-2 in human semen—A cohort study. Fertil. Steril. 114(2), 233–238 (2020).
Song, C. et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patientsdagger. Biol. Reprod. 103(1), 4–6 (2020).
Paoli, D. et al. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J. Endocrinol. Investig. 43(12), 1819–1822 (2020).
Klepinowski, T. et al. Does SARS-CoV-2 affect human semen? A systematic review and meta-analysis. Arch. Sex. Behav. 52(2), 669–677 (2023).
Borges, E. Jr. et al. Sperm DNA fragmentation is correlated with poor embryo development, lower implantation rate, and higher miscarriage rate in reproductive cycles of non-male factor infertility. Fertil. Steril. 112(3), 483–490 (2019).
Homa, S. T. et al. A comparison between two assays for measuring seminal oxidative stress and their relationship with sperm DNA fragmentation and semen parameters. Genes 10(3), 236 (2019).
Anifandis, G. et al. COVID-19 and fertility: A virtual reality. Reprod. Biomed. Online 41(2), 157–159 (2020).
Guo, T. H. et al. Semen parameters in men recovered from COVID-19. Asian J. Androl. 23(5), 479–483 (2021).
Koc, E. & Keseroglu, B. B. Does COVID-19 worsen the semen parameters? Early results of a tertiary healthcare center. Urol. Int. 105(9–10), 743–748 (2021).
Hu, B. et al. Evaluation of mid- and long-term impact of COVID-19 on male fertility through evaluating semen parameters. Transl. Androl. Urol. 11(2), 159–167 (2022).
Jimenez-Lopez, L. A. et al. Sperm concentrations do not correlate with semen parameters and hormone profiles in males recovered from COVID-19. Transl. Androl. Urol. 12(3), 353–363 (2023).
Ruan, Y. et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation. Andrology 9(1), 99–106 (2021).
Pazir, Y. et al. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: A prospective cohort study. Andrologia 53(9), e14157 (2021).
Wang, M. et al. Investigating the impact of SARS-CoV-2 infection on basic semen parameters and in vitro fertilization/intracytoplasmic sperm injection outcomes: A retrospective cohort study. Reprod. Biol. Endocrinol. 20(1), 46 (2022).
Gul, A. et al. Do SARS-CoV-2 infection (COVID-19) and the medications administered for its treatment impair testicular functions?. Urol. Int. 105(11–12), 944–948 (2021).
Hess, R. A. & Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 636, 1–15 (2008).
Cheng, C. Y. & Mruk, D. D. The biology of spermatogenesis: The past, present and future. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 365(1546), 1459–1463 (2010).
O’Donnell, L. et al. Spermiation: The process of sperm release. Spermatogenesis 1(1), 14–35 (2011).
Kawakami, Y. & Rosenberg, S. A. Human tumor antigens recognized by T-cells. Immunol. Res. 16(4), 313–339 (1997).
Leaver, R. B. Male infertility: An overview of causes and treatment options. Br. J. Nurs. 25(18), S35–S40 (2016).
Salam, A. P. & Horby, P. W. The breadth of viruses in human semen. Emerg. Infect. Dis. 23(11), 1922–1924 (2017).
Liu, W. et al. Viral threat to male fertility. Andrologia 50(11), e13140 (2018).
Teixeira, T. A. et al. Viral infections and implications for male reproductive health. Asian J. Androl. 23(4), 335–347 (2021).
Bhushan, S. et al. Testicular infection: Microorganisms, clinical implications and host-pathogen interaction. J. Reprod. Immunol. 83(1–2), 164–167 (2009).
Mac, L. J. Effect of chickenpox and of pneumonia on semen quality. Fertil. Steril. 2(6), 523–533 (1951).
Evenson, D. P. et al. Characteristics of human sperm chromatin structure following an episode of influenza and high fever: A case study. J. Androl. 21(5), 739–746 (2000).
Sergerie, M. et al. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil. Steril. 88(4), 970 e1–7 (2007).
Duarte-Neto, A. N. et al. Testicular pathology in fatal COVID-19: A descriptive autopsy study. Andrology 10(1), 13–23 (2022).
Carneiro, F. et al. Radiological patterns of incidental epididymitis in mild-to-moderate COVID-19 patients revealed by colour Doppler ultrasound. Andrologia 53(4), e13973 (2021).
Silva, E. J. R. et al. Lipopolysaccharide and lipotheicoic acid differentially modulate epididymal cytokine and chemokine profiles and sperm parameters in experimental acute epididymitis. Sci. Rep. 8(1), 103 (2018).
Basekim, C. C. et al. Mumps epididymo-orchitis: Sonography and color Doppler sonographic findings. Abdom. Imaging 25(3), 322–325 (2000).
Campos, R. K. et al. SARS-CoV-2 infects hamster testes. Microorganisms 9(6), 1318 (2021).
Selvaraj, K. et al. Testicular atrophy and hypothalamic pathology in COVID-19: Possibility of the incidence of male infertility and HPG axis abnormalities. Reprod. Sci. 28(10), 2735–2742 (2021).
Ma, L. et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J. Med. Virol. 93(1), 456–462 (2021).
Teixeira, T. A. et al. SARS-CoV-2 and multi-organ damage—What men’s health specialists should know about the COVID-19 pathophysiology. Int. Braz. J. Urol. 47(3), 637–646 (2021).
Lucio Carrasco, C. H. et al. SARS-CoV-2 nucleocapsid protein is associated with lower testosterone levels: An experimental study. Front. Physiol. 13, 867444 (2022).
Muciaccia, B. et al. Novel stage classification of human spermatogenesis based on acrosome development. Biol. Reprod. 89(3), 60 (2013).
Carlsen, E. et al. History of febrile illness and variation in semen quality. Hum. Reprod. 18(10), 2089–2092 (2003).
Sengupta, P., Leisegang, K. & Agarwal, A. The impact of COVID-19 on the male reproductive tract and fertility: A systematic review. Arab J. Urol. 19(3), 423–436 (2021).
Khalili, M. A. et al. Male fertility and the COVID-19 pandemic: Systematic review of the literature. World J. Men’s Health 38(4), 506–520 (2020).
Bao, J. et al. Semen parameters and sex hormones as affected by SARS-CoV-2 infection: A systematic review. Prog. Urol. 32(16), 1431–1439 (2022).
Bendayan, M. & Boitrelle, F. What could cause the long-term effects of COVID-19 on sperm parameters and male fertility?. QJM 114(4), 287 (2021).
Stigliani, S. et al. Semen parameters and male reproductive potential are not adversely affected after three or more months of recovery from COVID-19 disease. Front. Reprod. Health 4, 1114308 (2022).
Fathi, M. et al. Coronavirus disease and male fertility: A systematic review. Middle East Fertil. Soc. J. 26(1), 26 (2021).
Xie, Y. et al. SARS-CoV-2 effects on sperm parameters: A meta-analysis study. J. Assist. Reprod. Genet. 39(7), 1555–1563 (2022).
Tufvesson, K., Catalini, L. & Fedder, J. Semen parameters after SARS-CoV-2 infection: A literature review. Health Sci. Rep. 5(5), e745 (2022).
Lan, X. et al. A systematic review of the effect of COVID-19 on semen parameters. Heliyon 9(4), e14776 (2023).
Compiling Group of Chinese Experts on Routine Semen Analysis from the Reproductive Testing Branch of China Sexology Association. Chinese expert consensus on routine semen analysis. Chin. J. Androl. 37, 3–12 (2010).
World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen 5th edn. (World Health Organization, 2010).
World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen 6th edn. (World Health Organization, 2021).
International Organization for Standardization. ISO23162: 2021 Basic Semen Examination-Specification and Test Methods (ISO, 2021).
Cooper, T. G. et al. Ejaculate volume is seriously underestimated when semen is pipetted or decanted into cylinders from the collection vessel. J. Androl. 28(1), 1–4 (2007).
Jouannet, P. et al. Male factors and the likelihood of pregnancy in infertile couples. I. Study of sperm characteristics. Int. J. Androl. 11(5), 379–394 (1988).
Auger, J. et al. Sperm morphological defects related to environment, lifestyle and medical history of 1001 male partners of pregnant women from four European cities. Hum. Reprod. 16(12), 2710–2717 (2001).
Menkveld, R. & Kruger, T. F. Advantages of strict (Tygerberg) criteria for evaluation of sperm morphology. Int. J. Androl. 18(Suppl 2), 36–42 (1995).
Menkveld, R. et al. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: An effort towards standardization of in-vivo thresholds. Hum. Reprod. 16(6), 1165–1171 (2001).
Aziz, N. et al. The sperm deformity index: A reliable predictor of the outcome of oocyte fertilization in vitro. Fertil. Steril. 66(6), 1000–1008 (1996).
Aziz, N. et al. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 81(2), 349–354 (2004).
Slama, R. et al. Time to pregnancy and semen parameters: A cross-sectional study among fertile couples from four European cities. Hum. Reprod. 17(2), 503–515 (2002).
Acknowledgements
We thank the patients and their family members for their interest and cooperation. We thank LetPub (http://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the Sichuan Science and Technology Program (2023NSFSC1603), Sichuan Province Innovative Talent Funding Project for Postdoctoral Fellows (BX202208) and the Research Project of Chengdu Medical College (CYZYB22-12). The funding body was involved in designing and supervising the study experiments.
Author information
Authors and Affiliations
Contributions
G. Z. designed and supervised the study experiments. W. Z. collected the data and conducted the clinical evaluations of the participants. F. Y., D. X., and Y. Z analyzed the data. F. L., Y. Z., X. D., Y. W., M. H., J. L., and J. W., and Y. SL participated in data analysis and figure making. W. X., J. Z., S. C., and W. L reviewed and corrected the manuscript. All authors revised and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, G., Zhi, W., Ye, F. et al. Systematic analyses of the factors influencing sperm quality in patients with SARS-CoV-2 infection. Sci Rep 14, 8132 (2024). https://doi.org/10.1038/s41598-024-58797-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58797-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.