Abstract
To investigate the effect of COVID-19 infection or vaccine on IVF outcome. This is a multicenter retrospective study. Data were collected from all patients treated in the ART units between September and November 2021 after the vaccination of the general population began. Medical records of all patients who had IVF/intracytoplasmic sperm injection (ICSI) were retrospectively reviewed. Patients were categorized into four groups: previously infected by COVID-19, vaccinated by COVID vaccine, previously infected and vaccinated, or neither infected nor vaccinated. Total number of participants 151 (vaccinated only 66, infected only 18, vaccinated and previously infected 34, and control 33. Outcomes (ET on day of trigger, number of oocytes retrieved, quality of oocytes, number of fertilized oocytes, number and quality of embryos, number of embryos transferred, number of embryos frozen, implantation rate and clinical pregnancy rate) were compared between these four groups. Moreover, we compared the outcome before and post infection, as well as before and post vaccine in a group of patients. No evidence was found to suggest that COVID-19 disease or SARS-CoV-2 Vaccine adversely affects Clinical pregnancy rates (positive fetal heartbeat) (OR 0.9, CI 0.5–1.9, OR 1.8, CI 0.9–3.6, respectively) and the following parameters: fertilization rate, implantation rate, positive bHcg) (OR 0.9, CI 0.5–1.8, OR 1.5, CI 0.7–2.9, respectively). Although a limitation of our study is the small comparison groups, and the wide confidence intervals in the Odds Ratio estimates.
Similar content being viewed by others
Introduction
COVID-19 disease, caused by coronavirus SARS-CoV-2 was declared by World Health Organization as pandemic on 11th March 20201,2. More than 100 million cases have been recognized worldwide, and over 2.5 million people have died due to the disease3. Fever, headache, myalgia, cough, shortness of breath, diarrhea, and anosmia are the most common symptoms of COVID-194. SARS-CoV-2 enters into target host cells via the angiotensin converting enzyme 2 (ACE2), and needs cellular protease such as transmembrane protease serine (TMPRSS)5. Therefore, theoretically, organs with a high expression of ACE2 and TMPRSS2 are more vulnerable to infection. Results of immunohistochemistry and single-cell RNA sequencing data have indicated that there is high expression of ACE2 in the testis, and in the ovaries6,7,8,9. Therefore, the ovary is potentially vulnerable to SARS-CoV-2 infection. Furthermore, ACE 2 is expressed and Angiotensin- (1–7) Mas receptor-ACE2 axis is functioning in all stages of follicular maturation in the human ovary9. In addition, the rate of oocyte maturation is found to be related to the level of Angiotensin (1–7) in the follicular fluid10.
However, SARS- CoV-2 viral particles hasn’t been detected yet in the ovaries11,12,13,14. Yaakov Bentov et al. analyzed serum and follicular fluid for anti-COVID IgG, estrogen, and progesterone concentration, as well as the number and maturity of aspirated oocytes and previous estrogen and progesterone measurements. They found that both COVID-19 infection and vaccination with the BNT162b2 mRNA vaccine have no detrimental effect on follicular function15.
ACE2 has been detected as well in early embryos before the 8- cell stage in addition to the trophectoderm cells of late blastocysts, and TMPRSS2 is present in the late stage blastocyst, therefore peri-implantation embryos are highly susceptible to SARS-COV-2 infection16,17. Wang et al. analyzed assisted reproductive technology data and they found that SARS- CoV-2 infection didn’t affect female fertility and embryo development18. While, Raoul Ovrieto et al. found that couples infected with COVID-19 had lower proportion of top quality embryos but no impact was observed on patient’s performance and ovarian reserve as well. It worth to mention that the number included in this study was only 9 couples and in two of them the male partner who was infected rather than the female19. Furthermore, Yamila Herrero et al. compared the ovarian function of 34 patients who had never been infected versus 46 who had recovered from COVID-19. They found that patients with higher IgG levels in the follicular fluid had fewer retrieved oocytes. The authors of the study concluded that COVID-19 infection negatively affects the follicular microenvironment. It should be noted that the effect of this infection on the clinical outcome of IVF cycle was not investigated in this study. In addition, the study does not answer the question of how long does this effect last20.
ACE2 is present in the endometrium and the expression varies with the menstrual cycle phase, being stronger during the secretory phase3,22. The ACE2 has a vital role in endometrial proliferation and renewal. Accordingly, it is expected that the downregulation of ACE2 by SARS-CoV-2 could affect the endometrial stability and may impair implantation22,23,24,25,26. Whether this has a noxious effect on the endometrium due to COVID-19 infection or vaccination need to be clarified27. It has been reported that SARS-CoV-2 infection can cause transient menstrual cycle changes28. Another study found that the severity of viral infection is negatively associated with AMH Anti-Mullerian Hormone level29. Therefore, COVID-19 may cause ovarian injury and detrimentally affect ovarian reserve21.
In relation to COVID-19 vaccines, three main types are available in Jordan: mRNA vaccines, replication-defective live viral vectors based vaccines and virus inactivated vaccines30. The mRNA vaccine, BNT162b2, a Pfizer BionTech is the main vaccine that had been investigated. Myriam Safari et al. found that Pfizer BioNTech (BNT162b2) vaccine has no effect on intracytoplasmic sperm injection (ICSI) cycles outcome31. Their finding was confirmed by Raoul Orvieto et al.32. Moreover, it has been suggested that COVID-19 infection is unlikely to have long term effect on female reproductive tract however it need to be confirmed33. Recently, it has been published that SARS-Cov-2 spike protein sero-positivity from infection or vaccination does not prevent embryo implantation or early development34. Bowman and his team studied the effect on BNT162b2 vaccine on female fertility in rats. They found that it has no impact on fertility, ovarian or uterine parameters and embryo-fetal development26. Moreover, another study by Devora Ahron et al., they compared early IVF outcomes between 28 patients who received Pfizer vaccine, 37 patients received the Moderna vaccine and 328 unvaccinated patients who were a control group. They concluded that there was no association between COVID-19 vaccination and clinical pregnancy or current pregnancies35. Similarly, another study concluded that COVID-19 mRNA vaccine has no effect on ovarian response or pregnancy rate in patients who received the vaccine before IVF36. Furthermore, a very recent study showed that the number of retrieved oocyte, good quality embryos and percentage of clinical pregnancy rate were similar between 146 patients who received the inactivated SARS-CoV-2 vaccine (Sinopharm COVID-19 (BBIBP-CorV, COVILO) and in the 584 patients in the control group37.
In this study we set out to investigate whether COVID-19 infection or different types of vaccines(Pfizer(BioNTech), Oxford/AstraZeneca (ChAdOx1-S recombinant] vaccine) and/or Sinopharm (BBIBP-CorV)) affect on in-vitro fertilization (IVF) outcomes.
Methods
Data collection
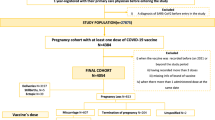
This study is a multicenter retrospective study, that was carried out at 2 assisted reproduction technology (ART) centers, in Jordan, Al Kindi IVF center in Amman and Irbid Specialty Hospital IVF center in Irbid. Data were collected from a convenient sample of patients who visited the units between September and November 2021, after the vaccination of the general population began in January 2021. Medical records of patients who underwent IVF/intracytoplasmic sperm injection (ICSI) were retrospectively reviewed. Patients were divided into four groups: those previously infected with COVID-19, vaccinated against the disease, previously infected and vaccinated, or neither infected nor vaccinated. Total number of participants 151 (vaccinated only 66, infected only 18, vaccinated and previously infected 34, and control 33. The data obtained in the groups were compared. The following parameters were included: patient demographics (age); number of previous IVF/ICSI cycles, duration of infertility, causes of infertility, protocol used, injections used, numbers days of stimulation, endometrial thickness at day of triggering, triggering method used, number of retrieved oocytes, number of MII oocytes, ICSI versus conventional IVF, number of oocytes fertilized, number and grade of day 3 embryos, number and grade of blastocysts, day of transfer, number of embryos transferred, number of embryos/blastocyst frozen, positive pregnancy test, and presence of OHSS. The embryos’ quality at day 3 was determined by cell number, symmetry and fragmentation according to the Society for Assisted Reproductive Technology (SART) grading guidelines, grading was good, fair or poor. In addition, the fact and the time of previous infection with COVID-19 and the history of vaccination against coronavirus were recorded. Specifically, the type of vaccine, timing, and number of doses received prior to the cycle were documneted. The type of vaccine could include one of the three vaccines available in Jordan, namely Pfizer(BioNTech), Oxford-Astrazeneca (ChAdOx1-S recombinant) and/or Sinopharm (BBIBP-CorV COVILO). The primary outcomes, which were compared between the four groups, included: fertilization rate, implantation rate and clinical pregnancy rate. Secondary outcomes included: number of oocytes retrieved; number of mature oocytes; and the number and quality of embryos at day 3. The IVF outcomes in a group of 50 patients who underwent IVF cycle before and after the pandemic, were also compared.
IVF protocol
Several protocols were used for controlled ovarian stimulation, short agonist protocol, flexible GnRH antagonist protocol and long agonist protocol. The starting dose of gonadotropin was decided according to patient’s age, ovarian reserve and BMI. Ovarian response was monitored by transvaginal ultrasound and gonadotropin dose changed accordingly. Once two leading follicles reaches a diameter of 17–18 mm or a dominant follicle 20 mm final oocyte maturation was triggered by human chorionic gonadotropin (HCG). Oocyte pickup was performed 35–37 h later. The oocytes were incubated for 2 h before the ICSI. The cumulus and corona cells were removed using enzymatic digestion by cumulase, in addition to utilizing denuding pipette for mechanical denudation. After 16 ± 2 h fertilization was assessed by looking for the 2 pronulcei (PN).
Luteal phase was supported by vaginal progesterone ± oral dydrogesterone 20 mg per day, started one day after pick up. Serum BhCG was measured 14 days after embryo transfer and value above 5 IU/ml was consider positive. Luteal phase support was continued until 10th week gestation. Embryo transfer was determined according to number and quality of embryos and the risk of ovarian hyper-stimulation syndrome.
Statistical analysis
All extracted data were summarized in a Microsoft Excel workbook and analyzed using Statistical Package for Social Sciences (SPSS) version 23. The study’s results were reported in the form of descriptive statistics. The study’s results were reported in the form of descriptive statistics. Categorical variables were summarized in the form of frequencies [n (%)], while continuous data were reported as means, medians (when applicable) and standard deviations. Categorical associations were evaluated using Chi-square test, while associations involving continuous data were assessed using Student’s t-test. Because the data failed to be normally distributed, nonparametric tests such as Kruskal–Wallis test were utilized to assess the study’s hypotheses and detect significant differences. Paired parameters were tested using Wilcoxon paired test. An alpha value of ≤ 0.05 (CI = 95%) was considered statistically significant.
Outcome measures and definitions
Fertilization rate is defined as the percentage of fertilized oocytes from the collected oocytes. Implantation rate is calculated as the number of gestational sacs observed per number of embryos transferred.
Ethical approval
Institutional Review Board approval was obtained from the Ethical Reviewing Board at the Faculty of Pharmacy and the Faculty of allied medical sciences at Al-Ahliyya Amman University. Approval number: (AAU/11/5/2020–2021). All research was performed in accordance with relevant guidelines/regulations, and in accordance with the declaration of Helsinki. Since it is a retrospective review of medical records, the informed consent was waived by the Ethical Reviewing Board at the Faculty of Pharmacy and the Faculty of allied medical sciences at Al-Ahliyya Amman University.
Results
General characteristics
Total number of cases included in this study was 151. Table 1 demonstrates general characteristics of the patients.
IVF parameters
Tables 2 and 3 show the difference in the results of IVF parameters among different groups of participants.
Previous COVID-19 infection
Our findings indicate that previous COVID-19 infection does not affect any of the following IVF outcomes including fertilization rate, implantation rate. Similarly there was no difference in the pregnancy rate as determined by positive BHcg or clinical pregnancy (OR 0.92, CI 0.463–1.827, OR 0.936, CI 0.462–1.897) respectively. Additionally, IVF parameters didn’t have a significant difference between those who had COVID-19 or not Table 2.
Moreover, the mean number of retrieved oocytes and the number, as well as class of embryos did not differ significantly before and after the COVID-19 infection (Table 4).
COVID-19 vaccine
We found no evidence thatSARS-Cov-2 vaccination adversly affected fertilization rate, implantation rate, positive bHcg (OR 1.460, CI 0.735–2.901) and clinical pregnancy (positive fetal heartbeat) (OR 1.786, CI 0.886–3.603). Furthermore, IVF parameters did not differ significantly between those who were or were not vaccinated but the number of embryos at the cleavage stage was significantly lower in the vaccinated group (Table 3).
Similarly, mean values of the number of retrieved oocytes, fertilized oocytes, and number and class of embryos did not significantly differ in women before and after the vaccination (Table 4).
Discussion
This study is consistent with already published results. As we found that, neither SARS-Cov-2 vaccine nor infection had a significant effect on IVF outcomes. Our results are in agreement with the currently available results of studies described in publications. Although the previously infected group had fewer embryos at the cleavage stage this didn’t affect the clinical outcome in terms of pregnancy. The latest study by Devora Aharon et al. in which they compared the IVF outcomes in 222 vaccinated patients versus 983 unvaccinated, were they found that there is no significant difference38. In addition to the previously mentioned study by Sarit Avraham et al. who came up with similar results36. Previously Bentov et al studied 32 IVF patients and found that follicular function was not altered by neither SARS-Cov-2 vaccine nor infection. Another study by Raoul Orvieto et al. among 36 patients came up with similar results15,32. Moreover, we couldn’t find any difference in the outcome between patients who received Sinopharm (BBIBP-CorV), Oxford-Astrazeneca or Pfizer(BioNTech) vaccine, in spite that the vaccines differ in their mechanism of action. Although, our sample size is small similar results was found in larger sample of over 100 women as mentioned earlier.
SARS-Cov-2 infection through the effect on angiotensin II can have a damaging effect on the ovaries and the endometrium. This infection can increase the circulating Angiotensin II due to reduction in ACE2 activity, so it can lead to changes in ovarian function, oocyte maturation and egg quality39. Moreover, Angiotensin II elevation may induce inflammation due to oxidative stress, consequently impairing reproductive ability7. TMPRSS4 is highly expressed in the endometrial cells in all phases of the menstrual cycle, especially during the window of implantation, furthermore, ACE2 expression increases in the mid secretory phase. Therefore, the secretory phase has a high risk of viral infectivity, although the evidence about the presence or absence of viral particles in the endometrial tissue is still lacking11. Accordingly, it could be anticipated that COVID-19 infection might has a harmful effect on female reproduction, and it has been proven by several studies that COVID-19 can detrimentally affect ovarian response and IVF outcomes19,20,21.
Furthermore, psychological stress due the pandemic might adversely affect reproductive system and fertility treatments outcome through its effect on the hypothalamic pituitary axis40. It has been hypothesized that stress increases reactive oxygen species (ROS) levels in the ovaries above acceptable physiological level, which may reduce the follicular growth. This appears to be more in infertile rather than the fertile women, though this hypothesis needs to be confirmed41,42,43. However, this can manifest itself during the peak of the pandemic, but further research is needed to investigate this effect.
Our study has several limitations, including relatively small sample size, and the retrospective design. Moreover. antibody levels were not assessed. However, what adds to the strength of our study is that we assessed the implantation rate, clinical pregnancy rate (using pregnancy test and positive fetal heart beat) which provides knowledge about the effect on early pregnancy. Moreover, we compared the outcome between different groups of IVF patients an addition to comparing the results in the same patients before and after the vaccination or infection. Further, more we looked at different types of vaccine.
Our study adds to the available evidence that COVID-19 vaccine is safe for patients planning to become pregnant. On the other hand, COVID-19 infection can have detrimental effects on a woman’s reproductive function. However, further research is needed to investigate the duration of the possible negative effect.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Tur-Kaspa, I., Tur-Kaspa, T., Hildebrand, G., Cohen, D. COVID-19 may affect male fertility but is not sexually transmitted: A systematic review. F&S Rev. (2021).
Singh, B. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its effect on gametogenesis and early pregnancy. Am. J. Reprod. Immunol. 84, e13351 (2020).
Delamuta, L. C., Monteleone, P. A., Ferreira-Filho, E. S., Heinrich-Oliveira, V., Soares-Júnior, J. M., Baracat, E. C. et al. Coronavirus disease 2019 and human reproduction: A changing perspective. Clinics 76 (2021).
Sheikhi, K., Shirzadfar, H. & Sheikhi, M. A review on novel coronavirus (Covid-19): Symptoms, transmission and diagnosis tests. Res. Infect. Dis. Trop. Med. 2, 1–8 (2020).
Lukassen, S. et al. SARS-CoV-2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39, e105114 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020).
Pan, P.-P., Zhan, Q.-T., Le, F., Zheng, Y.-M. & Jin, F. Angiotensin-converting enzymes play a dominant role in fertility. Int. J. Mol. Sci. 14, 21071–21086 (2013).
Goad, J., Rudolph, J. & Rajkovic, A. Female reproductive tract has low concentration of SARS-CoV2 receptors. PLoS ONE 15, e0243959 (2020).
Reis, F. M. et al. Angiotensin-(1–7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil. Steril. 95, 176–181 (2011).
Cavallo, I. K. et al. Angiotensin-(1–7) in human follicular fluid correlates with oocyte maturation. Hum. Reprod. 32, 1318–1324 (2017).
Chandi, A., Jain, N. State of ART in the COVID-19 era and consequences on human reproductive system. Biol. Reprod. (2021).
Barragan, M., Guillén, J., Martin-Palomino, N., Rodriguez, A. & Vassena, R. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum. Reprod. 36, 390–394 (2021).
Boudry, L. et al. P–274 Undetectable viral RNA in follicular fluid (FF), cumulus cells (CC) and endometrial tissue in SARS-CoV–2 positive patients. Hum. Reprod. 36, deab130-273 (2021).
Demirel, C. et al. Failure to detect viral RNA in follicular fluid aspirates from a SARS-CoV-2-positive woman. Reprod. Sci. 28, 1–3 (2021).
Bentov, Y., Beharier, O., Moav-Zafrir, A., Kabessa, M., Godin, M., Greenfield, C. et al. Ovarian follicular function is not altered by SARS-Cov-2 infection or BNT162b2 mRNA Covid-19 vaccination. MedRxiv (2021).
Cheng, G., Guo, S. & Zhou, L. Suggestions on cleavage embryo and blastocyst vitrification/transfer based on expression profile of ACE2 and TMPRSS2 in current COVID-19 pandemic. Mol. Reprod. Dev. 88, 211–216 (2021).
Chen, W. et al. SARS-CoV-2 entry factors: ACE2 and TMPRSS2 are expressed in peri-implantation embryos and the maternal–fetal interface. Engineering 6, 1162–1169 (2020).
Wang, M. et al. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: A retrospective cohort study. EClinicalMedicine 38, 101013 (2021).
Orvieto, R., Segev-Zahav, A. & Aizer, A. Does COVID-19 infection influence patients’ performance during IVF-ET cycle? An observational study. Gynecol. Endocrinol. 37, 895–897 (2021).
Herrero, Y. et al. SARS-CoV-2 infection negatively affects ovarian function in ART patients. Biochim. Biophys. Acta BBA Mol. Basis Dis. 1868, 166295 (2022).
Ding, T. et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: An observational study. Front. Med. 8, 286 (2021).
Vaz-Silva, J. et al. The vasoactive peptide angiotensin-(1–7), its receptor Mas and the angiotensin-converting enzyme type 2 are expressed in the human endometrium. Reprod. Sci. 16, 247–256 (2009).
Shan, T. et al. Effect of angiotensin-(1–7) and angiotensin II on the proliferation and activation of human endometrial stromal cells in vitro. Int. J. Clin. Exp. Pathol. 8, 8948 (2015).
Li, X.-F. & Ahmed, A. Dual role of angiotensin II in the human endometrium. Hum. Reprod. 11, 95–108 (1996).
Jing, Y. et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 26, 367–373 (2020).
Wang, M., Zhang, B. & Jin, L. Female fertility under impact of COVID-19 pandemic: A narrative review. Expert Rev. Mol. Med. 23, 1–21 (2021).
Henarejos-Castillo, I., Sebastian-Leon, P., Devesa-Peiro, A., Pellicer, A. & Diaz-Gimeno, P. SARS-CoV-2 infection risk assessment in the endometrium: Viral infection-related gene expression across the menstrual cycle. Fertil. Steril. 114, 223–232 (2020).
Li, K. et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod. Biomed. Online 42, 260–267 (2021).
Ding, T. et al. Potential influence of menstrual status and sex hormones on female severe acute respiratory syndrome coronavirus 2 infection: A cross-sectional multicenter study in Wuhan, China. Clin. Infect. Dis. 72, e240–e248 (2021).
Kamidani, S., Rostad, C. A. & Anderson, E. J. COVID-19 vaccine development: A pediatric perspective. Curr. Opin. Pediatr. 33, 144–151 (2021).
Safrai, M., Rottenstreich, A., Herzberg, S., Imbar, T., Reubinoff, B., Ben-Meir, A. Stopping the misinformation: BNT162b2 COVID-19 vaccine has no negative effect on women’s fertility. MedRxiv (2021).
Orvieto, R. et al. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle?. Reprod. Biol. Endocrinol. 19, 1–4 (2021).
Stanley, K. E., Thomas, E., Leaver, M. & Wells, D. Coronavirus disease (COVID-19) and fertility: Viral host entry protein expression in male and female reproductive tissues. Fertil. Steril. 114, 33–43 (2020).
Morris, R. S. SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F&s Rep. 2, 253–255 (2021).
Aharon, D. et al. mRNA covid-19 vaccines do not compromise implantation of euploid embryos. Fertil. Steril. 116, e77 (2021).
Avraham, S. et al. Coronavirus disease 2019 vaccination and infertility treatment outcomes. Fertil. Steril. 117, 1291–1299 (2022).
Huang, J. et al. No effect of inactivated SARS-CoV-2 vaccination on in vitro fertilization outcomes: A propensity score-matched study. J. Inflamm. Res. 15, 839 (2022).
Aharon, D. et al. (COVID-19) vaccination. Obstet. Gynecol. 2022, 10–1097 (2019).
Lee, W., Mok, A. & Chung, J. P. Potential effects of COVID-19 on reproductive systems and fertility; assisted reproductive technology guidelines and considerations: A review. Hong Kong Med. J. 27, 118–126 (2021).
Joseph, D. N. & Whirledge, S. Stress and the HPA axis: Balancing homeostasis and fertility. Int. J. Mol. Sci. 18, 2224 (2017).
Palomba, S. et al. Lifestyle and fertility: The influence of stress and quality of life on female fertility. Reprod. Biol. Endocrinol. 16, 1–11 (2018).
Prasad, S., Tiwari, M., Pandey, A. N., Shrivastav, T. G. & Chaube, S. K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 23, 1–5 (2016).
Li, R. et al. Potential risks of SARS-CoV-2 infection on reproductive health. Reprod. Biomed. Online 41, 89–95 (2020).
Acknowledgements
We would like to thank Mrs. Faten Ibrahim Diab, the Director of IVF Unit Lab at Irbid specialty hospital, for her great efforts in collecting the data.
Author information
Authors and Affiliations
Contributions
S.A.: conception and design, drafting the article, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Z.A.-A.: conception and design, critical revision, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. J.H.: conception and design, drafting of the article, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. H.A.: data acquisition, drafting of the article, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. H.Q.: conception and design, data acquisition, drafting of the article, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. M.A.N.: data acquisition, critical revision, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors agreed with the content and that all gave explicit consent to submit and that they obtained consent from the responsible authorities at the institute/organization where the work has been carried out, before the work is submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albeitawi, S., Al-Alami, Z.M., Hamadneh, J. et al. COVID-19 infection and vaccine have no impact on in-vitro fertilization (IVF) outcome. Sci Rep 12, 21702 (2022). https://doi.org/10.1038/s41598-022-25757-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25757-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.