Abstract
The crocodile monitor (Varanus salvator) is the most common monitor lizard in Thailand. Based on habitat and food, they have the potential to transmit zoonoses, with a high possibility of infecting ectoparasites and endoparasites. Diseases that could infect crocodile monitors and be transmitted to other animals, including humans. This research aims to identify and evaluate the phylogenetic relationships of Hepatozoon sp. and sheathed microfilaria in crocodile monitors. The phylogenetic analyses of Hepatozoon, based on 18S rRNA, and sheathed microfilaria, based on the COX1 gene, revealed that the Hepatozoon sp. were grouped with H. caimani, while sheathed microfilaria were grouped together with B. timori. This study provides insights into the genetic diversity and host-parasite interactions of hemoparasites in crocodile monitors in Thailand.
Similar content being viewed by others
Introduction
Crocodile monitors, which belong to the Varanidae family, are categorized into a singular genus called Varanus1,2. They can be found in various geological locations such as mainland and islands, spanning across Africa, Central Asia, the Middle East, the Arabian Peninsula, the Indo-Australian Archipelago, and South and Southeast Asia, which includes Thailand1,3,4,5,6. Since 1992, the crocodile monitor, known as V. salvator, has been classified as a “reserved wild animal” and listed in the Act of Animals Protection and Conservation of Thailand. They are predatory creatures that can be found in freshwater wetlands and urban waterways across the country. Limited research has been conducted to examine the microbial ecology of crocodile monitors, their role as hosts or reservoirs for pathogens transmitted by arthropods, and their interactions with ectoparasites3,4,7. However, parasitic infections in Varanus spp. have been investigated in Australia, Nigeria, Slovenia, South Africa, and Thailand8,9,10,11,12. Hepatozoon is a prevalent blood parasite species commonly found in the Asian crocodile monitor and various other reptiles. In South Africa, a prevalence of 25% was observed12, while Brazil ranged from 1.1 to 12.5%13,14, Iran had a prevalence of 39.72%15 and Australia had a high prevalence of 58.1%16.
Onchocercidae, Dirofilariinae, and Oswaldofilaria sp. have been reported in the abdominal cavity and pleural, peritoneal, and lung nodules of Varanus bengalensis (V. bengalensis), identified using a traditional blood smear preparation9. Nevertheless, there is a lack of comprehensive research on the crocodile monitor, specifically regarding the identification of parasites at the molecular level. In Thailand, only a single study has been conducted, which examined Hepatozoon sp. gamonts and reported their presence in less than 1% of the red blood cells (RBCs) of 43 crocodile monitors17. Hence, the objective of this study was to assess the phylogenetic distribution of hemogregarine and filarial nematodes in crocodile monitors from Thailand by comparing them to documented parasitic species found in diverse hosts and geographical regions.
Results
Morphological and morphometric analysis
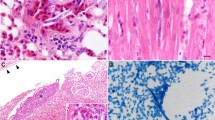
Out of the two free-living crocodile monitor specimens screened, one was found to be infected with Hepatozoon spp. and a filarial nematode, as shown in Fig. 1. The morphological and morphometric data analysis allowed identification of one morphotype of Hepatozoon spp. However, it was not possible to determine the species of the morphotype, and thus, it was classified as an undescribed species. The examination of blood smears revealed the presence of mature gamonts, as depicted in Figs. 1A,B.
In the blood smear, only mature gamonts were identified. These mature gamonts were found within a parasitophorous vacuole (PV) and had a rounded shape at both ends. The cytoplasm of the gamonts displayed a bluish-purple stain. The elongated nucleus exhibited purple-stained chromatin and occupied nearly half of the surface area of the parasite. Gamonts measured (mean ± standard deviation) 11.32 ± 0.886 in length and 4.25 ± 0.621 in width (n = 30) (Table 1).
Molecular amplification, sequencing, and similarity of Hepatozoon 18S rRNA and Brugia COX1 sequences
One captured crocodile monitor was observed with both hemogregarine and filarial infections (Fig. 1A,B). The polymerase chain reaction (PCR) products were sequenced for the 18S rRNA gene (hemogregarine) and COX1 (filarial). Three genes were amplified for filarial nematodes: the 18S rRNA, COX1, and 12S rRNA genes. Unfortunately, only the COX1 sequence was successfully amplified. All PCR amplicons were sequenced in both directions. Sequences were deposited in GenBank under the accession numbers OQ306503 (Hepatozoon sp. 18S rRNA gene sequence) and OQ338200 (Brugia sp.COX1 sequence). The basic local alignment search tool (BLAST) result for the 18S rRNA Hepatozoon sequence (917 base pairs [bps], Supplementary Fig. 1A) indicated 99% identity with Hepatozoon sp. from Philodryas patagoniensis (MN003368, Uruguay) and Tarentola deserti (KU680460, Morocco) and 98% identity with H. caimani (KU495923, Brazil) from caiman crocodiles. The COX1 sequence (651 bps, Supplementary Fig. 1B) demonstrated 96% identity with B. timori (AP017686), which was previously deposited in GenBank. The Thailand Hepatozoon sp. 18S rRNA sequence demonstrated 99.9% similarity with H. caimani (KU495923, Brazil), as shown in Table 2. The Brugia sp. COX1 sequence had 96.9% similarity with B. timori (AP017686, Japan) and 95.4% with B. timori (KP760173, Indonesia), as shown in Table 3.
Phylogenetic analysis of Hepatozoon 18S rRNA and Brugia COX1 sequences
The phylogenetic tree of Hepatozoon sp. 18S rRNA gene sequences demonstrated clustering in a monophyletic group together with sequences of H. caimani (KU495923) that were recently detected in caiman crocodiles in Brazil (Fig. 2). The phylogenetic tree was comprised of eight branches. The first branch included the Hepatozoon sp. sequence amplified in the present study and sequences retrieved from GenBank from other reptile taxa (e.g., snakes, lizards, geckos, and caiman crocodiles), rodents, and amphibians. Hepatozoon sequences amplified from tick (MG758137) and vulture (MF541372) were grouped in the second and third branches, respectively. The forth branch included the Hepatozoon sequences received from mammals (e.g. dogs, cats, lions and bears). In addition, the remaining branches comprising species from Karyolysus, Hemolivia, Haemogregarina and Dactylosoma. sp. Adelina dimidiate (DQ096835) and Adelina grylli (DQ096836) were used as the out-group (Fig. 2). The phylogenetic tree of Onchocercidae COX1 sequences were clustered in a monophyletic group comprising B. timori, B. malayi, Brugia sp, B. pahangi, W. bancrofti, Neofoleyellides sp., Breinlia boltoni, Dirofilaria sp., Dirofilaria sp. “hongkongensis,” D. lutrae, O. flexuosa, O. eberhardi, and O. japonica (Fig. 3). The amplified filarial sequence was clustered with sequences of B. timori (AP017686 and KP760173), recently detected in humans in Japan and Indonesia. Nematoda sp. and Setaria sp. were used as the out-groups (Fig. 3). Moreover, the reliability of bootstrap frequencies and Bayesian posterior probabilities of all phylogenies are displayed with the highest values on each branch.
Haplotype diversity
Nucleotide polymorphisms and DNA divergence between the sequences obtained in the present study and GenBank sequences were analyzed. Nucleotide polymorphism analysis of Hepatozoon sp. 18S rRNA and Brugia sp. COX1 sequences revealed 28 and 24 haplotypes, respectively (Table 4). The haplotype networks of these genes were obtained from the Templeton, Crandall, and Sing (TCS) Network tool (Figs. 4, 5). For the Hepatozoon sp. 18S rRNA gene, of the 28 haplotypes, haplotype 1 was detected in the crocodile monitor from the Nakhon Pathom provinces and in H. caimani in the caiman crocodile from Brazil. Haplotypes 1–23 were found in reptiles, rodents, and amphibians, while the remaining haplotypes were found in tick, canine, feline, and avian hosts from a range of countries (Table 3, Fig. 4). The haplotype network of Brugia sp. COX1 gene demonstrated that the sequence from the Thailand crocodile monitor was detected in haplotype 1, while the B. timori sequences from Japan and Indonesia were detected in haplotype 2 (Table 3, Fig. 5).
Discussion
Until now, the detection of Hepatozoon infections in crocodile monitors has been only reported in Bangkok, Thailand, by means of microscopy screening of blood samples17. Morphologic and morphometric studies could enable differentiation between Hepatozoon sp.18 However; a single parameter cannot be used to differentiate species using the microscopic technique. The need for an additional tool, such as the molecular technique, is needed to enable this. In addition, there is an apparent lack of relevant information regarding the genetic diversity of Hepatozoon sp. and microfilaria isolated from crocodile monitors in Thailand. The current study is the first investigation on the phylogeny of Hepatozoon and sheathed microfilaria isolated from crocodile monitors in Nakhon Pathom, Thailand.
The findings of this study show that crocodile monitors from Thailand can harbor various parasites, including both hemogregarines and filarial worms. The phylogenetic results revealed that Hepatozoon sp. in crocodile monitors had 99% similarity with H. caimani, and the Hepatozoon 18S rRNA gene was grouped in the same clade as crocodiles, reptiles, rodents, and amphibians. In this case, it is not possible for us to make a definitive determination about how the transmission occurs. However, it is plausible that transmission could occur either through prey-predator interactions or via vectors. This has been observed in African reptiles19 and described in the case of H. domerguei infection in native reptiles from Madagascar20. Prey-predator transmission occurs when a predator ingests infectious cysts present in its prey. Additionally, to confirm vector-borne transmission, it is necessary to ascertain and identify the developmental stages of arthropod vectors.
In this study, the oligonucleotide pairs HepF300/900 and HEMO1/HEMO2 were used to amplify the Hepatozoon 18S rRNA gene21,22. This method had already been successful in inferring phylogenetic relationships between Hepatozoon spp. from snakes23,24. Oligonucleotides 18S and 5.8S have also been used to successfully infer phylogenetic relationships between Hepatozoon spp. from reptiles, amphibians and mammals23,24,25,26. The utilization of oligonucleotides HEMO1 and HEMO2 enabled the identification of a new species of Hepatozoon in Coluber constrictor priapus and Thamnophis sauritus sackenii. Through their application, the researchers were able to establish the phylogenetic relationship among hemogregarine isolates originating from Florida27. Therefore, the 18S rRNA sequence is useful for characterization and comparing phylogenetic relationships generic affiliations without prior knowledge of the sporogonic development of parasites28.
The phylogenetic results for the sheathed microfilaria COX1 gene in crocodile monitors revealed that it closely related to B. timori in humans from Japan and Indonesia. However, little is known about phylogenetic relationships of filarial worms in crocodile monitors. The phylogenetic relationship of filarial worms in wild endemic reptiles from Madagascar has been previously described20. In Malaysia, a new genus, Malayfilaria, along with a new species, M. sofiani, was identified in common tree shrews using the COX1 and 12S rRNA genes and the ITS1 region29. M. sofiani appears most closely related to Wuchereria spp. and Brugia spp.; however, it differs in several morphological characteristics. Therefore, it is important to assess the real prevalence of this parasite and investigate its implication for the host, as filarial nematodes, such as B. malayi and B. timori, are known to cause lymphatic diseases in humans living in tropical areas, while B. pahangi infects carnivores and causes zoonotic diseases in humans29. Unfortunately, in the current study, the PCRs targeting the 18S and the 12S rRNA genes failed to amplify sheathed microfilaria DNA. However, parasitemia levels may have been lower, thereby potentially resulting in the failure to detect parasites using PCR. Therefore, the infection levels reported in previous studies that used different primers should be compared with caution.
Conclusions
This report presents the first findings on the molecular detection of Hepatozoon sp. 18S rRNA gene and sheathed microfilaria COX1 gene in crocodile monitors from Thailand. The results from the molecular analysis indicate that the evolutionary distance between the Hepatozoon sp. 18S rRNA gene and sheathed microfilaria COX1 gene is greater than the distance between the previously known species, H. caimani and B. timori, respectively. Consequently, further research focusing on the transmission, interactions between hosts and parasites, and distribution of vectors for these parasites is of utmost importance, particularly in crocodile monitors.
Materials and methods
Collection of blood samples and morphological study of the parasites
Two crocodile monitors (V. salvator) were captured in Nakhon Pathom province and restrained using a nose pole before being transported to a veterinary hospital. A veterinarian collected peripheral blood samples (n = 2) from the caudal tail vein of the crocodile monitors by using an 18-gauge needle and transferred them into EDTA-treated tubes. The samples were collected in accordance with applicable local guidelines. The blood samples were then submitted to the Vet Central Lab. The blood samples were used in blood smears for microscopic examination. Slides were air-dried, fixed with methanol, and stained with Giemsa30. Giemsa-stained thin blood smears were examined microscopically to assess the presence of Hepatozoon gamonts and microfilariae, as well as erythrocyte changes caused by the presence of parasites. To examine the intraerythrocytic parasite stages, digital images were obtained and measured using an Olympus CX31 biological microscope (Olympus, Japan) at 100× magnification. The measurements, in micrometers (µm), included the length and width of the parasite, with corresponding mean and standard deviation values (mean ± standard deviation). The remaining EDTA blood sample was preserved at − 20 °C for subsequent molecular analysis.
DNA extraction, amplification, and sequencing
The collected blood samples were used for DNA extraction. DNA samples were extracted using a genomic DNA blood kit (NucleoSpin® Blood, MACHEREY-NAGEL, Germany). This process was carried out according to the manufacturer’s instructions. Initially, the detection of Hepatozoon sp. DNA was achieved using primers targeting part of the 18S rRNA gene, namely HepF300, and HepR900. Positive samples then used the primers HEMO1 and HEMO2 to amplify a partially overlapping fragment of the 18S rRNA gene to obtain a longer gene portion, as shown in Table 1. Microfilariae were detected in blood smears, and three pairs of primers were used to taxonomically identify these parasites: the 18S rRNA, COX1, and 12S rRNA genes, as shown in Table 1. The amplification conditions involved 20 μL PCR reactions, containing DNA template (2 μL), 1X Gotaq® Green Master Mix (Promega, USA), forward and reverse primers (0.2 mM each), and nuclease-free water, and the reaction was performed in a thermal cycler (BIOER technology, China). A positive control for Hepatozoon sp. and microfilariae DNA was obtained from a naturally infected dog. Nuclease-free water was used as a negative control. The PCR products were stained with RedSafe™ Nucleic Acid Staining Solution (INtRON Biotechnology, Korea) and analyzed via gel electrophoresis using 1% agarose gels. A 100 bp DNA ladder (SibEnzyme®, Russia) was used as the standard for determining the molecular mass of the PCR products. The reaction products were purified using a PCR clean-up gel extraction kit (NucleoSpin® Gel and PCR Clean-up, MACHEREYNAGEL, Germany). Purified amplified DNA fragments were submitted for sequencing using Barcode Taq (BT) sequencing and used for subsequent phylogenetic and haplotype diversity analyses.
Phylogenetic analysis
Phylogenetic reconstructions were based on the DNA sequence alignment of positive samples. Comparisons with sequences deposited in GenBank used the nucleotide BLAST. The sequences were aligned with sequences published in GenBank using the Clustal W algorithm, available in the MEGA software, version 11.0.1331. Phylogenetic relationships were inferred using the Maximum Likelihood (ML) and the Bayesian inference (BI) methods in MrBayes, version 3.1.232. The reliability of inferred phylogenetic relationships was evaluated by the statistical calculation of 1000 replicates using the bootstrapping method33. A Bayesian Markov Chain Monte Carlo analysis was conducted with four Markov chains (three heated chains and one cold) for 50,000,000 generations, with the trees sampled every 1000 generations. The first 50% of the trees were discarded and the remaining samples were used to construct a Bayesian consensus tree and to infer the posterior probability. Genetic distances were assessed using distance matrices under the assumption of pairwise-distance34 and using the Kimura 2-parameter method35. Similarities were evaluated using the sequence identity matrix tool in the BioEdit program, version 7.0.5.336.
Haplotype diversity analysis
The DNA polymorphisms and haplotype information of Hepatozoon sp. and microfilariae sequences were determined using the DnaSP software, version 5.10.0137. Haplotype networks were established using the TCS network tool in the Population Analysis with Reticulate Trees (PopART) software38,39.
Ethical approval
This research project was approved by the Biosafety Committee of Chulalongkorn University, Faculty of Veterinary Science (IBC 2231037). The authors would like to confirm that the samples were collected in accordance with applicable local guidelines.
Data availability
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
References
Böhme, W. Checklist of the living monitor lizards of the world (family Varanidae). Zool. Verh. 34, 3–43 (2003).
Vidal, N. et al. Molecular evidence for an Asian origin of monitor lizards followed by tertiary dispersals to Africa and Australasia. Biol. Lett. 8(5), 853–855 (2012).
Lauprasert, K. & Thirakhupt, K. Species diversity, distribution and proposed status of monitor lizards (family Varanidae) in southern Thailand. Nat. Hist. J. Chulalongkorn Univ. 1(1), 39–46 (2001).
Cota, M., Chan-Ard, T., Mekchai, S. & Laoteaw, S. Geographical distribution, instinctive feeding behavior and report of nocturnal activity of Varanus dumerilii in Thailand. Biawak 2(4), 152–158 (2008).
Koch, A., Ziegler, T., Böhme, W., Arida, E. & Auliya, M. Pressing problems: distribution, threats, and conservation status of the monitor lizards (Varanidae: Varanus spp.) of Southeast Asia and the Indo-Australian Archipelago. Herpetol. Conserv. Biol. 8(3), 1–62 (2013).
Joshi, M., Das, S. K. & Sarma, K. Taxonomy, population status and ecology of Indian desert monitor lizard Varanus griseus koniecznyi Mertens 1954 in the Thar desert of Rajasthan. Saudi J. Biol. Sci. 28, 4542–4552 (2021).
Cota, M. Study and conservation of varanids of Thailand: Past achievements and future challenges. J. Wildl. Thailand 16, 11–12 (2009).
Vilcins, I. M., Ujvari, B., Old, J. M. & Deane, E. Molecular and morphological description of a Hepatozoon species in reptiles and their ticks in the Northern Territory, Australia. J. Parasitol. 95, 434–442 (2009).
Rataj, A. V., Lindtner-Knific, R., Vlahović, K., Mavri, U. & Dovč, A. Parasites in pet reptiles. Acta Vet. Scand. 53, 33. https://doi.org/10.1186/1751-0147-53-33 (2011).
Doornbos, K. et al. Rickettsia sp. closely related to Rickettsia raoultii (Rickettsiales: Rickettsiaceae) in an Amblyomma helvolum (Acarina: Ixodidae) tick from a Varanus salvator (Squamata: Varanidae) in Thailand. J. Med. Entomol. 50(1), 217–220 (2013).
Enabulele, E. E., Ozemoka, H. J., Awharitoma, A. O. & Aisien, M. S. O. Parasitic infections of the African dwarf crocodile (Osteolaemus tetraspis) and the ornate Nile monitor (Varanus ornatus) from Nigeria. Acta Parasitol. 58(2), 191–197 (2013).
Cook, C. A., Netherlands, E. C. & Smit, N. J. Redescription, molecular characterization and taxonomic re-evaluation of a unique African monitor lizard haemogregarine Karyolysus paradoxa (Dias, 1954) n. comb (Karyolysidae). Parasites Vectors 9, 347. https://doi.org/10.1186/s13071-016-1600-8 (2016).
Calil, P. R. et al. Hemogregarine parasites in wild captive animals, a broad study in São Paulo Zoo. J. Entomol. Zool. Stud. 5(6), 1378–1387 (2017).
Mendoza-Roldan, J. A. et al. Molecular detection of vector-borne agents in ectoparasites and reptiles from Brazil. Ticks Tick-borne Dis. 12, 101585. https://doi.org/10.1016/j.ttbdis.2020.101585 (2021).
Jameie, F., Nasiri, V. & Paykari, H. Morphological detection and molecular characterization of Hepatozoon spp. from venomous terrestrial snakes in Iran. Exp. Parasitol. 239, 108309. https://doi.org/10.1016/j.exppara.2022.108309 (2023).
Wicks, R. M. et al. Morphological and molecular characteristics of a species of Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina) from the blood of Isoodon obesulus (Marsupialia: Peramelidae) in Western Australia. Syst. Parasitol. 65, 19–25 (2006).
Salakij, C. et al. Quantitative and qualitative morphologic, cytochemical, and ultrastructural characteristics of blood cells in captive Asian water monitors. Vet. Clin. Pathol. 43(4), 538–546 (2014).
Moço, T. C. et al. Morphological, morphometric and molecular characterization of Hepatozoon spp. Apicomplexa, Hepatozoidae) from naturally infected Caudisona durissa terrifica. Parasitol. Res. 110, 1393–1401 (2012).
Tomé, B., Maia, J. P. M. C. & Harris, D. J. Hepatozoon infection prevalence in four snakes genera: influence of diet, prey parasitemia levels, or parasite type?. J. Parasitol. 98(5), 913–917 (2012).
Maia, J. P., Crottini, A. & Harris, D. J. Microscopic and molecular characterization of Hepatozoon domerguei (Apicomplexa) and Foleyella furcata (Nematoda) in wild endemic reptiles from Madagascar. Parasite 21, 47. https://doi.org/10.1051/parasite/2014046 (2014).
Ujvari, B., Madsen, T. & Olsson, M. High prevalence of Hepatozoon spp. (Apicomplexa, Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J. Parasitol. 90, 670–672 (2004).
Perkins, S. L. & Keller, A. K. Phylogeny of nuclear small subunit rRNA genes of hemogregarines amplified with specific primers. J. Parasitol. 87, 870–876 (2001).
Harris, D. J., Maia, J. P. & Perera, A. Molecular characterization of Hepatozoon species in reptiles from the Seychelles. J. Parasitol. 97, 106–110 (2011).
Maia, J. P. M. C., Harris, D. J. & Perera, A. Molecular survey of Hepatozoon species in lizards from North Africa. J. Parasitol. 97, 513–517 (2011).
Mathew, J. S. et al. Phylogenetic relationships of Hepatozoon (Apicomplexa, Adeleina) based on molecular, morphologic, and life-cycle characters. J. Parasitol. 86, 366–372 (2000).
O’Dwyer, L. H. et al. Description of three new species of Hepatozoon (Apicomplexa, Hepatozoidae) from rattlesnakes (Crotalus durissus terrificus) based on molecular, morphometric and morphologic characters. Exp. Parasitol. 135, 200–207 (2013).
Telford, S. R. Jr., Butler, J. F. & Telford, R. S. Hepatozoon polytopis n. sp. parasitic in two genera and species of colubrid snakes in Southern Florida. J. Parasitol. 91, 144–147 (2005).
Barta, J. R., Ogedengbe, J. D., Martin, D. S. & Smith, T. G. Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J. Euk. Microbiol. 59, 171–180 (2012).
Uni, S. et al. Morphological and molecular characteristics of Malayfilaria sofiani Uni, Mat Udin & Takaoka n. g., n. sp. (Nematoda: Filarioidea) from the common treeshrew Tupaia glis Diard & Duvaucel (Mammalia: Scandentia) in Peninsular Malaysia. Parasites Vectors 10, 194. https://doi.org/10.1186/s13071-017-2105-9 (2017).
Rosenblatt, J. E. Laboratory diagnosis of infections due to blood and tissue parasites. Clin. Infect. Dis. 49, 1103–1108 (2009).
Saitou, N. & Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987).
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001).
Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1985).
Nei, M. & Kumar, S. Molecular Evolution and Phylogenetics. (Oxford University Press, 2000).
Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120 (1980).
Hall, T. A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Librado, P. & Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism. Bioinformatics 25, 1451–1452 (2009).
Clement, M., Snell, Q., Walker, P., Posada, D. & Crandall, K. TCS: Estimating gene genealogies, in Parallel and Distributed Processing Symposium. International Proceedings, Vol. 2, 184 (2002). https://doi.org/10.1109/IPDPS.2002.1016585.
Leigh, J. W. & Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116 (2015).
Laidoudi, Y., Ringot, D., Watier-Grillot, S., Davoust, B. & Mediannikov, O. Cardiac and subcutaneous canine dirofilariosis outbreak in a kennel in central France. Parasites 26, 72. https://doi.org/10.1051/parasite/2019073 (2019).
Laidoudi, Y. et al. Development of a multiplexed qPCRs-based approach for the diagnosis of Dirofilaria immitis, D. repens, Acanthocheilonema reconditum. Parasites Vectors Dev. 13, 319. https://doi.org/10.1186/s13071-020-04185-0 (2019).
Laidoudi, Y. et al. Detection of canine vector-borne filariasis and their Wolbachia endosymbionts in French Guiana. Microorganisms 8, 770. https://doi.org/10.3390/microorganisms8050770 (2020).
Acknowledgements
The authors would like to thank the staff from Parasitology unit, Faculty of Veterinary Science and the Second Century Fund (C2F), Chulalongkorn University.
Funding
This research project is funded by the Thailand Science Research and Innovation Fund, Chulalongkorn University (FOODF67310022).
Author information
Authors and Affiliations
Contributions
W.J. contributed to study design, data analysis, and manuscript writing. P.K. contributed to PCR tests and organized the database. P.T. contributed to conception, supervision, funding acquisition and wrote, reviewed and edited the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Junsiri, W., Kamkong, P. & Taweethavonsawat, P. First molecular detection and genetic diversity of Hepatozoon sp. (Apicomplexa) and Brugia sp. (Nematoda) in a crocodile monitor in Nakhon Pathom, Thailand. Sci Rep 14, 3526 (2024). https://doi.org/10.1038/s41598-024-54276-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54276-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.