Abstract
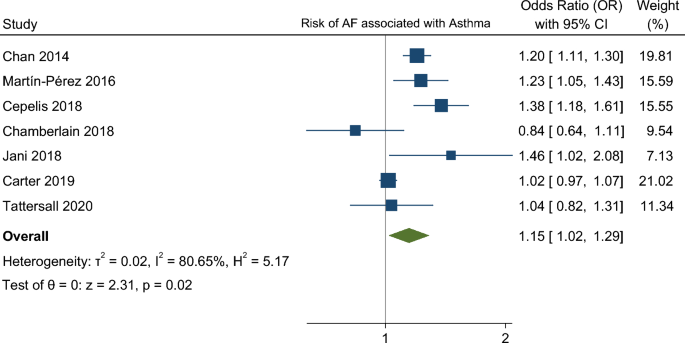
Respiratory disease and atrial fibrillation (AF) frequent coexist, but the risk of AF among asthma patients is less characterized. Growing evidence suggest that AF shares with asthma a systemic inflammation background and asthma treatments, such as beta agonists, have been associated with increased risk of cardiac arrhythmias. The aim of this systematic review was to assess the risk of AF in patients with asthma in observational studies. We search for longitudinal studies reporting AF outcome in asthma and control patients through MEDLINE, Cochrane Central Register of Controlled Trials and EMBASE. Pooled estimates of odds ratios (ORs) and 95% confidence intervals (CIs) were derived by random effects meta-analysis. Heterogeneity was assessed using the I2 test. The risk of bias of individual studies was evaluated using the ROBINS-E tool. The study protocol was registered at PROSPERO: CRD42020215707. Seven cohort/nested case–control studies with 1 405 508 individuals were included. The mean follow-up time was 9 years, ranging from 1 to 15 years. Asthma was associated with a higher risk of AF (OR 1.15. 95% CI 1.01–1.29). High heterogeneity (I2 = 81%) and overall “serious” risk of bias, lead to a very low confidence in in this result. Asthma was associated with an increased risk of AF. However, the high risk of bias and high heterogeneity reduces the robustness of these results, calling for further high-quality data.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is a supraventricular arrhythmia characterized by uncoordinated atrial electrical activation. It is the most common sustained arrhythmia worldwide and is associated with significant patients’ mortality and impacts quality of life1,2. However, AF can be silent, and the first clinical manifestation may be a stroke if this arrhythmia is not diagnosed before3,4. Therefore, it is important to recognize which are the clusters of patients that are more prone to develop AF to plan screening strategies.
Respiratory diseases and AF frequently coexist but the relationship with asthma is not well characterized5,6. Proposed mechanisms include an inflammatory pathway, particularly in obstructive sleep apnea which is one of the respiratory diseases most commonly associated with AF. Other respiratory diseases such as asthma are starting to show an association with AF7. It is now recognized that pathophysiology of AF is extremely heterogeneous8,9, sharing with asthma an inflammatory pathway. Systematic inflammation may conduct to atrial electrophysiology and structural remodelling, leading to increase vulnerability to AF10. Leukotrienes—inflammatory mediators produced in leukocytes—have systematic effects and their receptors are highly expressed in the heart11,12. Furthermore, asthma treatments, such as beta-2 adrenergic agonists, and airway obstruction with hypoxemia have been associated with increased risk of cardiac arrhythmias13,14, but some doubts still exist regarding this link.
The aim of the present systematic review was to evaluate the available evidence regarding the risk of atrial fibrillation in patients with asthma.
Methods
We performed a systematic review and meta-analysis following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis)15 and “The Meta-analysis of Observational Studies in Epidemiology”16 recommendations. The study protocol was registered with PROSPERO (CRD42020215707).
Data sources and search strategy
Potentially eligible studies were identified through an electronic search of bibliographic databases from inception to December 2020 (MEDLINE through PubMed, Cochrane Central Register of Controlled Trials and EMBASE). The search methods used are summarized in Supplementary Table 1. Additionally, we searched for relevant data by checking the reference lists of included studies. No dates or language restrictions were applied.
Eligibility criteria
For the purposes of our systematic review all longitudinal (prospective or retrospective) studies reporting atrial fibrillation outcome in both patients with asthma—defined by clinical signs/symptoms and/or functional tests, administrative codes or as defined by the physician/investigator—and matched controls were considered eligible.
Study selection and data extraction
Two authors (BG and MA) independently screened the title and abstracts of the citations retrieved in the electronic database search17. The full-text reports of all potentially relevant studies were obtained and the authors independently selected studies to be included in the review according to the predefined inclusion criteria. Doubts and disagreements were resolved by consensus. Reasons for the exclusion of articles were recorded.
Whenever available, data extracted included: study design, location, period of study, patient and control population characteristics, outcomes of interest and the adjustments of estimates.
Outcome
Atrial fibrillation was the primary outcome. It was defined as a supraventricular tachyarrhythmia with uncoordinated atrial electrical activation and consequently ineffective atrial contraction, with abnormal ECG activity (absence of P waves and irregular R–R intervals)18. Diagnosis made by the patient’s physician or corresponding administrative code were also acceptable for the definition of the patient’s condition.
Study-level risk of bias and meta-biases
Each study was evaluated independently by two authors (BG and MA) in each of the domains of bias contained in the ROBINS-E tool, accordingly to the algorithm19. Then, the overall risk of bias judgement was performed. Publication bias was evaluated through funnel plot evaluation and Egger test.
Assessment of confidence in the cumulative evidence
The evaluation of primary outcomes was performed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework regarding the study design, study quality, consistency, and directness20. The pooled evidence was then classified as having very low, low, moderate, or high confidence.
Data synthesis
We used STATA 17.0 to derive forest plots and to perform pooled analysis and related tests. Random-effects meta-analysis was performed with the DerSimonian-Laird model to estimate pooled odds ratio (OR) and 95% confidence intervals (95% CIs). Heterogeneity was defined as p value < 0,10 in the Chi-square test and the magnitude was reported through the I2 metric and was considered substantial if I2 > 50%21. Meta-regression was performed to evaluate the impact of age and follow-up.
Results
Included studies
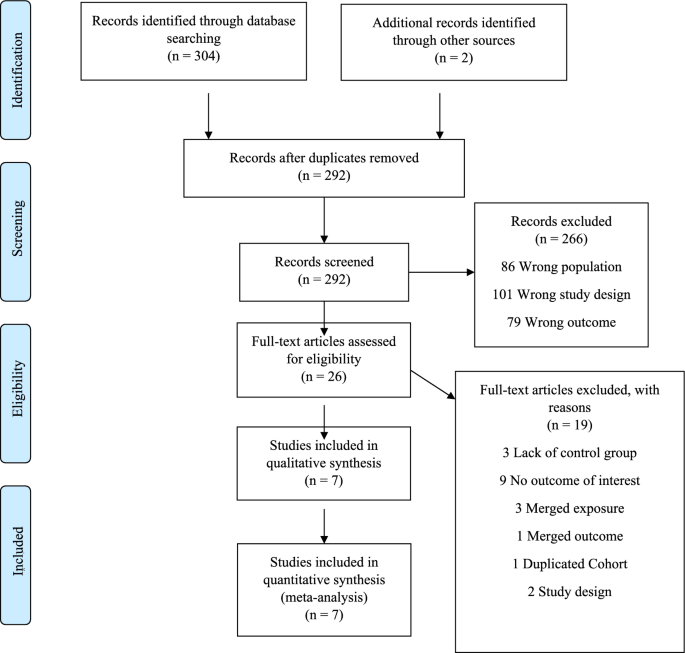
The search of the electronic databases yielded 292 studies after removal of duplicates. From those, 266 were excludes after titles and abstracts screening and full-text assessment was performed for 26 articles. Following our inclusion and exclusion criteria, we were able to include 7 studies for analysis (Fig. 1). The main reasons for excluding studies were lack of control group and no assessment of the outcome of interest.
Description of the studies
All studies had a prospective/retrospective cohort design7,22,23,24,25,26,27. Four studies were conducted in Europe, two in America and one in Asia. The mean follow-up time was 9 years, ranging from 1 to 15 years. Overall, 1,405,508 patients were included. Table 1 shows the main study characteristics.
Many studies did not report previous cardiovascular risk factors of the participants included (Supplementary Table 2). Asthma diagnosis methods were essentially based on codification systems and patient report. Almost all did not mention asthma severity and the type of medications for asthma control (Supplementary Table 3). Outcome adjustment for cardiovascular risk factors was present in only few studies (Supplementary Table 4).
Risk of bias
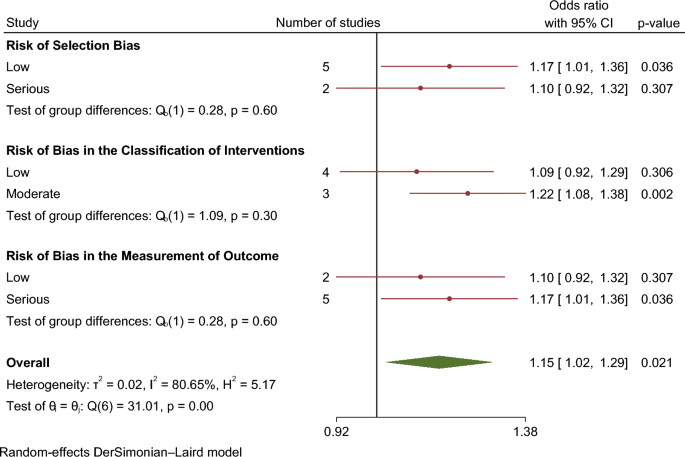
The risk of bias is serious mainly due to significant bias in the measurement outcome domain (Table 2). Diagnosis method of asthma vary from self-reported to detection through codification systems. In terms of AF diagnostic, it was also verified an important heterogeneity, being often based on codification systems but in some studies an ECG or Holter monitoring was required. Bias in selection of participants was classified as serious in two studies—Carter et al.22 and Marín-Pérez et al.28—since the participants are part of a hospitalized and heart failure population, respectively, which can influence the risk and enhance AF diagnosis. On the other hand, in these two studies the risk in measurement outcome was accepted as being low once this kind of populations usually performed ECG regularly.
Outcome: risk of atrial fibrillation
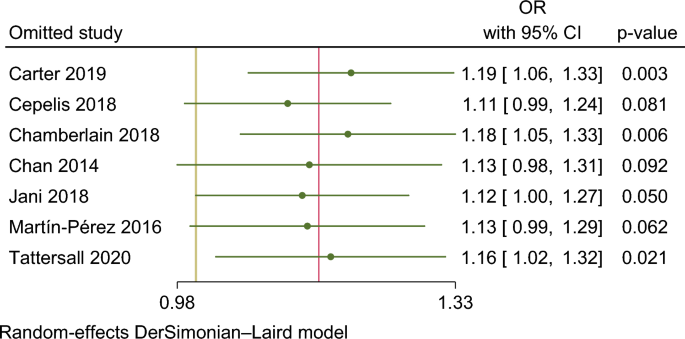
In our meta-analysis asthma was associated with a higher risk of AF (OR 1.15. 95% CI 1.01–1.29) (Fig. 2). The analysis showed very high statistical heterogeneity (I2 = 81%). A sensitivity analysis evaluating the result of the meta-analysis with exclusion of each single study was performed and the outcome remain consistent regarding the direction and magnitude of the effect, however the significance was lost with the exclusion of the studies Cepelis et al., Chan et al. and Martin-Pérez et al. which can be considered a robustness indicator (Fig. 3). Subgroup analysis according to the evaluation of risk of bias was also performed and the results between low and moderate or serious risk of bias regarding different domains, such as selection bias, classification of interventions and measurement outcome, were quite consistent (Fig. 4).
Meta regression results analyzing the effect of age and follow in the results did not find any significant estimates (p = 0.326 and p = 0.587, respectively) (Supplementary Fig. 1). Only few studies reported data according to asthma severity and asthma treatment.
The evaluation of the funnel plot and Egger regression test (p = 0.89) result did suggest the existence of publication bias (Supplementary Fig. 2).
Assessment of confidence in cumulative evidence
Applying GRADE criteria, the confidence in the evidence is very low, being the downgrading reasons mainly due to risk of bias and imprecision—Supplementary Table 5.
Discussion
Our analysis showed that asthma was significantly associated with a higher risk of AF, however with very low certainty according to GRADE criteria.
It is now recognized that pathophysiology of AF is complex and probably in association with systemic disease9,29. The initiation and maintenance of this supraventricular arrhythmia can be the result of the interaction between a trigger and the substrate, induced by an electrical and structural remodeling8. Emerging evidence suggest mechanisms that goes beyond the atrium, sharing with asthma an inflammatory pathway. Inflammation represents a trigger of AF and is also implicated in its perpetuation10. Different inflammatory cytokines mediate function of ion channels and atrial remodelling30,31. Important mediators of inflammation such as C reactive protein and interleukin-6, have been found to be high in patients with AF, and even their influence on the success of the AF ablation have been shown32,33.
Furthermore, asthma treatments, such as beta 2-agonists and corticosteroid therapy, in different ways can modulate the risk of cardiac arrythmias34. The beta 2-adrenergic receptor agonists, such as salbutamol or formoterol, have a positive chronotropic effect and also decrease the atrioventricular nodal, atrial, and ventricular refractoriness which can enhance the risk of both supraventricular and ventricular arrhythmias35. In the other hand, corticosteroids were found to be beneficial for the prevention of atrial fibrillation occurrence in patients undergoing cardiac surgery and AF ablation procedures, probably due to its anti-inflammatory activity36,37.
There is suggestion that the severity of asthma as a surrogate of inflammation and beta2 adrenergic agonists use can influence the incidence of AF. However, most of the studies included in our analysis did not report patients’ asthma severity or medication used. In the few studies reporting such data there was a higher risk for AF in participants with uncontrolled asthma (HR 1.74 [95%CI 1.26–2.42]) (Cepelis et al.25 study); persistent asthma, defined as asthmatics requiring controller medications, but not intermittent asthma was associated with a 1.5-fold increase in risk of AF in Tattersall et al.7; in Chan et al.24 bronchodilator therapy—corticosteroid and non-corticosteroid—was associated with an increased risk for AF (OR 2.13; 95% CI 1.226–3.701, p = 0.007 and OR 2.849; 95% CI 2.48–3.273, P < 0.001, respectively). The association between AF and the use of inhaled corticosteroids is still unclear and a possible explanation for these results is the relationship between these drugs use and asthma severity.
The strength of association between asthma and AF might be weaker due to the statistical and clinical heterogeneity among the included studies as well as the risk of bias which preclude robust conclusion. More evidence is needed to establish robustly this relationship, which can be of tremendous value concerning identification of individuals at higher risk in the community and, therefore, improving prevention and screening programmes for early AF detection and stroke prevention. These studies should be able to provide further evidence about the magnitude of the relationship between asthma and drugs used in the AF outcome.
Limitations
Our systematic review with meta-analysis has some limitations. The diagnosis of asthma was heterogenous in the included studies, varying from self-reported and codification systems and atrial fibrillation assessment was also variable (ECG or Holter monitoring and mainly based on codification system and patients’ reports) which represents a potential source of bias. The period of follow-up ranged from 1 to 15 years (mean follow-up time 7 years), which can influence the main outcome. One of the major drawbacks of most of the studies as well of this systematic review is that most of risk factors for AF were not adequately characterized which underpowers further exploratory analyses that were not performed due to lack of data (Supplementary Table 2).
The low confidence in the generated evidence was recognized by the GRADE assessment where the risk of bias due to confounding and risk the of bias due to the assessment and measurement of outcomes have an important role, and should be acknowledged in the planning of future studies.
Nevertheless, most of the outcomes were adjusted at least to some of these potential confounders, such as smoking, alcohol consumption, diabetes, lipid profile, arterial hypertension, obesity, and other cardiovascular diseases (Supplementary Table 4).
Conclusion
The risk of AF associated with asthma patients was significantly increased. However, more evidence is necessary to support these association and clarify etiologic mechanisms. Identifying individuals at higher risk of developing AF could facilitate screening programmes and earlier diagnose. Based on our data it is wise to recommend special attention in clinical evaluation of asthma patients, including a systematic revision of symptoms like palpitations, dyspnea and fatigue, as well as opportunistic evaluation of the pulse regularity, to prompt ECG recording (e.g. 12-lead ECG or Holter monitoring).
Data availability
The datasets generated and/or analysed during the current study are public. However, any data can be provided from the authors upon reasonable request by contacting Prof. Daniel Caldeira (dgcaldeira@hotmail.com), the corresponding author.
References
Hindricks, G. et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 42, 373–498 (2020).
Thrall, G., Lane, D., Carroll, D. & Lip, G. Y. H. Quality of life in patients with atrial fibrillation: A systematic review. Am. J. Med. 119, 448-e1 (2006).
Sgreccia, D. et al. Comparing outcomes in asymptomatic and symptomatic atrial fibrillation: A systematic review and meta-analysis of 81,462 patients. J. Clin. Med. 10, 3979 (2021).
Boriani, G. et al. Asymptomatic atrial fibrillation: Clinical correlates, management, and outcomes in the EORP-AF pilot general registry. Am. J. Med. 128(5), 509-518.e2 (2015).
Romiti, G. F. et al. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: A systematic review and meta-analysis of 4,200,000 patients. Eur. Heart J. 42, 3555–3557 (2021).
Huang, Q. et al. Risk factors for new-onset atrial fibrillation in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. PeerJ 2, 8 (2020).
Tattersall, M. C. et al. Persistent asthma is associated with increased risk for incident atrial fibrillation in the MESA. Circ. Arrhythm. Electrophysiol. 13, 121–130 (2020).
Wijesurendra, R. S. & Casadei, B. Mechanisms of atrial fibrillation. Heart 105(24), 1860–1867 (2019).
Wijesurendra, R. S. & Casadei, B. Atrial fibrillation: Effects beyond the atrium?. Cardiovasc. Res. 105(3), 238–247 (2015).
Hu, Y. F., Chen, Y. J., Lin, Y. J. & Chen, S. A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. [Internet] 12(4), 230–243 (2015).
Sanak, M. et al. Pharmacological inhibition of leukotriene biosynthesis: Effects on the heart conductance. J. Physiol. Pharmacol. 61(1), 53–58 (2010).
Sokołowska, B. et al. Influence of leukotriene biosynthesis inhibition on heart rate in patients with atrial fibrillation. Int. J. Cardiol. 145(3), 625–626 (2010).
Salpeter, S. R., Ormiston, T. M. & Salpeter, E. E. Cardiovascular effects of β-agonists in patients with asthma and COPD: A meta-analysis. Chest [Internet] 125(6), 2309–2321 (2004).
Hyunjae, K. et al. The relationship between chronic atrial fibrillation and reduced pulmonary function in cases of preserved left ventricular systolic function. Korean Circ. J. 39(9), 372–377 (2009).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 15, 339 (2009).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting—Meta-analysis of observational studies in epidemiology (MOOSE) Group B. JAMA Neurol. 283, 2008–2012 (2000).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan: A web and mobile app for systematic reviews. Syst. Rev. 5(1), 210 (2016).
Hindricks, G. et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 1–126 (2020).
Sterne, J. A. et al. The Risk of bias in non-randomized studies-of Interventions (ROBINS-I) assessment tool ROBINS-I tool (Stage I): At protocol stage. BMJ 355, 1–22 (2016).
Guyatt, G. H. et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J. Clin. Epidemiol. 64(4), 407–415 (2011).
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558 (2002).
Carter, P. et al. Association of cardiovascular disease with respiratory disease. J. Am. Coll. Cardiol. 73(17), 2166–2177 (2019).
Chamberlain, A. M. et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: A population-based study. Am. Heart J. 1(185), 74–84 (2017).
Chan, W. L. et al. The association of asthma and atrial fibrillation: A nationwide population-based nested case-control study. Int. J. Cardiol. 176(2), 464–469 (2014).
Cepelis, A. et al. Associations of asthma and asthma control with atrial fibrillation risk results from the Nord-Trøndelag health study (HUNT). JAMA Cardiol. 3(8), 721–728 (2018).
Yang, K. P., Chao, T. F., Huang, C. C. & Leu, H. B. Asthma and atrial fibrillation: A nationwide population-based study. In: Heart Rhythm S186–S215 (Elsevier, 2011).
Jani, B. D. et al. Multimorbidity and co-morbidity in atrial fibrillation and effects on survival: Findings from UK Biobank cohort. Europace 20(FI3), f329–f336 (2018).
Martin-Perez, M., Ruigomez, A., Michel, A. & Garcia Rodriguez, L. A. Incidence and risk factors for atrial fibrillation in patients with newly diagnosed heart failure. J. Cardiovasc. Med. 17(8), 608–615 (2016).
Ball, J., Carrington, M. J., McMurray, J. J. V. & Stewart, S. Atrial fibrillation: Profile and burden of an evolving epidemic in the 21st century. Int. J. Cardiol. 167, 1807–1824 (2013).
Liew, R. et al. Role of tumor necrosis factor-α in the pathogenesis of atrial fibrosis and development of an arrhythmogenic substrate. Circ. J. 77(5), 1171–1179 (2013).
Wakili, R., Voigt, N., Kääb, S., Dobrev, D. & Nattel, S. Recent advances in the molecular pathophysiology of atrial fibrillation. J. Clin. Investig. 121, 2955–2968 (2011).
Jiang, H., Wang, W., Wang, C., Xie, X. & Hou, Y. Associationofpre-ablation levelofpotential blood markers with atrial fibrillation recurrence after catheter ablation: A meta-analysis. Europace 19(3), 392–400 (2017).
da Silva, R. M. F. L. Influence of inflammation and atherosclerosis in atrial fibrillation. Curr. Atheroscler. Rep. 19, 1 (2017).
Warnier, M. J. et al. Cardiac arrhythmias in adult patients with asthma. J. Asthma 49(9), 942–946 (2012).
Kallergis, E. M. et al. Acute electrophysiologic effects of inhaled salbutamol in humans. Chest 127(6), 2057–2063 (2005).
Liu, L. et al. Effects of corticosteroids on new-onset atrial fibrillation after cardiac surgery A meta-analysis of randomized controlled trials. Medicine https://doi.org/10.1097/MD.0000000000025130 (2021).
de Vecchis, R. Promising effects of moderate-dose corticosteroid therapy in the blanking period for prevention of atrial fibrillation (AF) recurrences in patients undergoing AF ablation. Eur. J. Clin. Pharmacol. 75, 1179–80 (2019).
Acknowledgements
This work was supported by national funds, Fundação para a Ciência e a Tecnologia, reference number UIDB/00306/2020.
Author information
Authors and Affiliations
Contributions
B.G. and M.A. wrote the main manuscript. M.A. and D.C. performed statistical analysis and prepare figures. D.C. and F.J.P. contributed to manuscript discussion. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nogueira-Garcia, B., Alves, M., Pinto, F.J. et al. The association between asthma and atrial fibrillation: systematic review and meta-analysis. Sci Rep 14, 2241 (2024). https://doi.org/10.1038/s41598-023-50466-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50466-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.