Abstract
Understanding the response of plants to varied gravitational conditions is vital for developing effective food production in space bioregenerative life support systems. This study examines the impact of altered gravity conditions on the growth and morphological responses of Wolffia globosa (commonly known as “water lentils” or “duckweed”), assessing its potential as a space crop. Although an experiment testing the effect of simulated microgravity on Wolffia globosa has been previously conducted, for the first time, we investigated the effect of multiple gravity levels on the growth and morphological traits of Wolffia globosa plants. The plant responses to simulated microgravity, simulated partial gravity (Moon), and hypergravity environments were evaluated using random positioning machines and the large-diameter centrifuge. As hypothesized, we observed a slight reaction to different gravitational levels in the growth and morphological traits of Wolffia globosa. The relative growth rates (RGR) of plants subjected to simulated microgravity and partial gravity were reduced when compared to those in other gravity levels. The morphological analysis revealed differences in plant dimensions and frond length-to-width ratios under diverse gravity conditions. Our findings showed that Wolffia globosa is responsive to gravitational changes, with its growth and morphological adaptations being slightly influenced by varying gravitational environments. As for other crop species, growth was reduced by the microgravity conditions; however, RGR remained substantial at 0.33 a day. In conclusion, this study underscores the potential of Wolffia globosa as a space crop and its adaptability to diverse gravitational conditions, contributing to the development of sustainable food production and bioregenerative life support systems for future space exploration missions.
Similar content being viewed by others
Introduction
Wolffia globosa, commonly known as “water lentils” is a member of the Wolffoideae subfamily within the Lemnaceae family, shares similar biological traits with its Lemnoideae relatives: it is the smallest flowering plant and shows the fastest growth rate in the plant kingdom and lacks a pseudo root system1,2. Due to its rapid growth rate, high protein content, and nutritional value, it has emerged as a promising candidate for sustainable food production, particularly in agriculturally challenging regions3,4,5,6. This unique plant has recently garnered attention also as a potential bioregenerative life support system (BLSS) candidate7, offering traits that align with closed-loop, resource-efficient systems like those adopted for the ESA MELiSSA Loop8. Nevertheless, as for other candidate space crops9,10 to assess the suitability of Wolffia’s in space cultivation and its ability to recycle resources efficiently, further laboratory tests investigating growth, nutrition, and genetic responses under extreme conditions are fundamental7.
Studying the effects of various stimuli, including different gravity environments, radiations, and their interactions, on the growth and development of plants can be challenging and costly7,11. Space limitations within onboard test facilities, such as the International Space Station or orbiting vectors, often restrict the number of replicates, making studies under these conditions even more demanding12,13,14. However, cost-effective alternatives exist in the form of facilities that simulate microgravity and partial gravity levels by continuously altering the gravitational vector15,16,17. These facilities offer higher replicate numbers and serve as robust testbeds for experiments involving different gravity levels18,19.
Hypergravity, characterized by gravitational forces greater than Earth’s standard 1 g, is a significant factor in space exploration, affecting humans, and plant life20,21. Hypergravity is most prominent during the manuvers of take-off and landing phases of spacecraft. These brief yet intense episodes of increased gravitational force can influence various aspects of plant biology and growth21. Understanding how plants respond to hypergravity is essential for optimizing their cultivation in space environments and for Earth’s agriculture22,23. Furthermore, it provides valuable insights into the mechanisms plants employ to withstand and adapt to extreme gravitational conditions24,25.
Earlier studies under simulated microgravity have shown stable anatomical morphology in Wolffia plants26, possible effects of hypergravity on plant growth and reproduction have not yet been investigated. Testing plant reactions of potential space crops under varying gravity levels, including those experienced during take-off, landing, or in partial gravities, is reported as crucial27. Consequently, exploring the adaptability of Wolffia plants under different gravitational conditions significantly contributes to our understanding of their potential7.
Recent genome sequencing of Wolffia australiana has shed light on the gravity-sensing mechanisms and photomorphogenesis in Wolffia plants28,29. Due to the loss of gravity sensing genes in the sister species Wolffia australiana, we also hypothesised for Wolffia globosa a lack of the gravity sensing mechanisms, resulting in a reduced effect of the different gravity levels on the growth and morphological characteristics.
More specifically, we aim to study the effects of altered gravity conditions on the growth and morphology of Wolffia globosa. Leveraging the capabilities of random positioning machines and large-diameter centrifuges, we simulated microgravity, partial gravity (Moon), and hypergravity conditions to comprehensively explore the impact of varying gravitational environments on this plant species. These machines allow us to generate and study a range of gravity conditions, thereby elucidating Wolffia globosa's responses to these diverse gravity environments and assessing its potential utilisation in space-based food production systems.
Materials and methods
Plant material and cultivation
Plants of Wolffia globosa (9910) have been provided by Prof. Klaus Apperoth from the Department of Plant Physiology of the University of Jena, Germany. Upon receive, plant material was surface sterilised with 0.3% bleach/water solution for 5 min30. After 14 days from disinfection, plants were subcultured for 30 days in N-medium30 under axenic conditions. Therefore, plant material has been transferred under a laminar flow hood in sets of 6-well plates, each filled with 5 ml of N-medium solution and sealed with micropore® tape. Before the experimental run, plants were acclimatised for 24 h at 30 °C. After acclimatisation, an average of 20 ± 4 fronds were transferred in each well of a 6-multiwell plate previously filled with 5 ml of N-medium and 0.8% of Agar to achieve a semi-solid substrate. The experiment was conducted at 30 ± 0.5 °C average temperature with a photoperiod of 16/8 h light/dark and a total Photon Flux Density (PFD) of 72.37 ± 10.30 μmol m−2 s−1 for 168 h2. Carbon dioxide concentration has been monitored throughout the experiment, resulting in an average concentration of 303 ± 34 ppm.
Hardware description and dimensions
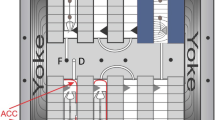
Experimental hardware has been developed to hold two multiwell plates in a setup that minimises the gravity gradient between the upper and lower multiwell plates. Furthermore, the experimental hardware had to be designed to fit in most test facilities, setting the lower constraints to an overall dimension of 15*15*15 cm of the smallest Random Positioning Machine (RPM) available.. Considering these constraints, we designed and developed the experimental hardware using the free available software SketchUp® (Fig. 1). Due to the constraints described before, multiwell plates had to be stacked vertically. This setup minimises the distance of the two centres of mass of both multiwells so that the difference in acceleration can be neglected. The experimental hardware has been equipped with LED white light (Goming LTD) to ensure proper plant development.
Experimental hardware: (A) In the Front view, the black arrows show the LED light strips, the star shapes marks the biological containers (multiwell plates), and the triangular shape marks the base plate of the experimental hardware. (B) Assembled hardware from a back view, the red arrows points at the 30 mm fan re-circulating air within the experimental hardware.
Furthermore, 3 cm 5 V fans have been added to the experimental hardware to increase uniformity between the temperature inside and outside the experimental hardware (Fig. 1). The prototype of the experimental hardware has been built in plywood. Plywood has been laser cut, and parts have been glued in place with vinyl glue. The template for laser cutting can be found in supplementary material Appendix A. Nevertheless, the experimental hardware can be 3D printed in any plastic material, and information for the 3D model can be found on the directory: GitHub.
The inner dimension of each experimental hardware is designed to fit a multiwell plate of standard dimensions (12.7 × 8.5 × 2.2 cm). From the multiwell lid to the lighting system, a fixed distance of 2.0 cm has been set up to guarantee optimal light intensity for plant growth. The experimental hardware's design and dimensions are reported below (Fig. 2).
Lighting system
Light treatment has been achieved utilising two 10 cm 5 V LED stripes illuminating each multiwell plate (Fig. 1). Via USB connector, the light was connected to a Wi-Fi USB switch that controlled the lighting system and photoperiod to a 16/8 light/dark cycle. Light emission spectrum has been determined with a spectroradiometer (SS-110, Apogee Instruments Inc.) measuring in six different positions within the experimental hardware (N = 6) in a wavelength range of 340–820 nm. A more detailed composition of the light spectrum is described in Table 1 and Fig. 3.
Gravity treatments
The study encompassed four different gravity treatments: simulated microgravity, simulated partial gravity (Moon), and two levels of hypergravity (2g ad 4g). In addition, a control group under Earth gravity conditions was included for comparison (Fig. 4).
Experimental hardware implementation. The different images show the implementation of the experimental hardware in the different gravity treatments. (A) RPM simulated partial gravity (Moon) (B) Simulated microgravity (sim-µg) (c) LDC gondola implemented with the experimental hardware within the LDC.
Simulated microgravity (sim-µg) was achieved using an RPM (Random Positioning Machine, original Dutch Space Leiden, NL; currently Yuri Space, Meckenbeuren, DE) that allowed the plants to be positioned within 10 cm from the centre of rotation. The RPM was set to a maximum random speed of 60 °/s, minimising residual acceleration31. RPM was also set to random interval and random direction.
Simulated Partial gravity (Moon) (sim-Moon) was simulated using a similar RPM, which was running a different program which does not provide a complete random rotation during the experiment but generates vector orientations and values that are biased such that the averaged resulted gravity vector simulates the Moon. This proprietary software generated motion can enabled the examination of plant responses to partial gravity ranging from nearly zero to 0.9 g levels27.
Hypergravity conditions were obtained using the large-diameter centrifuge (LDC)32. Two hypergravity levels were tested: 2g and 4g, corresponding to twice and four times the gravitational force on Earth, respectively. These conditions allowed investigation of how Wolffia globosa responded to increased gravitational forces.
A control group was included, where plants were grown under normal gravity conditions (1g) in a static gondola. This control group served as a baseline for comparing the growth and morphology of Wolffia globosa under different gravity treatments.
The experimental setup and the number of replicates for each treatment are summarised in Table 2:
Data collection and analysis
We collected data by imaging the multiwell plates at two-time points: the beginning of the experiment (t0) and after 168 h (t168).
Two imaging setups were used:
Camera Setup: a Sony Alpha II camera with an 18–70 mm objective was fixed to a camera stand. It captured top-down images of the multiwell plate placed on a lighting table with a white semi-transparent Polyvinyl chloride panel illuminated by an LED light panel, ensuring uniform lighting conditions. The images thaken with this setup were used to evaluate growth. Morespecificaly, the relative growth rate (RGR) was calculated by comparing the area occupied by the plants in pixels at the start of the experiment (t0) and after 168 h (t168). Doubling time (DT) was determined based on RGR.
Stereo Microscope Setup: detailed morphological analysis was performed using a Leica® MZ8 Stereo zoom Microscope. Images representing replicates for each treatment were captured. Images acquired with the steromicroscope were used to evaluate the morphological traits of Wolffia globosa were compared among gravity treatments. Morphological traits, such as the dimensions of the long and short axes of the mother fronds, were measured using Fiji® software. Ratios between the long and short axes of the mother fronds were also calculated to assess frond roundness.
Image analysis has been performed on the images gathered during the experimental run. Two types of analysis have been conducted: (1) Analysis of the growth parameters (RGR and DT) (2) Analysis of the morphological differences. RGR has been calculated by comparing the area expressed in pixels occupied by the plant at two-time intervals (N = 12). Images were analysed using the ilastik® software described by Romano et al.33. We calculated DT based on RGR as described by Naumann34. To perform morphological comparison among treatments, one hundred mother fronds have been randomly selected throughout the different wells of the same multiwell plate within the same treatment. For coherent results, a phenological standard was determined. We have measured only mother fronds that had a well-formed daughter frond still attached. Morphological traits have been evaluated by measuring the long and short side of the mother frond. Long and short side have been always measured as shown in Fig. 5. Furthermore, we have evaluated the existing ratios with respect to the long side between these two measurements (N = 100)26 (Fig. 5). Measurements have been taken utilising Fiji® software.
Image analysis data were processed and analysed using SPSS Statistics ver. 21 (IBM Corp.). Differences in growth and morphological traits of Wolffia globosa among gravity treatments was assessed by one-way ANOVA (p < 0.05). Pairwise comparisons were performed using Tukey's post-hoc test (P < 0.05) to identify differences among individual treatments.
IUCN policy statement
Experimental studies and field research on plants (cultivated or wild), including the collection of plant material, must comply with relevant institutional, national and international guidelines and legislation. We will strictly adhere to the IUCN Policy Statement on Research on Endangered Species and the Convention on Trade in Endangered Species of Wild Fauna and Flora. Prof. Klaus Apperoth provided specimens in this utilised in this study from the Department of Plant Physiology of the University of Jena, Germany and are available in the collection of the up mentioned university. We confirm compliance with the IUCN policy for plant.
Results
Relative growth rate (RGR)
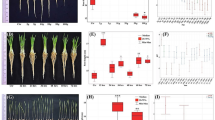
The analysis of variance (ANOVA) revealed a significant difference (F = 16.47, p < 0.001) for the RGR among the different gravity treatments. The Eta-squared and Epsilon-squared effect sizes indicated a large effect of the treatments on the RGR (Eta-squared = 0.54, Epsilon-squared = 0.51). The post hoc 'Tukey's test, conducted to determine the specific differences between the treatments, showed that the simulated microgravity treatment (sim-µg) (mean difference = 0.33, p < 0.001) had a significantly lower RGR (Fig. 6) compared to the control treatment (1 g) (mean difference = 0.38 ± 0.02). The simulated Moon partial gravity treatment (sim-Moon g) also exhibited a lower RGR than the control treatment, but the difference was not statistically significant (mean difference = 0.36, p = 0.25).
In contrast, hypergravity treatments (2 g and 4 g) showed higher RGR compared to the control treatment. However, there was no significant difference between the control and 2 g hypergravity treatments (mean difference = 0. 38, p = 0.99). The 4 g hypergravity treatment (4 g) showed the highest RGR among all treatments, however the difference was not statistically significant compared to the control treatment (mean difference = 0.39, p < 0.50). Furthermore, the doubling time is reported. The sim-µg treatment exhibited the longest doubling time of 2.18 days, followed by the sim-Moon g, 1 g, 2 g, and 4 g treatments with doubling times of 1.99, 1.83, 1.82, and 1.82 days, respectively (Fig. 7).
Morphological difference
Analysis of the morphological characteristics of Wolffia globosa fronds revealed differences in frond length among the gravity treatments. Resulting in a variation in the length of both short and long axes. Results of the ANOVA for the long side length showed a significant difference among gravity treatments (F = 17.34, p < 0.001). Results revealed that the fronds in the 1 g treatment exhibited the longest length (mean = 0.76 mm), followed by the sim-Moon g treatment (mean = 0.76 mm), the sim-µg treatment (mean = 0.75 mm), the 2 g treatment (mean = 0.73 mm), and the 4 g treatment (mean = 0.71 mm) (Fig. 8). Similarly, for the short side length, a significant main effect of gravity treatment was found (F = 5.349, p < 0.001). Post hoc tests showed that the fronds in the sim-µg treatment displayed the shortest length (mean = 0.56 mm), followed by the 4 g treatment (mean = 0.57 mm), the sim-Moon g treatment (mean = 0.57 mm), the 2 g treatment (mean = 0.58 mm), and the 1 g treatment (mean = 0.59 mm). These results suggest that the gravity environment significantly affects the morphological characteristics of Wolffia globosa fronds, resulting in shorter (long axis) plants for those that exhibited the highest growth and vice versa.
Comparison between average long and short sides across different treatments. Graphs show the average length in mm of the long and short sides of different mother fronds across different gravity treatments. Data refer to means ± SD of the means, N = 100. The letters on top of the bars describe the homogenous subsets calculated with Tukey's post-hoc analysis.
Analysis of the ratio in relation to the long side of Wolffia globosa fronds revealed significant differences among the different gravity treatments (F = 11.162, p < 0.001). Post hoc tests reported that the sim-µg treatment exhibited the highest ratio value (mean = 1.33), followed by the sim-Moon g treatment (mean = 1.32), the 1 g treatment (mean = 1.30), the 2 g treatment (mean = 1.27), and the 4 g treatment (mean = 1.26). These results suggest that the low gravity environment (sim-µg and sim-Moong) significantly impacts the ratio in relation to the long side of Wolffia globosa fronds (Fig. 9).
Discussion
The findings of this study shed light on the response of Wolffia globosa to altered gravitational conditions. While the results revealed some variations in growth and morphological traits among the different gravity treatments, these observations present exciting opportunities for further exploration and potential applications.
In the realm of plant cultivation for space exploration (on orbital platforms, the Moon, and Mars), a more profound comprehension of plant reactions to altered gravitational conditions is essential16. Previous studies have shown various effects of microgravity on the growth of several plant species, but research on the effects of partial gravity and hypergravity on plants has been limited15,18. The aid of facilities such as RPMs and LDC can be the perfect testing ground before experimenting with true microgravity18,19 and testing the effect of different gravity levels on the performances of novel space crop species7.
Plants grown under microgravity conditions manifest a slower growth rate compared to those grown under Earth’s gravity35. Results from our investigation show that simulated microgravity and simulated Moon gravity treatments resulted in slightly lower growth rates than the control treatment (Fig. 5). Although these differences, it is important to note that the relative growth rates observed in these treatments were still substantial, with the simulated microgravity treatment exhibiting a relative growth rate of 0.33 per day and the simulated Moon partial gravity treatment exhibiting a relative growth rate of 0.36 per day while it was 0.38 per day in the 1 g control plants. In addition, although reduced by the altered gravity conditions, growth rates of the Wolffia plants remain higher than those typically observed in other higher plant species36. Results from past experiments show the relative growth rate over a span of 40 days of lettuce grown under simulated microgravity, yielding a rate of 0.08/day37.
The effects of altered gravity, especially microgravity (whether real or simulated), on higher plant species have been the subject of numerous scientific studies38. Wolffia globosa is the smallest higher plant, and its morphology is entirely distinct from other higher plants39. It doesn’t exhibit the typical morphology of roots, stems, and leaves but only has a frond that cannot be associated with any of the three main organs39. The results of previous studies on the effects of altered gravity on plant growth are not directly comparable to this species. Most of the research conducted focuses on the interactions between microgravity and roots10,11,12,14,21. Wolffia globosa is famously known as a rootless plant39. To further complicate matters, their rootless nature makes comparison with what has been studied so far challenging. Additionally, while microgravity has often been reported as a limiting growth factor for plants40,41,42, results from tests performed under hypergravity are disagreeing. Studies on the nucleolar activity or on the elongation of hypocotyls in both Arabidopsis thaliana and Cucumis sativus, showed a decrease in growth rates43,44. At the same time, other results suggest that hypergravity might not affect growth45. In our experiment, the 2g and 4g hypergravity treatments manifest an increasing (although not statistically significant) trend in growth rates compared to the control treatment (Fig. 6). This possible positive response to hypergravity treatments opens up interesting possibilities for future studies and potential applications in space agriculture but requires new ad hoc tests, eventually applying higher levels of hypergravity to amplify the phenomenon. In such a scenario, hypergravity conditions could be leveraged to enhance plant productivity and biomass accumulation in controlled environments, such as space stations.
Considering that morphology plays a crucial role in plant growth, survival, and productivity46, we investigated the effect of different gravitational treatments on the shape of Wolffia globosa frond. Moreover, results revealed significant differences among the treatments for both the long and short sides. Worth mentioning that such an effect occurred on plants that can be considered genetically identical.
The study findings revealed that the fronds of Wolffia globosa were most extended in the 1 g treatment, both on the front and back sides. Subsequently, the sim-Moon g, sim-µg, 2 g, and 4 g treatments followed in terms of frond length. These results suggest that both simulated microgravity and simulated Moon’s partial gravity have don’t have an impact on frond elongation. Furthermore, when analysing the ratio in relation to the longer side, significant differences were observed among the gravity treatments, indicating distinct frond shapes. The sim-µg treatment resulted in the most elongated frond shape, followed by the sim-Moon g, 1 g, 2 g, and 4 g treatments. These findings provide compelling evidence supporting the significant influence of gravity on the morphological characteristics of Wolffia globosa fronds. The observed variations in frond length and shape among the treatments underscore the role of gravity in shaping plant growth and development. Specifically, the sim-µg treatment consistently induced the most pronounced changes, suggesting its potential as a key factor in determining frond morphology. These results contribute to our understanding of how different gravity environments impact the growth and shape of Wolffia globosa, highlighting the importance of considering gravity conditions in cultivating and manipulating these plants for various space applications. Understanding the underlying mechanisms driving these morphological changes could provide valuable insights into the plant's ability to perceive and respond to gravitational cues.
The adaptability of Wolffia globosa to altered gravity conditions suggested by our data hints at the potential use of plants from the genus Wolffia in space agriculture. These plants, characterised by rapid growth and high nutrient content, are strong candidates for food production in space, where resource constraints are critical7,13. Our findings further appraise the possible use of Wolffia globosa for space exploration and the establishment of sustainable agriculture in extraterrestrial environments. The robust growth and adaptability to altered gravity conditions can be added to the small size, rapid growth rate, and high nutrient content, confirming that this genus deserves consideration for cultivation in limited space and resource-constrained environments7.
Although Wolffia globosa exhibited a reduced growth rate in our experiment under simulated microgravity, previous research has seen growth enactment of Wolffia globosa under simulated microgravity26. As already mentioned by the authors of the up-mentioned work, the enhanced growth might be linked to better utilisation of dissolved oxygen and organic substances in the growth medium due to the type of cultivation system they have employed26. The varying responses observed among different gravity treatments in our experiment could be attributed to the interplay between genetic variability within the Lemnaceae family and the specific environmental conditions experienced by each individual plant. Notably, the genus Wolffia comprises numerous species with significant genetic diversity. Testing the effects of altered gravity on only one clone of Wolffia globosa, as done in our experiment, provides a valuable starting point but may not fully capture the entire range of responses within the genus Wolffia. Further investigations involving multiple Wolffia species and clones are warranted to explore the full extent of the species' reaction to altered gravity and better understand the role of genetic variability in shaping their growth patterns.
The recent sequencing of the genome of Wolffia australiana has provided valuable insights into the genetic characteristics of Wolffia species47. One intriguing finding is the absence of gravity-sensing genes commonly observed in higher plants. More specifically, new findings have shed light on the missing LAZY proteins in Wolffia australiana28. This genetic difference suggests that Wolffia species have evolved alternative mechanisms to perceive and respond to gravity cues, which may explain the distinct growth patterns exhibited under altered gravitational conditions. Furthermore, to some degree, the enhanced growth rate seen under the hypergravity treatment could be attributed to gravity resistance genes such as PTH248. Our results partially confirmed the expected limited reaction to gravity also of Wolffia globosa. The adaptability of Wolffia globosa to different gravitational conditions supported the hypothesis that the loss of gravity-sensing genes reported in the sister species Wolffia australiana might also occur in Wolffia globosa. The slight variations in growth rates and morphological traits observed among the treatments open up interesting avenues for future research and potential applications in space agriculture.
In addition to its implications for space agriculture, this research also contributes to our understanding of fundamental plant biology. The findings of our experiment might encourage further investigations to clarify the evolutionary pathways that shaped the peculiar adaptation to gravity associated to a return to an ancestral aquatic environment of Wolffia genus.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Landolt, E. The family of Lemnaceae—A monographic study. In Biosystematic Investigations in the Family of Duckweeds (Geobotanische Institut, 1986).
Ziegler, P., Adelmann, K., Zimmer, S., Schmidt, C. & Appenroth, K. J. Relative in vitro growth rates of duckweeds (Lemnaceae)—the most rapidly growing higher plants. Plant Biol. 17, 33–41. https://doi.org/10.1111/PLB.12184 (2015).
Paolacci, S., Stejskal, V. & Jansen, M. A. K. Estimation of the potential of Lemna minor for effluent remediation in integrated multi-trophic aquaculture using newly developed synthetic aquaculture wastewater. Aquac. Int. 29, 2101–2118. https://doi.org/10.1007/S10499-021-00736-Z/FIGURES/5 (2021).
Paolacci, S., Stejskal, V., Toner, D. & Jansen, M. A. K. Wastewater valorisation in an integrated multitrophic aquaculture system; assessing nutrient removal and biomass production by duckweed species. Environ. Pollut. 302, 119059. https://doi.org/10.1016/J.ENVPOL.2022.119059 (2022).
Petersen, F. et al. Influence of light intensity and spectrum on duckweed growth and proteins in a small-scale, re-circulating indoor vertical farm. Plants 11, 1010. https://doi.org/10.3390/PLANTS11081010/S1 (2022).
Stejskal, V., Paolacci, S., Toner, D. & Jansen, M. A. K. A novel multitrophic concept for the cultivation of fish and duckweed: A technical note. J. Clean Prod. 366, 132881. https://doi.org/10.1016/J.JCLEPRO.2022.132881 (2022).
Romano, L. E. & Aronne, G. The world smallest plants (Wolffia Sp.) as potential species for bioregenerative life support systems in space. Plants 10, 1896. https://doi.org/10.3390/PLANTS10091896 (1896).
Gòdia, F. et al. MELISSA: A loop of interconnected bioreactors to develop life support in space. J. Biotechnol. 99, 319–330. https://doi.org/10.1016/S0168-1656(02)00222-5 (2002).
Izzo, L. G. et al. Interaction of gravitropism and phototropism in roots of Brassica oleracea. Environ. Exp. Bot. 193, 104700. https://doi.org/10.1016/J.ENVEXPBOT.2021.104700 (2022).
Vandenbrink, J. P. & Kiss, J. Z. Space, the final frontier: A critical review of recent experiments performed in microgravity. Plant Sci. 243, 115–119. https://doi.org/10.1016/J.PLANTSCI.2015.11.004 (2016).
Izzo, L. G. et al. Chemotropic vs hydrotropic stimuli for root growth orientation in microgravity. Front. Plant Sci. https://doi.org/10.3389/FPLS.2019.01547 (2019).
Aronne, G., Romano, L. E. & Izzo, L. G. Subsequent inclusion/exclusion criteria to select the best species for an experiment performed on the ISS in a refurbished hardware. Life Sci. Space Res. 27, 19–26. https://doi.org/10.1016/J.LSSR.2020.07.002 (2020).
Aronne, G., Izzo, L. G., Romano, L. E., de Francesco, S., de Micco, V., de Pascale, S., Carrubba, E., Neri, G., Galoforo, G., Piccirillo, S. et al. Multitrop: The challenge of using refurbished hardware for an educational and scientific experiment on the ISS. In Proceedings of the International Astronautical Congress, IAC (2018).
Iovane, M., Izzo, L. G., Romano, L. E. & Aronne, G. Simulated microgravity affects directional growth of pollen tubes in candidate space crops. Front. Plant Sci. 14, 1186967. https://doi.org/10.3389/FPLS.2023.1186967 (2023).
Herranz, R. et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 13, 1–17. https://doi.org/10.1089/AST.2012.0876 (2013).
Kiss, J. Z., Wolverton, C., Wyatt, S. E., Hasenstein, K. H. & van Loon, J. J. W. A. Comparison of microgravity analogs to spaceflight in studies of plant growth and development. Front. Plant Sci. 10, 1577. https://doi.org/10.3389/FPLS.2019.01577/BIBTEX (2019).
Pardo, S. J. et al. Simulated microgravity using the random positioning machine inhibits differentiation and alters gene expression profiles of 2T3 preosteoblasts. Am. J. Physiol. Cell Physiol. 288, 1211–1221. https://doi.org/10.1152/AJPCELL.00222.2004/ASSET/IMAGES/LARGE/ZH00060524430005.JPEG (2005).
van Loon, J. J. W. A. Centrifuges for microgravity simulation. The reduced gravity paradigm. Front. Astron. Space Sci. 3, 21. https://doi.org/10.3389/FSPAS.2016.00021/BIBTEX (2016).
Aronne, G. et al. A novel device to study altered gravity and light interactions in seedling tropisms. Life Sci. Space Res. 32, 8–16. https://doi.org/10.1016/J.LSSR.2021.09.005 (2022).
Jamšek, M. et al. Effects of simulated microgravity and hypergravity conditions on arm movements in normogravity. Front. Neural Circuits 15, 750176. https://doi.org/10.3389/FNCIR.2021.750176/BIBTEX (2021).
Hosamani, R., Swamy, B. K., Dsouza, A. & Sathasivam, M. Plant responses to hypergravity: A comprehensive review. Planta 257, 1–17. https://doi.org/10.1007/S00425-022-04051-6 (2022).
Swamy, B. K. et al. Novel hypergravity treatment enhances root phenotype and positively influences physio-biochemical parameters in bread wheat (Triticum aestivum L.). Sci. Rep. 11, 1–16. https://doi.org/10.1038/s41598-021-94771-8 (2021).
Allen, J. et al. Gravity control of growth form in Brassica rapa and Arabidopsis thaliana (Brassicaceae): Consequences for secondary metabolism. Am. J. Bot. 96, 652–660. https://doi.org/10.3732/AJB.0800261 (2009).
Hosamani, R., Swamy, B. K., Dsouza, A. & Sathasivam, M. Plant responses to hypergravity: A comprehensive review. Planta 257, 1–17. https://doi.org/10.1007/S00425-022-04051-6/TABLES/1 (2023).
Waldron, K. W. & Brett, C. T. Effects of extreme acceleration on the germination, growth and cell wall composition of pea epicotyls. J. Exp. Bot. 41, 71–77. https://doi.org/10.1093/JXB/41.1.71 (1990).
Yuan, J. & Xu, K. Effects of simulated microgravity on the performance of the duckweeds Lemna aequinoctialis and Wolffia globosa. Aquat. Bot. 137, 65–71. https://doi.org/10.1016/J.AQUABOT.2016.11.010 (2017).
Manzano, A. et al. Novel, moon and mars, partial gravity simulation paradigms and their effects on the balance between cell growth and cell proliferation during early plant development. npj Microgravity 4, 1–11. https://doi.org/10.1038/s41526-018-0041-4 (2018).
Michael, T. P. et al. Genome and time-of-day transcriptome of Wolffia australiana link morphological minimization with gene loss and less growth control. Genome Res. 31, 225–238. https://doi.org/10.1101/GR.266429.120 (2021).
Park, H. et al. Characterization of the complete chloroplast genome sequence of Wolffia globosa (Lemnoideae) and its phylogenetic relationships to other Araceae family. Mitogenome Announc. 5, 1905–1907. https://doi.org/10.1080/23802359.2020.1754948 (2020).
Appenroth, K. J., Teller, S. & Horn, M. Photophysiology of turion formation and germination in Spirodela polyrhiza. Biol. Plant. 38, 95–106. https://doi.org/10.1007/BF02879642/METRICS (1996).
van Loon, J. J. W. A. Some History and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 39, 1161–1165. https://doi.org/10.1016/J.ASR.2007.02.016 (2007).
van Loon, J. J. W. A. et al. The Large diameter centrifuge, LDC, for Life and physical sciences and technology. Life Space Life Earth 553, 92 (2008).
Romano, L. E., Iovane, M., Izzo, L. G. & Aronne, G. A machine-learning method to assess growth patterns in plants of the family Lemnaceae. Plants 11, 1910. https://doi.org/10.3390/PLANTS11151910/S1 (2022).
Naumann, B., Eberius, M. & Appenroth, K. Growth rate based dose-response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. Clone St. J. Plant Physiol. 164, 1656–1664. https://doi.org/10.1016/J.JPLPH.2006.10.011 (2007).
Cowles, J. R., Scheld, H. W., Lemay, R. & Peterson, C. Growth and lignification in seedlings exposed to eight days of microgravity. Ann. Bot. 54, 33–48. https://doi.org/10.1093/oxfordjournals.aob.a086865 (1984).
Sree, K. S., Sudakaran, S. & Appenroth, K. J. How fast can angiosperms grow? Species and clonal diversity of growth rates in the genus Wolffia (Lemnaceae). Acta Physiol. Plant https://doi.org/10.1007/S11738-015-1951-3 (2015).
Rivera, C. M. et al. Influence of simulated microgravity on growth, yield, and quality of leafy vegetables: Lettuce and rocket. Eur. J. Hortic. Sci. 71, 45 (2006).
Sathasivam, M., Hosamani, R., Swamy, B. K. & Kumaran, G. S. Plant responses to real and simulated microgravity. Life Sci. Space Res. 28, 74–86. https://doi.org/10.1016/J.LSSR.2020.10.001 (2021).
Yang, J., Zhao, X., Li, G., Hu, S. & Hou, H. Frond architecture of the rootless duckweed Wolffia globosa. MC Plant Biol. https://doi.org/10.1186/s12870-021-03165-5 (2021).
Kiss, J. Z. Mechanisms of the early phases of plant gravitropism. CRC Crit. Rev. Plant Sci. 19, 551–573. https://doi.org/10.1080/07352680091139295 (2010).
Medina, F. J., Manzano, A., Villacampa, A., Ciska, M. & Herranz, R. Understanding reduced gravity effects on early plant development before attempting life-support farming in the moon and mars. Front. Astron. Space Sci. 8, 729154. https://doi.org/10.3389/FSPAS.2021.729154/BIBTEX (2021).
De Micco, V., Aronne, G., Joseleau, J. P. & Ruel, K. Xylem development and cell wall changes of soybean seedlings grown in space. Ann. Bot. 101, 661. https://doi.org/10.1093/AOB/MCN001 (2008).
Manzano, A. I., Herranz, R., Manzano, A., van Loon, J. J. W. A. & Medina, F. J. Early effects of altered gravity environments on plant cell growth and cell proliferation: Characterization of morphofunctional nucleolar types in an Arabidopsis cell culture system. Front. Astron. Space Sci. https://doi.org/10.3389/FSPAS.2016.00002 (2016).
Kasahara, H., Shiwa, M., Takeuchi, Y. & Yamada, M. Effects of hypergravity on the elongation growth in radish and cucumber hypocotyls. J. Plant Res. 108, 59–64. https://doi.org/10.1007/BF02344306 (1995).
Takakura, T., Goto, E. & Tanaka, M. The effect of gravity on plant germination. Adv. Space Res. 18, 255–258. https://doi.org/10.1016/0273-1177(95)00886-J (1996).
Shepard, K. A. & Purugganan, M. D. The genetics of plant morphological evolution. Curr. Opin. Plant Biol. 5, 49–55. https://doi.org/10.1016/S1369-5266(01)00227-8 (2002).
Park, H. et al. Genome of the world’s smallest flowering plant, Wolffia australiana, helps explain its specialized physiology and unique morphology. Commun. Biol. 4, 1–12. https://doi.org/10.1038/s42003-021-02422-5 (2021).
Hattori, T., Soga, K., Wakabayashi, K. & Hoson, T. An Arabidopsis PTH2 gene is responsible for gravity resistance supporting plant growth under different gravity conditions. Life https://doi.org/10.3390/LIFE12101603 (2022).
Acknowledgements
All authors would like to express immense gratitude to Leonardo Surdo and Sebastien Vincent-Bonnieu for their invaluable support provided during the Superfood for Space research project. Additionally, special thanks are extended to Alan Dowson for his support within the ESA-LIS laboratory.
Funding
This experiment has been conducted in the framework of the Superfood for Space research project financed by the European space agency (ESA Contract No. 4000133778/21/NL/CBi). This work was partially possible by Grant #4000136280/21/NL/KML/rk from ESA to J.J.W.A. van Loon.
Author information
Authors and Affiliations
Contributions
L.E.R. and G.A. conceived the experiment L.E.R. and J.J.W.A. planned and executed the experiment, LER wrote the manuscript. M.I., L.G.I., G.A., J.J.W.A. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Romano, L.E., van Loon, J.J.W.A., Izzo, L.G. et al. Effects of altered gravity on growth and morphology in Wolffia globosa implications for bioregenerative life support systems and space-based agriculture. Sci Rep 14, 410 (2024). https://doi.org/10.1038/s41598-023-49680-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49680-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.