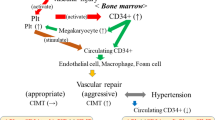
Abstract
Endothelial progenitor cells (EPCs), expressing markers for stemness (CD34), immaturity (CD133) and endothelial maturity (KDR), may determine the extent of post-stroke vascular repair. Given the prevalence of stroke in elderly, this study explored whether variations in plasmatic availability of certain EPC subtypes could predict the severity and outcome of disease in older patients. Blood samples were collected from eighty-one consented patients (≥ 65 years) at admission and days 7, 30 and 90 post-stroke. EPCs were counted with flow cytometry. Stroke severity and outcome were assessed using the National Institutes of Health Stroke Scale, Barthel Index and modified Rankin Scale. The levels of key elements known to affect EPC characteristics were measured by ELISA. Diminished total antioxidant capacity and CD34 + KDR + and CD133 + KDR + counts in early phases of stroke were associated with disease severity and worse functional outcome at day 90 post-stroke. Baseline levels of angiogenic agent PDGF-BB, but not VEGF, positively correlated with CD34 + KDR + numbers at day 90. Baseline LDL-cholesterol levels were inversely correlated with CD34 + KDR+, CD133 + KDR + and CD34 + CD133 + KDR + numbers at day 90. Close correlation between baseline CD34 + KDR + and CD133 + KDR + counts and the outcome of stroke proposes these particular EPC subtypes as potential prognostic markers for ischaemic stroke.
Similar content being viewed by others
Introduction
Ischaemic stroke (IS) develops through an interference with blood supply to the brain and continues to be one of the leading causes of mortality and morbidity in the world1. The prevalence of stroke doubles every 10 years after the age of 55 and about two-third of all stroke patients are older than 65 years1,2. Although various mechanisms can initiate and exacerbate the development of atherosclerotic disease throughout chronological ageing, endothelial dysfunction (ED), accompanied by impaired vascular relaxation and inflammation, is widely considered as a key pathology behind the structural and functional alterations in the vascular system3.
The endothelium is important in preserving vascular homeostasis4,5. Endothelial progenitor cells (EPCs), released from bone marrow, play an instrumental role in maintaining appropriate endothelial function by re-endothelialisation of cerebral blood vessels after IS6,7. Similar to embryonic angioblasts, EPCs are equipped with an inherent capacity to proliferate, migrate and differentiate. Hence, non-haematopoietic cells (CD45-) expressing a variety of markers for stemness (CD34+), immaturity (CD133+) and endothelial cell maturity (KDR+) are regarded as distinct EPC subtypes in circulation7,8. Circulating EPCs can also negate the deleterious effects of cerebral ischaemia by inducing angiogenesis and vasculogenesis through secretion of various trophic factors9. However, the mobilisation, survival and the reparative capacity of EPCs are negatively affected by chronological ageing in that age-related changes in the availability of circulatory cytokines, chemokines and growth factors, appear to play an instrumental role10. Besides, advancing age renders EPCs susceptible to internal changes and environmental factors such as oxidative stress which, markedly influences the number of circulating EPCs and contributes to age-mediated gradual loss of endothelium in cerebrovasculature and to the pathogenesis of IS11.
Oxidative stress represents a common pathology during the acute phase of an IS and is characterised by the excessive availability of reactive oxygen species (ROS)12. Both the enhanced production and reduced metabolism of ROS may account for this phenomenon. Exposure to excessive levels of ROS irreversibly damages macromolecules, including DNA and proteins, exacerbates ED and may adversely affect EPC characteristics13.
In light of the above, we hypothesised that variations in numbers of different EPC subtypes might correlate with the severity and outcome of IS and therefore serve as prognostic markers for stroke. In addition, the study also investigated the correlation between the plasmatic profile of inflammatory cytokines, growth factors and angiogenic factors known to affect EPC characteristics and disease severity and outcome.
Materials and methods
Study participants
Data from eighty-one older (≥ 65 years old) IS patients recruited for The Dunhill Medical Trust EPC study (DMT EPC study, NCT02980354) between February 2017 and November 2019 were used for this substudy. IS was defined as a sudden focal neurological deficit persisting longer than 24 h with no evidence of cerebral haemorrhage on imaging. The DMT EPC study was a single-centre, prospective, observational, case-controlled study, performed in accordance with the ethical standards for human experimentation established by the Declaration of Helsinki. Details of the study have already been reported elsewhere14. The study protocol was reviewed and approved by West Midlands-Coventry & Warwickshire Research Ethics Committee (REC number: 16/WM/0304). Written informed consent was obtained from all participants for their anonymised information to be published in this study.
National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS) and Barthel Index (BI) were used to measure patients’ neurological and functional status on admission (baseline, BL) and days 7, 30 and 90 post-stroke. As all patients received guideline-based medical therapy and underwent an appropriate rehabilitation programme, patients’ EPC counts and neurological outcomes were unlikely to be differently affected by these treatments.
Blood sampling and flow cytometry
Blood samples (~ 30 mL) were collected from participants at the abovementioned time points to cover acute (within the first 48 h of stroke, baseline, BL), subacute (day 7, D7) and chronic (days 30 and 90, D30 and D90, respectively) phases of stroke. Six mL of blood samples were used to count circulating EPC subtypes by flow cytometry where non-haematopoietic cells (CD45-) co-expressing two or more cell surface markers for stemness (CD34 +), immaturity (CD133 +) and endothelial maturity (KDR +), i.e., CD45-CD34 + CD133 +, CD45-CD34 + KDR +, CD45-CD133 + KDR + and CD45-CD133 + CD34 + KDR +, were counted. To determine the percentage of each EPC subpopulation 1 million total events were acquired. The researchers who carried out the experiments remained blinded to subject characteristics throughout the study to avoid bias.
Biochemical profile assessment
The biochemical profile of patient plasma samples was scrutinised for major angiogenic promoters and inhibitors, namely VEGF (DVE00), PDGF-BB (DBB00), thrombospondin-1 (DTSP10), thrombospondin-2 (DTSP20), endostatin (Abcam, ab100508) and angiostatin (Abcam, ab99973) and inflammatory cytokines and chemokines, namely TNF-α (DTA00D), SDF-1 (DSA00), G-CSF (DCS50), using specific ELISAs. Changes in total antioxidant capacity (TAC), determined by the sum of endogenous and food-derived antioxidants, were also measured in plasma using a commercial kit, TAC assay kit (Abcam, ab65329). Unless otherwise stated, all kits mentioned were from R&D systems.
Statistical analysis
The statistical analyses were performed using SPSS package version 26 (SPSS Inc., USA) and GraphPad Prism 8.0 statistical software package (GraphPad Software Inc). Spearman correlation analysis was performed to capture the association between all the variables. Spearman’s correlation coefficients (r) were utilised to summarise the relationship between all the variables. p < 0.05 was considered as significant.
Ethics approval
The Dunhill Medical Trust EPC study protocol was reviewed and approved by West Midlands—Coventry & Warwickshire Research Ethics Committee (16/WM/0304).
Results
Study population
Blood samples were collected from a total of 81 subjects with lacunar (n = 38) or cortical (n = 43) stroke on the basis of clinical syndrome and neuroimaging of whom 69% were male (n = 56). The median age at the diagnosis was 76 years. More than half of the patients had a history of smoking (n = 48), consumed alcohol (n = 51) and were hypertensive (n = 49). Over a quarter of all patients had atrial fibrillation (AF, n = 23) and were hyperlipidaemic (n = 24). Over 15% of all patients also had a previous history of transient ischaemic attack (TIA, n = 15) and IS (n = 12) and were diabetic (n = 17). Hence, over 40% of patients were on statins (n = 38), ACE inhibitors (n = 34) and anti-platelets (n = 33). The use of calcium channel blockers (n = 17), beta blockers (n = 15) and glucose-lowering (n = 13) agents amongst patients was frequent (Table 1).
Association of NIHSS, mRS and BI scores at D90 to the numbers of circulating EPC subtypes pinpointed substantial correlations between decreased BL numbers of CD34 + KDR + and stroke severity on D90 (NIHSS: r: − 0.281; p: 0.043; mRS: − 0.343; p: 0.01) (Fig. 1). In line with these findings, lower BL number of CD133 + KDR + cells were also associated with higher mRS on D90 (r: − 0.2664; p: 0.0493), suggesting a worse stroke outcome. Despite observation of a similar trend between BL CD34 + CD133 + KDR + numbers and D90 mRS score, the correlation remained insignificant (r: − 0.2563; p = 0.0589).
Correlation between baseline CD34 + CD133 + (A), CD34 + KDR + (B), CD133 + KDR + (C) and CD34 + CD133 + KDR + (D) counts and post-stroke severity and outcome on day 90. NIHSS National Institutes of Health Stroke Scale, BI Barthel index, mRS modified Rankin Score, CD cluster differentiation, r correlation coefficients, p p value.
Similar to BL values, lower number of CD34 + KDR + cells on D7 were also associated with higher NIHSS (r: − 0.356; p: 0.015) and mRS (r: − 0.286; p: 0.049) at D90. In contrast, only reductions in D30 CD133 + KDR + (r: − 0.397; p: 0.006) and CD34 + CD133 + KDR + (r: − 0.399; p: 0.005) cell numbers were correlated with stroke severity on D90 (Supplementary Table 1). The variations in numbers of specific EPC subtypes per mL blood sample are documented in Supplementary Table 2.
Analyses of correlation between biochemical parameters and stroke severity and outcome
Assessment of the plasmatic biochemical profile of IS patients showed no correlation between the BL levels of angiogenic modulators VEGF, PDGF-BB, thrombospondin-1/2, angiostatin and endostatin and the severity and outcome of stroke at D90 (Fig. 2). Similarly, the BL levels of TAC and plasmatic SDF-1 and G-CSF had no impact on any of the neurological and functional parameters. However, a negative correlation was detected by BL TNF-α levels and D90 NIHSS (r: − 0.343; p: 0.014).
Correlation between baseline levels of angiogenic factors, total anti-oxidant capacity, chemokines and cytokines and post-stroke severity and outcome on day 90. The size of bubbles indicates the magnitude of correlation coefficient. While red and blue bubbles successively indicate significant (p < 0.05) and insignificant (p > 0.05) correlations, the symbols + and − indicate positive and negative correlations, respectively. NIHSS National Institutes of Health Stroke Scale, BI Barthel index, mRS modified Rankin Score, CD cluster differentiation, G-CSF granulocyte colony-stimulating factor, PDGF-BB platelet-derived growth factor, SDF-1 stromal cell-derived factor-1, TAC total anti-oxidant capacity, TNF-α tumour necrosis factor-α, THR-1 thrombospondin-1, THR-2 thrombospondin-2, VEGF vascular endothelial growth factor.
Scrutiny of the correlation between circulating EPC subtypes and the levels of key elements capable of affecting their counts showed that only BL level of angiogenic factor PDGF-BB (r: 0.334; p: 0.043) increased CD34 + KDR + numbers on D90 after stroke. In contrast, no correlation between any EPC subtype and BL levels of VEGF, thrombospondin-1/2 and endostatin were observed (Fig. 3). Again, no correlation between BL levels TAC, G-CSF, SDF-1 and TNF-α and EPC counts were observed D90 post-stroke.
Correlation between baseline levels of angiogenic factors, total anti-oxidant capacity, chemokines and cytokines and the number of circulating EPC subtypes on day 90. The size of bubbles indicated the magnitude of correlation coefficient. While red and blue bubbles successively indicate significant (p < 0.05) and insignificant (p > 0.05) correlations, the symbols + and − indicate positive and negative correlations, respectively. CD cluster differentiation, G-CSF granulocyte colony-stimulating factor, PDGF-BB platelet-derived growth factor, SDF-1 stromal cell-derived factor-1, TAC total anti-oxidant capacity, TNF-α tumour necrosis factor-α, THR-1 thrombospondin-1, THR-2 thrombospondin-2, VEGF vascular endothelial growth factor.
Analysis of correlations between D7 and D30 levels of the same biochemical elements and the severity and outcome of stroke on D90 revealed negative correlations between D7 TAC and D90 NIHSS (r: − 0.318; p: 0.028) and between D30 thrombospondin-2 and D90 mRS (r: − 0.336; p: 0.045) scores. Interestingly, D30 thrombospondin-1 levels appeared to correlate positively with the extent of disability on D90 (r: 0.459; p: 0.007) as attested by the BI score (Supplementary Table 3). The actual level of biomedical parameters at BL, D7 and D30 and the neurological and functional scores on D90 are documented in the Supplementary Tables 4 and 5, respectively.
Attempts to identify prognostic marker(s) for IS
Assessment of biological variables, that are known to be involved in the pathogenesis as well as severity and outcome of stroke, including body mass index (BMI), systolic blood pressure and blood triglyceride, glucose and LDL-cholesterol levels, and the circulating EPC numbers showed an inverse correlation between BL cholesterol levels and D90 CD34 + KDR + numbers (r: − 0.34 p: 0.016). The level of LDL-cholesterol was inversely correlated with D90 numbers CD34 + KDR + (r: − 0.308; p: 0.035), CD133 + KDR + (r: − 0.301; p: 0.04) and CD34 + CD133 + KDR + (r: − 0.305; p: 0.037) (Fig. 4).
Correlation between baseline levels of biological variables, including body mass index (BMI), blood pressure (BP) and blood triglyceride, glucose and LDL- and HDL-cholesterol and the number of circulating EPC subtypes on day 90. The size of bubbles indicated the magnitude of correlation coefficient. While red and blue bubbles successively indicate significant (p < 0.05) and insignificant (p > 0.05) correlations, the symbols + and − indicate positive and negative correlations, respectively.
Scrutiny of the correlations between BL variables and biochemical parameters showed LDL-cholesterol were inversely correlated with D90 endostatin (r: − 0.36; p: 0.01) (Fig. 5). In contrast, BL glucose and LDL-C levels positively correlated with the level of VEGF (r: 0.412; p: 0.011) and TAC (r: 0.31; p: 0.036), respectively. However, LDL-C and HDL-C negatively correlated with TNF-α (r: − 0.378; p: 0.039; r: − 0.441; p: 0.012, respectively) levels on D90. No correlation was observed between any BL variable and the remaining cytokines, chemokines and angiogenesis regulators.
Correlation between baseline levels of biological variables, including body mass index (BMI), blood pressure (BP) and blood triglyceride, glucose and LDL- and HDL-cholesterol and the levels of angiogenic factors, total anti-oxidant capacity, cytokines and chemokines on day 90. The size of bubbles indicated the magnitude of correlation coefficient. While red and blue bubbles successively indicate significant (p < 0.05) and insignificant (p > 0.05) correlations, the symbols + and − indicate positive and negative correlations, respectively. G-CSF granulocyte colony-stimulating factor, PDGF-BB platelet-derived growth factor, SDF-1 stromal cell-derived factor-1, TAC total anti-oxidant capacity, TNF-α tumour necrosis factor-α, THR-1 thrombospondin-1, THR-2 thrombospondin-2, VEGF vascular endothelial growth factor.
Discussion
Alterations in endothelial integrity and function play a critical role in the pathogenesis of stroke and develop in otherwise healthy older individuals as part of physiological ageing process15,16. Besides physical damage and endothelial cell senescence, diminished availability of EPCs may also contribute to age- and stroke-mediated ED and associated vascular and functional abnormalities. Substantial decreases reported in the number of circulating EPCs expressing markers specifically for stemness and immaturity in healthy individuals over 65 years of age versus those between 18 and 64 years confirm the influence of ageing on distinct EPC subtypes10. In light of these and further findings implying the value of circulating EPCs for endothelial repair6,11,17 and as potential prognostic biomarkers18, the present study hypothesised that changes in circulating numbers of certain EPC subtypes, defined as cells co-expressing a variety of markers for stemness (CD34 +), immaturity (CD133 +) and endothelial cell maturity (KDR +), may explain the differences reported in the severity and/or outcome of ischaemic stroke in older patients. Observation of a significant correlation in the current study between higher CD34 + KDR + and CD133 + KDR + numbers during the acute phase of stroke and better neurological and functional outcome on D90 post-stroke corroborate this hypothesis and attribute a key role to these particular EPC subtypes in neurovascular repair19,20. Moreover, these findings also indicate the capacity of these particular cells to serve as prognostic markers for IS patients21. Since one-fourth of all mild ischaemic stroke patients manifest functional changes between days 30 and 90 after stroke, it was important to assess the level of recovery and correlate the potential changes in various biochemical compounds to recovery on D90 in this study. Indeed, neurological and functional assessments performed on D7 and D30 appear to insufficiently represent long-term recovery in mild stroke22.
The capacity of CD34 + KDR + cells to fully differentiate into mature endothelial cells has long been recognised23. Considering that the vasculoprotective effects of CD34 + cells may be explained by the paracrine effect rather than the ability of these cells to differentiate into fully functional endothelial cells, CD34 + cells continue to face scrutiny as markers of EPCs24. In contrast, mature endothelial cells do not express a great deal of CD133 antigens. The cells that co-express CD133 + and KDR + represent a phenotypically and functionally distinct subset of circulating endothelial cells and are widely regarded as true EPCs25. Observation of intracellular CD133 expression in EPCs and the diminished post-ischemic revascularisation in a nude mouse model with hind-limb ischemia following the administration of OECs transfected with specific siRNA (siCD133-EPCs) help reconcile the discrepancies regarding CD133 positivity and ontogeny in endothelial progenitors26.
Major growth factors such as VEGF27 and PDGF-BB28 and anti-angiogenic elements, including thrombospondin-1/229, endostatin30 and angiostatin31 that regulate post-ischaemic vasculogenesis and angiogenesis may also determine the extent of neurological recovery. Unlike a recent study correlating BL plasma endostatin levels with increased risk of mortality and severe disability at 3 months, no correlation was observed between the BL plasma levels of these elements and NIHSS, mRS and BI scores at D90 in this study32. Although genetic variations between the two study populations, European vs Chinese, may somewhat account for the differences noted, the dichotomy in data cast doubt on the capacity of these agents to serve as highly specific and reliable prognostic markers for acute IS.
Environmental changes evoked by advanced age and ischaemic injury may suppress the production of EPCs and therefore exacerbate patients’ dependence and disability10,11. Oxidative stress represents one such change and evokes cerebrovascular dysfunction by not only disrupting major cellular components but also neutralising nitric oxide, a potent anti-atherogenic agent33,34. Albeit insignificant, the presence of a correlation between higher levels of plasma TAC and better post-stroke neurological outcome substantiates the disruptive effects of ROS on neurovasculature and pinpoints the importance of TAC in maintaining neurovascular equilibrium. Normalisation of vascular tone in dysfunctional arterial segments by ROS scavengers and antioxidant vitamins further confirm the crucial role of oxidative stress in ED35,36. Under normal conditions, EPCs are better protected against oxidative injury due to possession of high TAC37,38, but prolonged exposure to oxidative stress adversely affect their ability to proliferate, differentiate, self-renew and secrete agents with vasculoprotective capacity such as SDF-1 and VEGF9,12,20,39.
In addition to production, the function of EPCs may also be adversely affected by the ageing process in that suppression of systemic elements known to affect neovasculogenesis may play a crucial role17,40. Indeed, observation of a positive correlation between acute plasma level of PDGF-BB and CD133 + KDR +, CD34 + KDR + numbers supports this notion. The steady increases in plasma levels of PDGF-BB are likely to trigger proliferation and directed migration of EPCs to the site of injury during chronic phases of IS to counter ischaemic damage. The absence of a correlation between early SDF-1 levels and EPC counts during chronic phases of IS may be instrumental, in this context, to keep vascular growth in check41,42. Although SDF-1 strongly mobilizes the release of EPCs from the bone marrow43, the seemingly unaltered levels of SDF-1 after IS and age-mediated potential decreases in SDF-1 receptor, CXCR4 levels may explain the abovementioned lack of correlation18. Overall, the timing and the degree of variations observed for the parameters studied here rule out their use as reliable diagnostic markers in clinical settings.
Considering that all study participants enrolled to the study were ≥ 65 years of age, the presence of additional vascular risk factors alongside the use of certain medicines e.g. anti-platelets and anti-hypertensives may somewhat account for the differences observed in EPC counts and patients’ outcome44,45. Co-analyses of EPC counts with a large number of BL demographic and medical variables showed negative correlations between BL LDL-C and most EPC subtypes and specifically between BL cholesterol and CD34 + KDR + cells, confirming the point that statins significantly enhance circulating EPC numbers and pinpointing the importance of a therapy targeting cholesterol levels in stroke patients46. However, a recent study revealing decreases in plasma VEGF levels after statin therapy necessitate a cautious approach as regards the use of statins while signifying the importance of the type of statin used and the length of treatment received47. In contrast, a positive correlation between BL glucose and D90 endostatin levels indicate a prerequisite for the close monitoring of the blood glucose levels in IS patients. Positive correlations between glucose levels and endostatin levels have been established in other diseases such as diabetes and coronary artery disease48.
This study investigated how differences in the plasmatic availability of certain EPC subtypes during acute phase of IS might correlate with severity of disease, patients’ functional outcome and the level of agents known to affect their bioavailability. Since the numbers of patients recruited for the DMT EPC study (81 patients) and retained until D90 (62 patients) were below the originally planned number of recruits (100 patients), it is possible that the reduced sample size could have resulted in lower statistical power and might have somewhat biased the results. A bigger sample size would have been useful to better compare the differences in a large number of parameters studied. Besides, given that the majority of patients enrolled for the study had significant recovery on D90 after stroke, it is important to further investigate the correlations established in this study in patients who continue to show serious morbidity on D90.
In conclusion, circulating baseline levels of CD34 + KDR + and to a lesser degree CD133 + KDR + closely associate with the outcome of IS and may be considered as prognostic markers for IS. A future study enrolling larger numbers of patients with different degrees of morbidity is required to dis/prove this point.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Feigin, V. L. et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20(10), 795–820. https://doi.org/10.1016/S1474-4422(21)00252-0 (2021).
King, D. et al. The future incidence, prevalence and costs of stroke in the UK. Age Ageing 49(2), 277–282. https://doi.org/10.1093/ageing/afz163 (2020).
Kadir, R. R. A. et al. Outgrowth endothelial cell conditioned medium negates TNF-α-evoked cerebral barrier damage: A reverse translational research to explore mechanisms. Stem Cell Rev. Rep. https://doi.org/10.1007/s12015-022-10439-4 (2022).
Ya, J., Kadir, R. R. A. & Bayraktutan, U. Delay of endothelial cell senescence protects cerebral barrier against age-related dysfunction: Role of senolytics and senomorphics. Tissue Barriers 11, 2103353. https://doi.org/10.1080/21688370.2022.2103353 (2022).
Guo, S. et al. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Acad. Sci. USA 105(21), 7582–7587. https://doi.org/10.1073/pnas.0801105105 (2008).
Kadir, R. R. A., Alwjwaj, M. & Bayraktutan, U. Treatment with outgrowth endothelial cells protects cerebral barrier against ischemic injury. Cytotherapy https://doi.org/10.1016/j.jcyt.2021.11.005 (2022).
Abdulkadir, R. R. et al. Outgrowth endothelial cells form a functional cerebral barrier and restore its integrity after damage. Neural Regen. Res. 15(6), 1071–1078. https://doi.org/10.4103/1673-5374.269029 (2020).
Chambers, S. E. J. et al. Current concepts on endothelial stem cells definition, location, and markers. Stem Cells Transl. Med. 10(S2), S54–S61. https://doi.org/10.1002/sctm.21-0022 (2021).
Alwjwaj, M., Kadir, R. R. A. & Bayraktutan, U. The secretome of endothelial progenitor cells: A potential therapeutic strategy for ischemic stroke. Neural Regen. Res. 16(8), 1483–1489. https://doi.org/10.4103/1673-5374.303012 (2021).
Kadir, R. R. A. et al. Inhibition of oxidative stress delays senescence and augments functional capacity of endothelial progenitor cells. Brain Res. 1787, 147925. https://doi.org/10.1016/j.brainres.2022.147925 (2022).
Kukumberg, M. et al. Characterization and functional assessment of endothelial progenitor cells in ischemic stroke patients. Stem Cell Rev. Rep. https://doi.org/10.1007/s12015-020-10064-z (2020).
Höhn, A. et al. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 11, 482–501. https://doi.org/10.1016/j.redox.2016.12.001 (2017).
Satoh, M. et al. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis 198(2), 347–353. https://doi.org/10.1016/j.atherosclerosis.2007.09.040 (2008).
Rakkar, K. et al. Endothelial progenitor cells, potential biomarkers for diagnosis and prognosis of ischemic stroke: Protocol for an observational case-control study. Neural. Regen. Res. 15(7), 1300–1307. https://doi.org/10.4103/1673-5374.269028 (2020).
Martinez-Majander, N. et al. Endothelial dysfunction is associated with early-onset cryptogenic ischemic stroke in men and with increasing age. J. Am. Heart Assoc. 10(14), e020838. https://doi.org/10.1161/JAHA.121.020838 (2021).
Kadir, R. R. A., Alwjwaj, M. & Bayraktutan, U. MicroRNA: An emerging predictive, diagnostic, prognostic and therapeutic strategy in ischaemic stroke. Cell. Mol. Neurobiol. https://doi.org/10.1007/s10571-020-01028-5 (2020).
Chang, E. I. et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1α stabilization during ischemia. Circulation 116(24), 2818–2829. https://doi.org/10.1161/CIRCULATIONAHA.107.715847 (2007).
Rakkar, K. et al. Evaluation of endothelial progenitor cell characteristics as clinical biomarkers for elderly patients with ischaemic stroke. Stem Cell Rev. Rep. https://doi.org/10.1007/s12015-023-10544-y (2023).
Chu, K. et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 39(5), 1441–1447. https://doi.org/10.1161/STROKEAHA.107.499236 (2008).
Bayraktutan, U. Endothelial progenitor cells: Potential novel therapeutics for ischaemic stroke. Pharmacol. Res. 144, 181–191. https://doi.org/10.1016/j.phrs.2019.04.017 (2019).
Camps-Renom, P. et al. Endothelial progenitor cells count after acute ischemic stroke predicts functional outcome in patients with carotid atherosclerosis. J. Stroke Cerebrovasc. Dis. 30(12), 106144. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106144 (2021).
Gardener, H. et al. Functional status at 30 and 90 days after mild ischaemic stroke. Stroke Vasc. Neurol. 7(5), 375–380. https://doi.org/10.1136/svn-2021-001333 (2022).
Pelosi, E. et al. Identification of the hemangioblast in postnatal life. Blood 100(9), 3203–3208. https://doi.org/10.1182/blood-2002-05-1511 (2002).
Popa, E. R. et al. Circulating CD34+ progenitor cells modulate host angiogenesis and inflammation in vivo. J. Mol. Cell Cardiol. 41(1), 86–96. https://doi.org/10.1016/j.yjmcc.2006.04.021 (2006).
Peichev, M. et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95(3), 952–958 (2000).
Rossi, E. et al. Human endothelial colony forming cells express intracellular CD133 that modulates their vasculogenic properties. Stem Cell Rev. Rep. 15(4), 590–600. https://doi.org/10.1007/s12015-019-09881-8 (2019).
Abumiya, T. et al. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J. Cereb. Blood Flow Metab. 19(9), 1038–1050. https://doi.org/10.1097/00004647-199909000-00012 (1999).
Di Santo, S. et al. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE 4(5), e5643. https://doi.org/10.1371/journal.pone.0005643 (2009).
Lin, T. N. et al. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke 34(1), 177–186. https://doi.org/10.1161/01.str.0000047100.84604.ba (2003).
Skovseth, D. K. et al. Endostatin dramatically inhibits endothelial cell migration, vascular morphogenesis, and perivascular cell recruitment in vivo. Blood 105(3), 1044–1051. https://doi.org/10.1182/blood-2004-03-1164 (2005).
Ji, W. R. et al. Characterization of kringle domains of angiostatin as antagonists of endothelial cell migration, an important process in angiogenesis. Faseb J. 12(15), 1731–1738. https://doi.org/10.1096/fasebj.12.15.1731 (1998).
Zhang, C. et al. Endostatin as a novel prognostic biomarker in acute ischemic stroke. Atherosclerosis 293, 42–48. https://doi.org/10.1016/j.atherosclerosis.2019.11.032 (2020).
Abdullah, Z. & Bayraktutan, U. NADPH oxidase mediates TNF-α-evoked in vitro brain barrier dysfunction: Roles of apoptosis and time. Mol. Cell Neurosci. 61, 72–84. https://doi.org/10.1016/j.mcn.2014.06.002 (2014).
Allen, C. L. & Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 4(6), 461–470. https://doi.org/10.1111/j.1747-4949.2009.00387.x (2009).
Ülker, S., McKeown, P. P. & Bayraktutan, U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension 41(3), 534–539. https://doi.org/10.1161/01.HYP.0000057421.28533.37 (2003).
Ulker, S. et al. Antioxidant vitamins C and E ameliorate hyperglycaemia-induced oxidative stress in coronary endothelial cells. Diabetes Obes. Metab. 6(6), 442–451. https://doi.org/10.1111/j.1462-8902.2004.00443.x (2004).
He, T., Joyner, M. J. & Katusic, Z. S. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc. Res. 78(3), 447–452. https://doi.org/10.1016/j.mvr.2009.08.009 (2009).
Liu, Y. et al. Proteomic analysis of endothelial progenitor cells exposed to oxidative stress. Int. J. Mol. Med. 32(3), 607–614. https://doi.org/10.3892/ijmm.2013.1419 (2013).
Wang, C. et al. MeCP2 mediated dysfunction in senescent EPCs. Oncotarget 8(45), 78289–78299 (2017).
Xia, W. H. et al. Age-related decline in reendothelialization capacity of human endothelial progenitor cells is restored by shear stress. Hypertension 59(6), 1225–1231. https://doi.org/10.1161/HYPERTENSIONAHA.111.179820 (2012).
Capillo, M. et al. Continuous infusion of endostatin inhibits differentiation, mobilization, and clonogenic potential of endothelial cell progenitors. Clin. Cancer Res. 9(1), 377 (2003).
Meijles, D. N. et al. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci. Signal. 10(501), 1784. https://doi.org/10.1126/scisignal.aaj1784 (2017).
Cun, Y. et al. Role of the stromal cell derived factor-1 in the biological functions of endothelial progenitor cells and its underlying mechanisms. Exp. Ther. Med. 21(1), 39. https://doi.org/10.3892/etm.2020.9471 (2021).
Churdchomjan, W. et al. Comparison of endothelial progenitor cell function in type 2 diabetes with good and poor glycemic control. BMC Endocr. Disord. 10(1), 5. https://doi.org/10.1186/1472-6823-10-5 (2010).
Fadini, G. P. et al. Gender differences in endothelial progenitor cells and cardiovascular risk profile. Arterioscler. Thromb. Vasc. Biol. 28(5), 997–1004. https://doi.org/10.1161/ATVBAHA.107.159558 (2008).
Deng, Y. et al. Mobilization of endothelial progenitor cell in patients with acute ischemic stroke. Neurol. Sci. 39(3), 437–443. https://doi.org/10.1007/s10072-017-3143-y (2018).
Sahebkar, A. et al. Does statin therapy reduce plasma VEGF levels in humans? A systematic review and meta-analysis of randomized controlled trials. Metabolism 64(11), 1466–1476. https://doi.org/10.1016/j.metabol.2015.08.002 (2015).
Sodha, N. R. et al. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am. J. Physiol. Heart Circ. Physiol. 296(2), H428-434. https://doi.org/10.1152/ajpheart.00283.2008 (2009).
Acknowledgements
The graphical abstract was created with BioRender.com.
Funding
This work was supported by a grant from The Dunhill Medical Trust (R459/0216).
Author information
Authors and Affiliations
Contributions
R.R.A.K. analysed the data and wrote the manuscript. K.R. and O.O. processed the blood samples. N.S. and P.B. were the DMT grant co-applicants and performed manuscript revision. U.B. was the chief investigator of the DMT study. He designed the study, interpreted the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadir, R.R.A., Rakkar, K., Othman, O.A. et al. Analysis of endothelial progenitor cell subtypes as clinical biomarkers for elderly patients with ischaemic stroke. Sci Rep 13, 21843 (2023). https://doi.org/10.1038/s41598-023-48907-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48907-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.