Abstract
Atlantoaxial joint is a possible affected site during rheumatoid arthritis (RA) and, in this work, we evaluated its occurrence and associated characteristics in a “real-life” cohort. By a medical records review study of RA patients longitudinally followed-up, the occurrence of severe atlantoaxial joint involvement was estimated (incidence proportion and incidence rate per 1000 person-years at risk). Regression analyses were also exploited to evaluate possible associated factors. Based on these findings, a prospective recruitment was performed to build a descriptive cross-sectional study in evaluating a subclinical atlantoaxial joint involvement in patients with the same clinical characteristics. Retrospectively, 717 patients (female 56.6%, age 64.7 ± 12.3 years) were studied. The incidence proportion of severe atlantoaxial joint involvement was 2.1% [1.5–2.5], occurring in 15 out of 717 patients, and identified by both MRI and CT scan. Considering over 3091 person-years, an incidence rate of 5.2 × 1000 [2.9–8.3] person-years was estimated. Regression analyses suggested that male gender, a longer disease duration, ACPA positivity and extra-articular manifestations resulted to be significantly associated with a severe atlantoaxial joint involvement. Given these findings, 30 asymptomatic patients were selected according to these clinical characteristics and underwent MRI of cervical spine. To date, almost 50% of these asymptomatic patients showed a subclinical atlantoaxial joint involvement. The occurrence of the severe atlantoaxial joint involvement in RA patients was estimated in a “real-life” setting. Male gender, ACPA positivity, long disease duration, and extra-articular manifestations could be associated with the severe atlantoaxial joint involvement in RA. MRI could provide a useful clinical tool to early evaluate the atlantoaxial joint involvement in RA, also in asymptomatic patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease associated with a significant morbidity and mortality1,2. Clinically, RA manifests as a chronic, symmetrical articular disease, typically affecting the small joints, typically proximal interphalangeal and metacarpophalangeal joints1. However, any synovial joint may be involved, and the cervical spine may be another possible affected site of the disease1. As early as 1890, Garrod AE reported the first description of 178 patients with cervical spine involvement in a series of 500 patients with RA, reporting a prevalence around 30%3. Subsequently, many works investigated this issue with different and partially conflicting results4,5,6,7,8,9,10,11, highlighting the need of further studies to fully elucidate this topic. In fact, a highly different prevalence of cervical spine involvement is reported in RA patients, ranging between 25 and 88%4,5,6,7,8. During RA, the atlas-axis cervical vertebrae 1 and 2 (C1 and C2) articulation may be typically involved, between the transverse ligaments of the atlas and the posterior side of the odontoid. At this level, hypertrophic synovitis, juxta-articular bone erosions, joint ankylosis, and local osteoporosis may induce a late spinal instability, especially when involving the transverse ligament provoking a ligamentous laxity and a consequent cranial migration of the odontoid4,5,6. From a clinical point of view, in initial stages, patients may be asymptomatic; usually, the neck pain is the presentation of the cervical involvement in RA5,6,9. Subsequently, the compression of the cranial nerves may lead to other clinical features, such as occipital headache, migraines, and neck, mastoid, ear, or facial pain4,5,6,7. In later and more severe stages, patients may experience a cervical instability, generally reporting a clinical picture of crepitation associated with a sensation of their head falling forward upon flexion5,6,8. In this context, the diagnostic role of computed tomography (CT) and magnetic resonance imaging (MRI) has been recently highlighted in an accurate recognition of this manifestation7,8. A prompt identification of such feature is of importance to timely manage these patients. In fact, when the patient is still asymptomatic, a therapeutic strategy may be of crucial importance to induce a complete resolution of this feature5,6. Conversely, when the patient begins to complain the first symptoms of atlantoaxial joint involvement, a severe radiological picture is usually present, which often requires a more aggressive and less frequently successful treatment. However, presently, international validated recommendations are still lacking about the timing as well as the management of atlantoaxial joint involvement in RA patients. In addition, a few studies specifically focused on severe atlantoaxial joint involvement, when a clinically relevant picture and radiologic damage are present7,9,10,11. Furthermore, predictive factors are not entirely clarified to suggest patients at higher risk of developing such involvement in patients with RA.
On these bases, in this work, we aimed at evaluating the occurrence of a more severe atlantoaxial joint involvement in RA by a medical records review study in a “real-life” cohort in a first phase of the study. Furthermore, we exploited a clinical risk profile to identify some factors associated with a more severe atlantoaxial joint involvement in this first retrospective phase. After that, a prospective recruitment was performed to build a cross-sectional study to descriptively assess a possible subclinical atlantoaxial joint involvement by MRI. This second phase of the study involved asymptomatic patients but all characterised by derived associated factors from the analysis of the retrospective phase.
Materials and methods
Study design, setting, patients, and variables to be assessed in retrospective phase
In this study, we performed a medical records review study of consecutive patients with RA longitudinally followed-up at Rheumatologic Clinics of University of L’Aquila, L’Aquila, Italy, and of University of Campus Biomedico of Rome, Rome, Italy, between January 2010 and December 2020. Patients who met the ACR/EULAR 2010 criteria were assessed12. Each patient clinical chart was reviewed to assess the occurrence of a severe atlantoaxial joint involvement. The latter was defined as a clinically relevant picture in association with the presence of one or more of these radiologic features on atlantoaxial joint: large bone oedema, extensive synovitis, bone erosions, spinal cord compression, and/or atlantoaxial instability (subluxation, impaction). In addition, symptoms of atlantoaxial joint involvement were investigated and collected including occipital headache, migraines or and neck, mastoid, ear, facial pain, tinnitus, vertigo, and loss of equilibrium. In the present evaluation, these additional variables were also registered: age, gender, body mass index (BMI), smoking habit, disease duration, rheumatoid factor (RF), anticitrullinated protein antibodies (ACPA), and administered therapies. Glucocorticoids (GCs), synthetic-, biological-, and target synthetic disease modifying anti-rheumatic drugs (DMARDs) were recorded. GCs therapy was also categorised, either high dosage or low dosage, as previously reported13. All patients with atlantoaxial joint symptoms were investigated by both MRI and CT scan to elucidate a possible severe involvement due to RA. After that possible associated factors of severe atlantoaxial joint involvement were derived by regression analyses to exploit a clinical risk profile about this issue.
The local Ethics Committee (Comitato Etico Azienda Sanitaria Locale 1 Avezzano/Sulmona/L'Aquila, L'Aquila, Italy, protocol number 0095184/20) approved the study, which was performed according to the Good Clinical Practice guidelines and the Declaration of Helsinki. In reporting the results, we followed the STROBE guidelines.
Data sources, bias, and study size in retrospective phase
Relevant data were collected by a review of clinical charts of patients attending the involved centres between 2010 and 2020. Considering the retrospective design of this analysis, we tried to minimize possible biases with a careful definition of each variable to be assessed. Patients with concomitant neurological diseases, history of cervical trauma, and previous pathologies of the cervical spine were excluded from the assessment. A specific sample size was not estimated since this would be “real-life” evaluation of atlantoaxial joint involvement in patients with RA in our two cohorts.
Prospective recruitment of the study to build a cross-sectional study to assess a possible subclinical atlantoaxial joint involvement by MRI
In addition, according to the derived associated factors with the severe atlantoaxial joint involvement in retrospective study, a prospective recruitment was carried out that served to build a cross-sectional study to assess a possible subclinical atlantoaxial joint involvement by MRI (Fig. 1). Between January 2021 and December 2022, consecutive asymptomatic RA patients, who were characterized by the same associated factors (at least 3 out of 4 derived clinical variables) obtained by our analysis, underwent a cervical MRI to evaluate a possible subclinical atlantoaxial joint involvement. This prospective recruitment was performed to have a 2:1 matching with patients with a severe atlantoaxial joint involvement, who were assessed in the retrospective phase of this study.
MRI and CT scan definitions
In the retrospective phase, instrumental imaging evaluation of the atlantoaxial joint was performed in all patients with clinical suspicion of involvement. MRI examinations were performed on a 1.5 T scanner (Discovery MR750w, GE Healthcare), acquiring T1, T2, and STIR (short-tau inversion recovery) sequences on sagittal, coronal, and axial planes. CT examinations were performed on a multidetector 320-row CT scanner (Aquilion One, Toshiba) acquired with a thin collimation; soft tissue or bone algorithms were applied for image data reconstruction and analysis. Image analysis was performed by two expert radiologists dedicated to musculoskeletal and spine imaging. In particular, MRI images were examined to depict the presence of thickened synovium around the odontoid process, bone marrow oedema, and spinal cord changes, whereas CT images provided a superior visualization of bone erosion and atlanto-axial or sub-axial subluxation. In the prospective recruitment, the same MRI protocol was similarly used to highlight a possible subclinical atlantoaxial joint involvement. In both phases, experienced radiologists evaluated the collected images.
Statistical methods
In retrospective phase, statistics provided descriptive analysis of the data. Normally distributed continuous variables were expressed as mean ± standard deviation (SD), otherwise as median and range interquartile (IQR), as appropriate. Patients with significant missing data were removed. In retrospective phase, occurrence of severe atlantoaxial joint involvement was also estimated by incidence proportion and incidence rate per 1000 person-years at risk. In addition, Cox regression analyses were exploited to evaluate possible associated factors of severe atlantoaxial joint involvement in this phase of our study. Based on the number of retrieved patients codified as having this feature only univariate analyses were performed following the general rule that 10 events of the outcome of interest are required for each variable in the model including the exposure of interest (i.e. 10:1 events per variable)14. Thus, possible “sparse data” biases, related to multivariate analysis in small cohort of patients, were minimised15. These univariate analyses had the main purposes to provide the basis of arranging a specific prospective recruitment in which we selected patients, who could be considered at higher risk according to these features, to assess a possible subclinical atlantoaxial joint involvement by MRI. The derived results of this second section were descriptively assessed. Similarly to the retrospective phase, normally distributed continuous variables were expressed as mean ± SD, otherwise as median and IQR.
The Statistics Package for Social Sciences (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL, USA) was used for all analyses.
Ethics approval and consent to participate
The local Ethics Committee (Comitato Etico Azienda Sanitaria Locale 1 Avezzano/Sulmona/L’Aquila, L’Aquila, Italy; protocol number 0095184/20) approved the study, which was performed according to the Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent to participate before data collection.
Results
Descriptive characteristics and incidence of severe atlantoaxial joint involvement in our cohort in the retrospective phase of the study
In this evaluation, 717 patients were assessed (Table 1), mostly female (56.6%) with a mean age of 64.7 ± 12.3 years. The median disease duration was 10 years (range interquartile 28) and 68.3% displayed the positivity for RF and/or ACPA. During the follow-up (6.2 ± 3.3 years), 78.2% of patients were treated with low dose of GCs, 51.9% with methotrexate (MTX), and 45.7% with biologic DMARDs. Moreover, 48 out of 717 (6.7%) had extra-articular manifestations, 26 patients were affected by a secondary Sjogren’s syndrome. Interstitial lung disease (ILD) was identified in 16 patients whereas 3 had a leukocytoclastic vasculitis. Finally, 3 patients had rheumatoid nodules.
In this cohort, the cumulative incidence proportion of severe atlantoaxial joint involvement was 2.1% [1.5–2.5], occurring in 15 out of 717 patients. Considering over 3019 person-years, an incidence rate of 5.2 × 1000 [2.9 − 8.3] person-years was also estimated.
Characteristics of patients with atlantoaxial joint involvement in the retrospective phase of the study
The mean age of 15 patients with atlantoaxial joint involvement was 64.7 ± 12.3 years and most patients were female, 7 reported a smoking habit. At the beginning, all these patients experienced a mild pain of the cervical spine with an insidious onset. After that, over few weeks, out of 15 patients, 10 become strongly symptomatic, experiencing an intense neck pain, 3 reported vertigos, 2 described loss of equilibrium, and 1 complained tinnitus. The atlantoaxial joint involvement was confirmed by both MRI and CT scan.
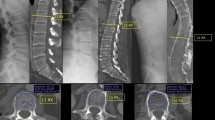
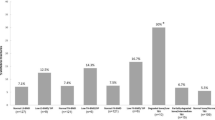
MRI images reported a synovial hypertrophy in 100% of these patients, whereas 86.7% showed a spinal cord compression (Fig. 2). The presence of bone oedema was recognized in 73.3% of patients, and 53.3% were characterized by bone erosions, confirmed by CT scan (Fig. 3). Out of assessed patients, 2 also showed cervical instability (Fig. 4). The presence of ACPA was reported in all these patients. None of these patients was in clinical remission at the time of diagnosis of severe atlantoaxial joint involvement onset. Furthermore, this manifestation occurred within a mean of 5 years from the onset of the disease, and 10 out of 15 had were previously treated with short and low doses of GCs whereas 11 patients were treated with synthetic DMARDs and 3 with biologic DMARDs. Extra-articular manifestations were associated with severe atlantoaxial joint involvement, 4 patients presented ILD and 2 presented rheumatoid nodules. None of these patients reported a previous cervical trauma. After the diagnosis of severe atlantoaxial joint involvement, all these patients were treated with MTX and high doses of intravenous GCs, 9 out of 15 were also treated with TNF inhibitors (TNFi) (7 infliximab, 1 certolizumab-pegol, 1 etanercept). This therapeutic strategy resulted in a complete resolution of the inflammatory synovitis in all but two patients. These developed a cervical spinal instability resulting in neurological progression and required a subsequent surgical management.
Clinical factors associated with of severe atlantoaxial joint involvement in our cohort of patients in retrospective phase of the study
As shown in Table 2, considering the relatively low incidence of severe atlantoaxial joint involvement in our study, only univariate explorative HR analyses were exploited to assess possible factors associated with such manifestation in this retrospective phase of the study. Male gender (HR 10.97, 95% CI 2.47–48.81, p = 0.002) and a longer disease duration (HR 1.06, 95% CI 1.01–1.10, p = 0.022) resulted to be significantly associated with a severe atlantoaxial joint involvement. Similarly, ACPA positivity (HR 4.87, 95% CI 1.10–21.60, p = 0.037) and extra-articular manifestations (HR 7.83, 95% CI 2.81–21.81, p < 0.0001) were associated with a severe atlantoaxial joint involvement in our cohort in this retrospective phase of the study.
Clinical features and results obtained in the prospective recruitment of the study
In the prospective recruitment of this work, 30 consecutively asymptomatic RA patients were selected and assessed among those attending the involved centres to build a cross-sectional study in evaluating a possible subclinical atlantoaxial joint involvement by MRI (Table 3). They were characterized by at least 3 of the 4 associated factors identified by regression analyses in the retrospective phase. Specifically, they were mostly females (80.0%), with a mean age of 62.8 ± 11.7 years, and 33.3% of them had a smoking habit. The positivity for RF and/or ACPA was recognised in 90.0% of these patients, long disease duration in 80.0%, and extra-articular manifestations in 66.7%. At the time of assessment, 56.7% of patients were treated with low dose of GCs, 46.7% with MTX, and 76.7% with biologic DMARDs.
Out of 30, 13 patients (43.3%) showed a subclinical atlantoaxial joint involvement documented by MRI. A synovial hypertrophy was reported in 100% of these patients, even if a milder involvement than previously reported in the retrospective phase (Fig. 5). Furthermore, MRI showed that 26.7% had bone oedema (Fig. 6), 13.3% a spinal cord compression, and 6.7% bone erosions. When compared with retrospective phase, a lower percentage of these MRI findings was reported in these patients. Among these patients with subclinical atlantoaxial joint involvement, 69.2% were characterized by extra-articular manifestations and 84.6% showed positivity for RF and/or ACPA. These RA patients with subclinical atlantoaxial joint involvement, although asymptomatic, were immediately evaluated for a possible change of therapeutic strategies. Based on MRI findings, the dose of daily GCs was increased, and, in biologic DMARD treated patients, a possible switch to another biologic agent was considered.
Discussion
In our study, in the retrospective phase, the occurrence of a severe atlantoaxial joint involvement in RA patients was estimated in a “real-life” setting with an incidence proportion of 2.1% (1.5–2.5%) and an incidence rate of 5.2 × 1000 (2.9–8.3) person-years, respectively. Male gender, a longer disease duration, presence of ACPA, and extra-articular manifestations resulted to be associated with such severe manifestation in this first phase the study. Subsequently, in 30 asymptomatic RA patients, who were characterized by these clinical features, a subclinical atlantoaxial joint involvement was found in almost half of these patients in the second cross-sectional descriptive phase.
In the retrospective phase of this study, a severe atlantoaxial joint involvement was identified following the onset of mild cervical pain, with a subtle symptomatology at the beginning, which subsequently evolved, over few weeks, in a more severe clinical picture. In this setting, MRI and CT scan showed to be sensitive and specific imaging modalities for an accurate determination of an atlantoaxial joint involvement in RA7,16,17. Comparing our results with previous experiences3,5,7,9,18,19, we retrieved a lower occurrence of severe atlantoaxial joint involvement in patients with RA. This possible discrepancy with our results could be attributed to different study designs and target populations. In fact, we focused our study on more severe patients with a high suspicion clinical picture of atlantoaxial joint involvement. In our cohort, the factors associated with a severe atlantoaxial joint involvement were also exploratively assessed. Our analysis suggested that male gender could predict the occurrence of this feature, as reported in previous studies5,7,9,18. Although conflicting available results, a less favourable RA outcome in men could be observed20,21,22. Furthermore, the presence of ACPA was also associated with atlantoaxial joint involvement. Generally, ACPA positivity is associated with the extent of joint destruction and with a more destructive RA course23. Furthermore, synovial fluid from RA joints may contain citrullinated proteins, which in turn would increase the local inflammation, correlating with radiographic damage and progression24. In our patients, a longer disease duration was also associated with the severe atlantoaxial joint occurrence, which could be related with a longer exposition to an active inflammatory process. Long-standing RA could less likely respond to the treatment, even the administration of biological DMARDs could not completely suppress the disease activity in a large percentage of patients24. Thus, a long duration of the disease could influence the presence of atlantoaxial joint involvement due to a persistent inflammation over time25. In addition, atlantoaxial joint involvement was also associated with the presence of extra-articular manifestations in our patients. These features are related to poor long-term outcomes25,26,27. Taking together all these observations, patients with atlantoaxial joint involvement could be considered as having a more severe subset of RA2,7,9.
In the descriptive cross-sectional phase of this study , we evaluated the subclinical atlantoaxial involvement in asymptomatic consecutive RA patients, characterized by the associated factors identified in the retrospective part. Among asymptomatic patients who underwent MRI, almost half showed a subclinical atlantoaxial joint involvement, pointing out some clinically silent imaging abnormalities which could anticipate the occurrence of a severe clinical picture. Thus, a very early recognition of the atlantoaxial joint involvement may be allowed by MRI. The latter is a non-ionizing imaging technique with an excellent soft-tissue visualisation, a multiplanar large anatomical coverage, and it may allow a possible centralised reading28. Furthermore, MRI may accurately visualise and evaluate in detail soft tissue changes of synovitis, which are not detectable by conventional clinical assessment and standard radiographic methods29,30,31. Importantly, MRI is a safe technique, without ionising radiations and no risk increase of malignancies. Taking together these observations, a sophisticated imaging modality of atlantoaxial joint involvement may detect clinically silent lesions, identifying those features of the preclinical phases which could be completely reversed by an appropriate therapeutic strategy. Based on these findings, further studies may be held to evaluate the evolution of the atlantoaxial joint involvement and the most appropriate management of these patients. Exploratively comparing MRI results of the retrospective and the prospective phases of our study, we observed that these patients were similarly characterized by a synovial hypertrophy in the atlantoaxial joint. However, in patients with subclinical atlantoaxial joint involvement, a less prominent synovial involvement and with a less percentage of spinal cord compression were observed. In addition, in these patients with subclinical atlantoaxial joint involvement a lower percentage of bone oedema and bone erosions was observed. Thus, the occurrence of these MRI features may be associated with the development of the clinical picture, associated with severe atlantoaxial joint involvement. These findings parallel what observed in peripheral joints, when the appearance of bone erosions is a marker of a more progressive disease and joint damage32.
As far as the treatment of atlantoaxial joint involvement is concerned, conflicting information is available without specific recommendations. In any case, the early recognition of the atlantoaxial joint involvement in RA may allow the clinicians to administer an appropriate and aggressive therapeutic strategy, considering that it may cause a severe neurologic damage9,13,16. A combination therapy between GCs and csDMARDs, or csDMARDs and biologic DMARDs, mainly infliximab, is suggested in reducing the development as well as the progression of cervical spine lesions in RA patients33,34,35. In this context, also the administration of high dosage of GCs is also proposed to manage more severe patients34, even if a possible confounding by indication bias may be reported in some studies4,18,36. Taking together all these observations, patients with atlantoaxial joint involvement may need a timely management devoted to an early recognition and a properly inflammation reversal as the major therapeutic target to be achieved. In addition, RA patients with a severe atlantoaxial joint involvement should be considered for a possible surgical management of the associated cervical instability5,6,8,9. Since these patients are often treated with a combination of immunosuppressive agents, they should be carefully evaluated to reduce the risk of serious post-operative infections37. In any case, it must be pointed out that further studies are required to elucidate a safe therapeutic immunosuppressive strategy in patients who may need a surgical management.
Given the retrospective analysis of the first part of this work, our study may be subjected to possible biases and our results should be cautiously generalised. The medical records were evaluated assessing those patients with clear symptoms and underwent the cervical spine diagnostics, including both MRI and CT. Thus, a relatively small number of patients were retrieved with this manifestation which is associated with another weakness of this section. In fact, following generally accepted methodological considerations14,15, only univariate explorative analyses were performed in deriving the associated clinical factors with severe atlantoaxial joint involvement in RA patients. In addition, a causal relationship could not be fully established by these explorative analyses since it needs properly conducted prospective studies. Concerning the prospective recruitment that served to build the descriptive cross-sectional evaluation, a limitation of our study is the relatively small number of assessed patients. Furthermore, considering the lack of accurate predictive models about the evolution toward a severe atlantoaxial joint involvement, our descriptive findings could be considered as “hypothesis-generating” to arrange specific powered studies to fully elucidate this unmet need of the management of patients with RA also assessing possible predictive biomarkers38,39,40.
Conclusions
In conclusion, the occurrence of atlantoaxial joint involvement in RA patients was estimated in a “real-life” setting, possibly causing severe neurologic damage, and requiring a timely management. Male gender, a longer disease duration, presence of ACPA, and extra-articular manifestations resulted to be associated with such manifestation. MRI could provide a useful clinical tool to early evaluate the atlantoaxial joint involvement in RA, also in asymptomatic patients. An early recognition and treatment of such manifestation is of crucial importance to improve the outcome of these patients reducing the consequent associated morbidity.
Data availability
All data relevant to the study are included in the article.
Abbreviations
- RA:
-
Rheumatoid arthritis
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- BMI:
-
Body mass index
- RF:
-
Rheumatoid factor
- ACPA:
-
Anticitrullinated protein antibodies
- GCs:
-
Glucocorticoids, -, biological-, and
- DMARDs:
-
Disease modifying anti-rheumatic drugs
- csDMARDs:
-
Conventional synthetic-disease modifying anti-rheumatic drugs
- bDMARDs:
-
Biological-disease modifying anti-rheumatic drugs
- tsDMARDs:
-
Target synthetic-disease modifying anti-rheumatic drugs
- STIR:
-
Short-tau inversion recovery
- SD:
-
Standard deviation
- MTX:
-
Methotrexate
- ILD:
-
Interstitial lung disease
- TNFi:
-
TNF inhibitors
References
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388, 2023–2038 (2016).
Scott, D. L., Wolfe, F. & Huizinga, T. W. Rheumatoid arthritis. Lancet 376, 1094–1108 (2010).
Garrod, A. E. A Treatise on Rheumatism and Rheumatoid Arthritis (Griffin’s Medical Series, 1890).
Shlobin, N. A. & Dahdaleh, N. S. Cervical spine manifestations of rheumatoid arthritis: A review. Neurosurg. Rev. 44, 1957–1965 (2021).
Kim, H. J., Nemani, V. M., Riew, K. D. & Brasington, R. Cervical spine disease in rheumatoid arthritis: Incidence, manifestations, and therapy. Curr. Rheumatol. Rep. 17, 9 (2015).
Chattopadhyay, A., Samanta, J., Jindal, A. & Jain, S. Neck pain in rheumatoid arthritis: A look beyond cervical spine involvement. Rheumatology (Oxford) 60, 471 (2021).
Carotti, M., Salaffi, F., Di Carlo, M., Sessa, F. & Giovagnoni, A. Magnetic resonance imaging of the craniovertebral junction in early rheumatoid arthritis. Skelet. Radiol. 48, 553–561 (2019).
Drosos, A. A., Pelechas, E. & Voulgari, P. V. Radiological findings of the cervical spine in rheumatoid arthritis: What a rheumatologist should know. Curr. Rheumatol. Rep. 22, 19 (2020).
Kaito, T. et al. Incidence and risk factors for cervical lesions in patients with rheumatoid arthritis under the current pharmacologic treatment paradigm. Mod. Rheumatol. 27, 593–597 (2017).
Montemerani, M. et al. Involvement of atlanto-axial joint in rheumatoid arthritis: Rare or frequent?. Clin. Rheumatol. 13, 459–463 (1994).
Zhang, T. & Pope, J. Cervical spine involvement in rheumatoid arthritis over time: Results from a meta-analysis. Arthritis Res. Ther. 17, 148 (2015).
Aletaha, D. et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588 (2010).
Duru, N. et al. EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann. Rheum. Dis. 72, 1905–1913 (2013).
Peduzzi, P., Concato, J., Kemper, E., Holford, T. R. & Feinstein, A. R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 49, 1373–1379 (1996).
Greenland, S., Schwartzbaum, J. A. & Finkle, W. D. Problems due to small samples and sparse data in conditional logistic regression analysis. Am. J. Epidemiol. 1(151), 531–539 (2000).
Joaquim, A. F., Ghizoni, E., Tedeschi, H., Appenzeller, S. & Riew, K. D. Radiological evaluation of cervical spine involvement in rheumatoid arthritis. Neurosurg. Focus 38, E4 (2015).
Castro, S. et al. Cervical spine involvement in rheumatoid arthritis: A clinical, neurological and radiological evaluation. Clin. Exp. Rheumatol. 12, 369–374 (1994).
Baek, I. W., Joo, Y. B., Park, K. S. & Kim, K. J. Risk factors for cervical spine instability in patients with rheumatoid arthritis. Clin. Rheumatol. 40, 547–555 (2021).
Kotecki, M., Gasik, R., Głuszko, P. & Sudoł-Szopińska, I. Radiological evaluation of cervical spine involvement in rheumatoid arthritis: A cross-sectional retrospective study. J. Clin. Med. 10, 4587 (2021).
Hyrich, K. L., Watson, K. D., Silman, A. J., Symmons, D. P., British Society for Rheumatology Biologics Register. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 45, 1558–1565 (2006).
Kvien, T. K., Uhlig, T., Ødegård, S. & Heiberg, M. S. Epidemiological aspects of rheumatoid arthritis: The sex ratio. Ann. N. Y. Acad. Sci. 1069, 212–222 (2006).
Mancarella, L. et al. Good clinical response, remission, and predictors of remission in rheumatoid arthritis patients treated with tumor necrosis factor-alpha blockers: The GISEA study. J. Rheumatol. 34, 1670–1673 (2007).
Kocijan, R., Harre, U. & Schett, G. ACPA and bone loss in rheumatoid arthritis. Curr. Rheumatol. Rep. 15, 366 (2013).
Katchamart, W., Koolvisoot, A., Aromdee, E., Chiowchanwesawakit, P. & Muengchan, C. Associations of rheumatoid factor and anti-citrullinated peptide antibody with disease progression and treatment outcomes in patients with rheumatoid arthritis. Rheumatol. Int. 35, 1693–1699 (2015).
Symmons, D. et al. Patients with stable long-standing rheumatoid arthritis continue to deteriorate despite intensified treatment with traditional disease modifying anti-rheumatic drugs—Results of the British Rheumatoid Outcome Study Group randomized controlled clinical trial. Rheumatology (Oxford) 45, 558–565 (2006).
Turesson, C. Extra-articular rheumatoid arthritis. Curr. Opin. Rheumatol. 25, 360–366 (2013).
Conforti, A. et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun. Rev. 20, 102735 (2021).
Bruno, F. et al. New advances in MRI diagnosis of degenerative osteoarthropathy of the peripheral joints. Radiol. Med. 124, 1121–1127 (2019).
Østergaard, M. & Boesen, M. Imaging in rheumatoid arthritis: The role of magnetic resonance imaging and computed tomography. Radiol. Med. 124, 1128–1141 (2019).
Barile, A. et al. Computed tomography and MR imaging in rheumatoid arthritis. Radiol. Clin. North Am. 55, 997–1007 (2017).
Bruno, F. et al. MR imaging of the upper limb: Pitfalls, tricks, and tips. Radiol. Clin. North Am. 57, 1051–1062 (2019).
Buch, M. H. Defining refractory rheumatoid arthritis. Ann. Rheum. Dis. 77, 966–969 (2018).
Kauppi, M. J. et al. Rheumatoid atlantoaxial subluxation can be prevented by intensive use of traditional disease modifying antirheumatic drugs. J. Rheumatol. 36, 273–278 (2009).
Kaito, T. et al. Effect of biological agents on cervical spine lesions in rheumatoid arthritis. Spine (Phila Pa 1976) 37, 1742–1746 (2012).
Kanayama, Y. et al. Radiographic progression of cervical lesions in patients with rheumatoid arthritis receiving infliximab treatment. Mod. Rheumatol. 20, 273–279 (2010).
Wasserman, B. R., Moskovich, R. & Razi, A. E. Rheumatoid arthritis of the cervical spine—Clinical considerations. Bull. NYU Hosp. Jt. Dis. 69, 136–148 (2011).
Cipriani, P. et al. Biologic therapies and infections in the daily practice of three Italian rheumatologic units: A prospective, observational study. Clin. Rheumatol. 36, 251–260 (2017).
Giacomelli, R. et al. Guidelines for biomarkers in autoimmune rheumatic diseases—Evidence based analysis. Autoimmun. Rev. 18, 93–106 (2019).
Giacomelli, R. et al. International consensus: What else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren’s syndrome)?: The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun. Rev. 16, 911–924 (2017).
Giacomelli, R. et al. The growing role of precision medicine for the treatment of autoimmune diseases; Results of a systematic review of literature and Experts’ Consensus. Autoimmun. Rev. 20, 102738 (2021).
Acknowledgements
The authors thank Mrs. Federica Sensini for her technical assistance.
Author information
Authors and Affiliations
Contributions
C.D.M.: Conceptualization of the study; clinical data curation and collection; formal analysis of collected data; project administration; writing of the original draft; writing, review and editing of the final draft. A.C.: Conceptualization of the study; clinical data curation and collection; formal analysis of collected data; writing of the original draft; writing, review and editing of the final draft. F.B.: Radiological data curation and collection; writing of the original draft; writing, review and editing of the final draft. D.C.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. O.B.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. L.N.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. V.P.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. I.D.C.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. A.B.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. S.D.D.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. A.M.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. S.L.: Clinical data curation and collection; writing of the original draft; writing, review and editing of the final draft. F.U.: Clinical data curation and collection; methodology and supervision of the project; writing of the original draft; writing, review and editing of the final draft. A.B.: Radiological data curation and collection; methodology and supervision of the project; writing of the original draft; writing, review and editing of the final draft. C.M.: Radiological data curation and collection; methodology and supervision of the project; writing of the original draft; writing, review and editing of the final draft. P.C.: Conceptualization of the study; clinical data curation and collection; formal analysis of collected data; methodology and supervision of the project; writing of the original draft; writing, review and editing of the final draft. R.G.: Conceptualization of the study; clinical data curation and collection; formal analysis of collected data; methodology and supervision of the project; writing of the original draft; writing, review and editing of the final draft. P.R.: Conceptualization of the study; clinical data curation and collection; formal analysis of collected data; methodology and supervision of the project; writing of the original draft; writing, review and editing of the final draft. All authors made substantial contributions to the conception or design of the work, the acquisition, and interpretation of data. All authors read and approved the final version of the manuscript. All the authors agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Muzio, C., Conforti, A., Bruno, F. et al. The assessment of atlantoaxial joint involvement in patients with rheumatoid arthritis, results from an observational “real-life” study. Sci Rep 13, 20146 (2023). https://doi.org/10.1038/s41598-023-46069-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46069-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.