Abstract
To evaluate the differential associations of high-risk plaque characteristics (HRPC) with resting or hyperemic physiologic indexes (instantaneous wave-free ratio [iFR] or fractional flow reserve [FFR]), a total of 214 vessels from 127 patients with stable angina or acute coronary syndrome who underwent coronary computed tomography angiography (CCTA) and invasive physiologic assessment were investigated. HPRC were classified into quantitative (minimal luminal area < 4 mm2 or plaque burden ≥ 70%) and qualitative features (low attenuation plaque, positive remodeling, napkin ring sign, or spotty calcification). Vessels with FFR ≤ 0.80 or iFR ≤ 0.89 had significantly higher proportions of HRPC than those with FFR > 0.80 or iFR > 0.89, respectively. FFR was independently associated with both quantitative and qualitative HRPC, but iFR was only associated with quantitative HRPC. Both FFR and iFR were significantly associated with the presence of ≥ 3 HRPC, and FFR demonstrated higher discrimination ability than iFR (AUC 0.703 vs. 0.648, P = 0.045), which was predominantly driven by greater discriminating ability of FFR for quantitative HRPC (AUC 0.832 vs. 0.744, P = 0.005). In conclusion, both FFR and iFR were significantly associated with CCTA-derived HRPC. Compared with iFR, however, FFR was independently associated with the presence of qualitative HRPC and showed a higher predictive ability for the presence of ≥ 3 HRPC.
Similar content being viewed by others
Introduction
Since a fundamental goal of coronary revascularization is relieving myocardial ischemia and symptoms caused by flow-limiting epicardial coronary stenosis, fractional flow reserve (FFR) and instantaneous wave free ratio (iFR) are currently used to guide revascularization1. However, another important goal of evaluating patients with coronary artery disease is to identify, and ultimately reduce, the risk of future cardiac events such as acute coronary syndrome (ACS) and sudden cardiac death. Since postmortem studies provided insights into morphological features of high-risk vulnerable plaque as the major cause of ACS and sudden cardiac death2,3, several imaging modalities such as intravascular imaging or coronary computed tomography angiography (CCTA) have identified high-risk plaque characteristics (HRPC) that predict future ACS and cardiac death4,5,6,7.
Recent studies linking the functional significance of a coronary stenosis as assessed by FFR to HRPC observed on CCTA3,8,9,10,11,12 may, in part, explain the observed reduction in spontaneous myocardial infarction (MI) in patients undergoing FFR-guided percutaneous coronary intervention (PCI)13,14. Unlike for FFR, there are limited data describing a relationship between iFR and HRPC10. Yet, there are reasons to consider that such a relationship might be different. On one hand, stenosis assessment with FFR is performed under conditions of maximal hyperemic flow, while iFR assesses stenosis severity using resting or non-hyperemic flow. On the other, iFR correlates better with estimates of microcirculatory flow modulation like coronary flow reserve than FFR15. Both maximal hyperemic flow and autoregulation affect epicardial vessel biology through mechanisms like modification of wall shear stress16, thus play a role in atheromatous plaque destabilization.
To address this void of knowledge, in the present study we investigated the association between CCTA-derived HRPC and invasively measured FFR and iFR. We further examined whether any differences in the predictive ability of the invasive physiologic indexes were related to quantitative or qualitative HRPC.
Methods
Study population
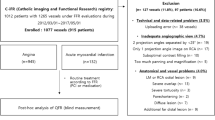
The current study was retrospectively conducted based on the international, multicenter registry which enrolled 361 vessels from 237 patients who underwent CCTA followed by invasive coronary angiography with physiologic assessments for stable angina or ACS at tertiary medical centers in Korea, Japan, Australia, and Spain, where CCTA and physiologic assessments have been routinely done in daily practice for the evaluation of coronary artery disease. The registry was established to examine diagnostic accuracy of CCTA-derived FFR values calculated based on the novel computational fluid dynamics method, compared with invasively measured physiologic indexes. Among them, 86 vessels without both FFR and iFR measurements were excluded. Then, vessels with poor CCTA image quality (n = 24), insufficient CCTA images for core laboratory analysis (n = 14), severe image artifact (n = 14), or prior stents or coronary artery bypass graft (n = 9) were excluded. Following these exclusions, a total of 214 vessels from 127 patients were analyzed in the current study. The study protocol was approved and the requirement for informed consent of the individual patients was waived by the Institutional Review Board at Samsung Medical Center, South Korea due to retrospective nature of the study. The study protocol was in accordance with the Declaration of Helsinki.
Coronary computed tomography angiography and high-risk plaque characteristics
All patients underwent CCTA with 64 or higher detector row scanner platforms. In accordance with the Society of Cardiovascular Computed Tomography guidelines17, the CCTA images were analyzed at a core laboratory (Seoul National University Bundang Hospital, Seongnam, Korea) in a blinded fashion. Plaque in the most severe stenosis within the target vessel was selected for per-vessel analyses. Quantitative features of target vessels and lesions were analyzed, including minimal luminal area (MLA), plaque burden in MLA segment, total aggregated plaque volume (TAPV), and percent TAPV (TAPV/total vessel volume X 100). Based on previous invasive imaging4,5,18 and CCTA-based studies 6,8,18,19,20, MLA < 4 mm2 and plaque burden ≥ 70% were considered to be quantitative HRPC. Qualitative features of plaques were identified based on previous studies which reported definitions and predictive values of 4 qualitative HRPC for subsequent adverse clinical outcomes6,8,18,19,20: low attenuation plaque, positive remodeling, napkin ring sign, and spotty calcification. Briefly, plaque density was assessed semi-automatically using a dedicated cardiac workstation (Intellispace Portal, Philips Healthcare, Cleveland, OH, USA)19. Low attenuation plaque was defined as a plaque with an average density ≤ 30 Hounsfield units (HU) from 3 random region-of-interest in noncalcified portion of the plaque8. Positive remodeling was defined as a remodeling index ≥ 1.1, a ratio of maximal diameters between lesion and proximal reference vessel3,6. Napkin ring sign was characterized as a low attenuating plaque core surrounded by a ring-like area of higher attenuation8,20. Spotty calcification was defined as an intralesional calcification with an average density > 130 HU, diameter < 3 mm in any direction, length < 1.5 times the vessel diameter, and width of the calcification less than two-thirds of the vessel diameter3,8.
Consequently, HRPC was defined if there was any quantitative (MLA < 4 mm2 or plaque burden ≥ 70%) or qualitative feature (low attenuation plaque, positive remodeling, napkin ring sign, or spotty calcification) noted, and the presence of ≥ 3 HRPC from any combination of quantitative and/or qualitative HRPC features was considered to be significant, since it was found to be independently associated with adverse outcomes8.
Angiographic analysis and quantitative coronary angiography
Coronary angiography was performed using standard techniques and median interval between CCTA and coronary angiogram was 15.5 days (interquartile range: 5–30 days). Angiographic views were obtained after administration of intracoronary nitrate (100–200 μg). All angiograms were analyzed at a core laboratory in a blinded fashion. Quantitative coronary angiography was performed in optimal projections with validated software (CAAS II, Pie Medical System, Maastricht, The Netherlands). Minimal lumen diameter, reference vessel size, percent diameter stenosis (%DS), and lesion length were measured.
Invasive physiologic assessments
All coronary physiologic indexes were measured after diagnostic angiography. After a pressure wire sensor was zeroed and equalized to the aortic pressure, it was positioned at the distal segment of a target vessel. Intracoronary nitrate (100–200 µg) was administered before each set of physiologic measurements. For the physiologic assessment, a ratio between proximal (Pa) and distal coronary pressure (Pd) was obtained during resting and maximal hyperemia. iFR was calculated as a resting Pd/Pa measured during the wave-free period of diastole21. If iFR was not obtained during the procedure, it was calculated using resting pressure tracings in post-hoc manner (15.4%; N = 33/214), as previously described22. FFR was calculated as the lowest average of mean Pd/Pa from 3 consecutive beats during maximal hyperemia. All coronary physiologic measurements were analyzed at a core laboratory in a blinded fashion.
Statistical analysis
Data were analyzed on a per-vessel basis and generalized estimating equation (GEE) models with independent correlation structures were used to adjust for intrasubject variability among vessels from the same patient and participating center8. CCTA-measured characteristics of target vessels and HRPC were compared between groups according to FFR or iFR with 0.80 and 0.89 as cutoff values, respectively. In addition, the CCTA-derived characteristic and number of HRPC (0, 1, 2, and ≥ 3) were compared among 4 groups classified by FFR and iFR values. Associations between physiologic indexes and the presence of ≥ 3 HRPC were evaluated using multivariable GEE model with logistic regression. Covariables were age, sex, diabetes mellitus, presentation with ACS, current smoking, dyslipidemia, and plaque in proximal segment of a target vessel. To investigate independent associations, presence of quantitative HRPC was further adjusted when looking at the associations between physiologic indexes and qualitative HRPC, and vice versa. In addition, the associations of physiologic indexes with probability of the presence of ≥ 3 HRPC were graphically presented with penalized splines with a degree of freedom of 3. Discrimination abilities of FFR and iFR for the presence of ≥ 3 HRPC as well as quantitative and qualitative HRPC were compared using receiver operating characteristic (ROC) curve and area under the curve (AUC). All probability values were 2-sided and p-values < 0.05 were considered statistically significant.
Results
Characteristics of patients and target lesions
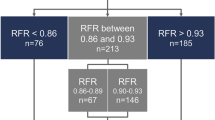
Tables 1 and 2 show general characteristics of the study population and target vessels, respectively. In brief, mean age was 64.9 ± 10.9 years and 74.0% were men. Most patients (88.2%) presented with stable angina. Mean FFR was 0.81 ± 0.13 and 44.9% had positive FFR ≤ 0.80. Mean iFR was 0.88 ± 0.13 and 40.2% had positive iFR ≤ 0.89. There was a significant correlation between FFR and iFR (r = 0.767, P < 0.001) and discordance between FFR- and iFR-based classifications occurred in 20.6% (Fig. 1).
CCTA-derived characteristics and physiologic indexes
Table 2 demonstrates CCTA-derived characteristics of target vessels and lesions according to FFR and iFR. Compared to the lesions with negative FFR > 0.80 or iFR > 0.89, those with positive FFR ≤ 0.80 or iFR ≤ 0.89 had significantly higher incidence of quantitative and qualitative HRPC as well as lesions with ≥ 3 HRPC, respectively (FFR: 21.2% vs. 41.7%, respectively, P = 0.001; iFR: 24.2% vs. 39.5%, respectively, P = 0.017). In addition, there were significant differences in FFR or iFR values according to the number of HRPC (P < 0.001 and P = 0.001, respectively; Supplementary Fig. 1), and FFR and iFR values were inversely associated with the number of HRPC (P < 0.001 for both; Supplementary Fig. 2).
CCTA-derived characteristics according to agreement between FFR and iFR
Table 3 shows CCTA-derived characteristics of target vessels and lesions according to the 4 groups classified by FFR and iFR. There was significant difference in the proportion of lesions with ≥ 3 HRPC among the 4 groups. Group 1 with concordantly negative FFR and iFR had the lowest proportion of lesions with ≥ 3 HRPC and group 4 with concordantly positive FFR and iFR had the highest proportion of lesions with ≥ 3 HRPC (overall P < 0.001). Furthermore, group 4 showed a consistently higher number of lesions with quantitative or qualitative HRPC than the other groups. Among the discordant groups, group 3 with positive FFR but negative iFR showed a numerically higher proportion of lesions with ≥ 3 HRPC than group 2 with negative FFR but positive iFR (Table 3 and Fig. 2).
Proportion of High-Risk Plaque Characteristics Among 4 Groups, Classified by FFR and iFR. Proportions of number of HRPC (0, 1, 2, and ≥ 3) are compared according to the 4 groups classified by FFR and iFR with cutoff values of 0.80 and 0.89, respectively. Abbreviations HRPC, high-risk plaque characteristics; other abbreviations as in Fig. 1.
Associations between CCTA-derived HRPC and physiologic indexes
After adjusting for various patient- and vessel-related characteristics including age, sex, diabetes mellitus, presentation with ACS, current smoking, dyslipidemia, and plaque in proximal segment of a target vessel, both FFR and iFR were significantly associated with the presence of ≥ 3 HRPC (per 0.01 decrease in FFR: OR 1.043, 95% CI 1.018–1.069, P = 0.001; per 0.01 decrease in iFR: OR 1.032, 95% CI 1.008–1.057, P = 0.008) (Table 4). After adjusting for quantitative HRPC, FFR was independently associated with qualitative HRPC, and vice versa. In contrast, iFR was independently associated with quantitative HRPC after adjusting for qualitative HRPC, but not with qualitative HRPC after adjusting for quantitative HRPC (Table 4). When the probability of ≥ 3 HRPC was plotted according to FFR or iFR values, both physiologic indexes were significantly associated with the probability of ≥ 3 HRPC (P = 0.006 and 0.010, respectively) (Fig. 3).
Association of FFR or iFR with Probability of ≥ 3 High-Risk Plaque Characteristics. Probabilities of the presence of ≥ 3 HRPC are plotted according to FFR and iFR values. Abbreviations as in Fig. 2.
Discrimination ability of physiologic indexes for quantitative and qualitative HRPC
To evaluate the discrimination ability of FFR and iFR for HRPC, ROC curves and AUC values were compared (Fig. 4). FFR showed a higher discrimination ability for the presence of ≥ 3 HRPC than iFR (AUC 0.703 vs. 0.648, respectively; P = 0.045). This difference was mainly driven by a higher discrimination ability of FFR for the presence of quantitative HRPC (AUC 0.832 vs. 0.744, respectively; P = 0.005). Among qualitative HRPC, FFR showed higher discrimination abilities to predict positive remodeling and napkin ring sign than iFR (P = 0.021 and 0.028, respectively), but no differences were seen for low attenuation plaque and spotty calcification (Supplementary Fig. 3).
Comparison of Discrimination Ability for Quantitative or Qualitative High-Risk Plaque Characteristics between FFR and iFR. The receiver operating characteristic curves of FFR (in red) and iFR (in blue) to discriminate the presence of (A) overall HRPC ≥ 3, (B) quantitative HRPC, and (C) qualitative HRPC are presented. Quantitative HRPC included minimal luminal area < 4 mm2 and plaque burden ≥ 70%. Qualitative HRPC included low attenuation plaque, positive remodeling, napkin ring sign, and spotty calcification. Abbreviations AUC, area under the curve; other abbreviations as in Fig. 2.
Discussion
The current study evaluated the association between CCTA-derived HRPC and invasively measured FFR and iFR. There were a number of observations. First, vessels with FFR ≤ 0.80 or iFR ≤ 0.89 had significantly higher number of both quantitative and qualitative HRPC. Second, both FFR and iFR were inversely associated with the number of HRPC. Even after adjusting for quantitative HRPC, FFR was independently associated with qualitative HRPC, but iFR was not. Third, although FFR showed a significantly higher predictability of the presence of ≥ 3 HRPC than iFR, the difference was mainly derived from a greater discrimination ability of FFR for the presence of quantitative HRPC (Central illustration).
Quantitative and qualitative adverse plaque characteristics in coronary artery disease
Previous studies using intravascular ultrasound have identified 3 independent predictors of major adverse cardiac events (MACE): (1) small MLA ≤ 4.0 mm2; (2) large plaque burden ≥ 70%; and (3) virtual histology-defined thin-cap fibroatheroma (TCFA)4,5,23. Interestingly, no event occurred from untreated segments with a plaque burden < 40% in the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study, suggesting an importance of quantitative plaque features4. Motoyama et al.6 reported higher event rates in patients with CCTA-derived qualitative HRPC (positive remodeling and/or low attenuation plaque), irrespective of the presence of significant stenosis ≥ 70%, representing an importance of qualitative plaque features. These results suggested that both quantitative and qualitative HRPC are important in risk stratification of patients for future adverse cardiac events.
Association between coronary physiology and plaque vulnerability
The current standard decision-making for revascularization has been guided by invasive physiologic indexes as surrogates of coronary blood flow to define functional significant coronary stenosis. A recent meta-analysis demonstrated that FFR-guided PCI reduced the composite of cardiac death or MI compared with medical therapy alone14. In addition, the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) 2 trial demonstrated that FFR-guided strategy reduced the risk of spontaneous MI13. These results raised an important question as to how functionally significant lesions determined by physiologic indexes are related to high-risk plaques prone to cause ACS.
In fact, recent studies have shown that HRPC were closely related to FFR, and lesions with low FFR were likely to have more HRPC3,8,9,10,11,12. The current study also demonstrated that per-vessel FFR was inversely associated with the number of HRPC and the presence of quantitative and qualitative HRPC. There may be 2 potential mechanistic explanations for the relationship between FFR and HRPC. First, low FFR represents a high-pressure gradient across the lesion which can cause hemodynamic stress on a plaque, resulting in an adverse transformation of the plaque into more vulnerable form8,19,24,25. Among hemodynamic stress, wall shear stress (WSS) has been extensively studied. Park et al. demonstrated the significant association between lower FFR and higher WSS, which was also linked with the higher probability of CCTA-derived adverse plaque characteristics19. Second, the presence of HRPC could impair the local vasodilator capacity of the affected coronary segment via oxidative stress and inflammation, whereas that of an unaffected coronary segment is relatively preserved9. This differential vasodilatory capacity might induce a higher pressure gradient under maximal hyperemia and thereby decrease FFR.
Hyperemic and non-hyperemic physiologic indexes and high-risk plaque characteristics
Based on the 2 representative randomized clinical trials, non-hyperemic pressure ratios (NHPRs) such as iFR have been used in clinical practice as alternatives to FFR26,27. However, unlike FFR and its previously described association with HRPC, there is limited evidence regarding the relationship between NHPRs and plaque vulnerability. A recent study by Driessen et al. reported that there might be a difference between hyperemic and non-hyperemic physiologic indexes in their relationship with CCTA-derived HRPC10. In their study, CCTA-derived qualitative HRPC, especially positive remodeling and spotty calcification, were independently associated with FFR, but not with iFR10. One potential explanation suggested by the authors was that a positively remodeled coronary segment containing lipid-rich plaques might not be able to dilate as much as an unaffected segment during maximal hyperemia, which could result in more obvious pressure gradient across the lesion during hyperemia than at rest10. Therefore, FFR could be more sensitive to the presence of HRPC, especially lipid-rich necrotic core and positive remodeling, than iFR. These results suggested that the effect of hyperemia on pressure-derived physiologic indexes might depend not only on quantitative stenosis severity but also on qualitative plaque characteristics10. The results of the current study also showed that FFR was independently associated with qualitative HRPC after adjusting for quantitative HRPC, but iFR was not. Furthermore, FFR showed a greater discrimination ability to predict positive remodeling than iFR, which could further support the above hypothesis.
Interestingly, the current study showed that FFR also had a higher discrimination ability for the presence of ≥ 3 HRPC than iFR, which was mainly driven by a higher sensitivity of FFR for the presence of quantitative HRPC. These findings may be related to the prior observation on FFR being more sensitive to anatomical stenosis severity than iFR, likely due to an increased flow separation and pressure loss across the stenosis during hyperemia than at rest21. Although the association between coronary physiology and plaque vulnerability would be far more complex, the current study provides evidence for the potential difference between hyperemic and non-hyperemic physiologic indexes, and their relationship with HRPC. Nonetheless, clinical significance of these differences is not clear given clinical trials showing comparable outcomes between FFR- and iFR-guided revascularization strategies26,27 and relatively low risk among lesions with discordant FFR and iFR values28. Rather, the current study should be regarded as an effort to enhance our understanding of complex interplay between hemodynamic and anatomic plaque characteristics.
In addition, it should be noted that the current study showed neither FFR nor iFR could be a perfect predictor for the presence of qualitative HRPC. These results support the complementary role of invasive physiologic indexes and CCTA-defined HRPC for risk stratification of patients with coronary artery disease. Considering the solid evidence for prognostic implication of invasive physiologic indexes, future studies investigating the incremental value of CCTA-derived HRPC over invasive physiologic indexes for predicting clinical outcomes are warranted. Furthermore, a clinical trial (The Preventive Coronary Intervention on Stenosis with Functionally Insignificant Vulnerable Plaque [PREVENT]; NCT02316886) is underway to test whether treating hemodynamically insignificant lesions with HRPC would provide any benefits, which may confirm or refute potential role of combining these complementary tools in the treatment of patients with coronary artery disease29.
The current study has several limitations. First, since it was conducted as a post hoc analysis, the influence of selection bias could not be fully excluded. Second, prognostic impact of the differential association between FFR and iFR for the presence of CCTA-derived HRPC is beyond the scope of the current study. Further study with clinical outcome is warranted. Third, other reference modalities to assess plaque characteristics, such as intravascular ultrasound or optical coherence tomography, were not systematically performed. Fourth, most patients included in the current study had stable angina. Therefore, our results could not be applied to non-culprit lesions in patients with ACS. Fifth, reproducibility of CCTA analysis was not specifically assessed in the current study, although it was done at the same core laboratory participated in the prior study30. Sixth, although physiologic disease pattern (focal vs. diffuse) can affect the discordance between FFR and iFR, it was not able to be considered in current study due to lack of pullback information.
In conclusion, both FFR and iFR were significantly associated with CCTA-derived HRPC. Compared with iFR, FFR was independently associated with the presence of qualitative HRPC and showed a higher predictive ability for the presence of ≥ 3 HRPC due primarily to a greater ability to predict quantitative HRPC. Further research is warranted to investigate the potentially incremental prognostic value of these observations.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- CCTA:
-
Coronary computed tomography angiography
- FFR:
-
Fractional flow reserve
- HRPC:
-
High risk plaque characteristics
- iFR:
-
Instantaneous wave-free ratio
- NHPR:
-
Non-hyperemic pressure ratio
References
Neumann, F. J. et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165. https://doi.org/10.1093/eurheartj/ehy394 (2019).
Virmani, R., Burke, A. P., Farb, A. & Kolodgie, F. D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 47, C13-18. https://doi.org/10.1016/j.jacc.2005.10.065 (2006).
Park, H. B. et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: A direct comparison to fractional flow reserve. JACC Cardiovasc. Imaging 8, 1–10. https://doi.org/10.1016/j.jcmg.2014.11.002 (2015).
Stone, G. W. et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364, 226–235. https://doi.org/10.1056/NEJMoa1002358 (2011).
Cheng, J. M. et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur. Heart J. 35, 639–647. https://doi.org/10.1093/eurheartj/eht484 (2014).
Motoyama, S. et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J. Am. Coll. Cardiol. 66, 337–346. https://doi.org/10.1016/j.jacc.2015.05.069 (2015).
Puchner, S. B. et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 64, 684–692. https://doi.org/10.1016/j.jacc.2014.05.039 (2014).
Lee, J. M. et al. Prognostic implications of plaque characteristics and stenosis severity in patients with coronary artery disease. J. Am. Coll. Cardiol. 73, 2413–2424. https://doi.org/10.1016/j.jacc.2019.02.060 (2019).
Ahmadi, A. et al. Lesion-specific and vessel-related determinants of fractional flow reserve beyond coronary artery stenosis. JACC Cardiovasc. Imaging 11, 521–530. https://doi.org/10.1016/j.jcmg.2017.11.020 (2018).
Driessen, R. S. et al. Adverse plaque characteristics relate more strongly with hyperemic fractional flow reserve and instantaneous wave-free ratio than with resting instantaneous wave-free ratio. JACC Cardiovasc. Imaging 13, 746–756. https://doi.org/10.1016/j.jcmg.2019.06.013 (2020).
Gaur, S. et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur. Heart J. 37, 1220–1227. https://doi.org/10.1093/eurheartj/ehv690 (2016).
Driessen, R. S. et al. Effect of plaque burden and morphology on myocardial blood flow and fractional flow reserve. J. Am. Coll. Cardiol. 71, 499–509. https://doi.org/10.1016/j.jacc.2017.11.054 (2018).
Xaplanteris, P. et al. Five-year outcomes with PCI guided by fractional flow reserve. N. Engl. J. Med. 379, 250–259. https://doi.org/10.1056/NEJMoa1803538 (2018).
Zimmermann, F. M. et al. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: Meta-analysis of individual patient data. Eur. Heart J. 40, 180–186. https://doi.org/10.1093/eurheartj/ehy812 (2019).
Petraco, R. et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: Results of the JUSTIFY-CFR study (Joined coronary pressure and flow analysis to determine diagnostic characteristics of basal and hyperemic indices of functional lesion severity-coronary flow reserve). Circ. Cardiovasc. Interv. 7, 492–502. https://doi.org/10.1161/CIRCINTERVENTIONS.113.000926 (2014).
Siasos, G. et al. Local low shear stress and endothelial dysfunction in patients with nonobstructive coronary atherosclerosis. J. Am. Coll. Cardiol. 71, 2092–2102. https://doi.org/10.1016/j.jacc.2018.02.073 (2018).
Taylor, A. J. et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation appropriate use criteria task force, the society of cardiovascular computed tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 56, 1864–1894. https://doi.org/10.1016/j.jacc.2010.07.005 (2010).
Maurovich-Horvat, P., Ferencik, M., Voros, S., Merkely, B. & Hoffmann, U. Comprehensive plaque assessment by coronary CT angiography. Nat. Rev. Cardiol. 11, 390–402. https://doi.org/10.1038/nrcardio.2014.60 (2014).
Park, J. B. et al. Computational fluid dynamic measures of wall shear stress are related to coronary lesion characteristics. Heart 102, 1655–1661. https://doi.org/10.1136/heartjnl-2016-309299 (2016).
Lee, J. M. et al. Identification of high-risk plaques destined to cause acute coronary syndrome using coronary computed tomographic angiography and computational fluid dynamics. JACC. Cardiovasc. Imaging https://doi.org/10.1016/j.jcmg.2018.01.023 (2018).
Lee, J. M. et al. Exploring coronary circulatory response to stenosis and its association with invasive physiologic indexes using absolute myocardial blood flow and coronary pressure. Circulation 136, 1798–1808. https://doi.org/10.1161/CIRCULATIONAHA.117.029911 (2017).
Lee, J. M. et al. Physiological and clinical assessment of resting physiological indexes. Circulation 139, 889–900. https://doi.org/10.1161/CIRCULATIONAHA.118.037021 (2019).
Calvert, P. A. et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: The VIVA (VH-IVUS in vulnerable atherosclerosis) study. JACC. Cardiovasc. Imaging 4, 894–901. https://doi.org/10.1016/j.jcmg.2011.05.005 (2011).
Kumar, A. et al. High coronary shear stress in patients with coronary artery disease predicts myocardial infarction. J. Am. Coll. Cardiol. 72, 1926–1935. https://doi.org/10.1016/j.jacc.2018.07.075 (2018).
Choi, G. et al. Coronary artery axial plaque stress and its relationship with lesion geometry: Application of computational fluid dynamics to coronary CT angiography. JACC Cardiovasc. Imaging 8, 1156–1166. https://doi.org/10.1016/j.jcmg.2015.04.024 (2015).
Davies, J. E. et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N. Engl. J. Med. 376, 1824–1834. https://doi.org/10.1056/NEJMoa1700445 (2017).
Gotberg, M. et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N. Engl. J. Med. 376, 1813–1823. https://doi.org/10.1056/NEJMoa1616540 (2017).
Lee, J. M. et al. Clinical outcomes according to fractional flow reserve or instantaneous wave-free ratio in deferred lesions. JACC Cardiovasc. Interv. 10, 2502–2510. https://doi.org/10.1016/j.jcin.2017.07.019 (2017).
Ahn, J. M. et al. Preventive PCI or medical therapy alone for vulnerable atherosclerotic coronary plaque: Rationale and design of the randomized, controlled PREVENT trial. Am. Heart J. 264, 83–96. https://doi.org/10.1016/j.ahj.2023.05.017 (2023).
Lee, J. M. et al. Identification of high-risk plaques destined to cause acute coronary syndrome using coronary computed tomographic angiography and computational fluid dynamics. JACC Cardiovasc. Imaging 12, 1032–1043. https://doi.org/10.1016/j.jcmg.2018.01.023 (2019).
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: D.S., J.M.L., H.S.; data collection: D.S., J.M.L., S.H.L., K.H.C., S.M.K., E.J.C., K.Y.L., D.H., S.G.A., A.J.B., H.M.-R., A.L., D.M., K.C., T.K., J.E., H.S.; analysis and interpretation of results: D.S., J.M.L., H.S.; draft manuscript preparation: D.S., J.M.L., H.S.; critically revising it for important intellectual contents: D.S., J.M.L., S.H.L., K.H.C., S.M.K., E.J.C., K.Y.L., D.H., S.G.A., A.J.B., H.M.-R., A.L., D.M., K.C., T.K., J.E., H.S. All authors reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Dr. Joo Myung Lee received an Institutional Research Grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. Dr. Samady received research funding from Philips Volcano, St. Jude Medical (Abbott Vascular), and Medtronic, Inc.; is a consultant for Philips Volcano, Abbott Vascular; and is co-founder and equity holder in Covanos Inc. All other authors declare that there is no conflict of interest relevant to the submitted work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J.M., Shin, D., Lee, S.H. et al. Differential predictability for high-risk plaque characteristics between fractional flow reserve and instantaneous wave-free ratio. Sci Rep 13, 16005 (2023). https://doi.org/10.1038/s41598-023-43352-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43352-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.