Abstract
18F-FP-CIT is a high-resolution imaging marker of nigrostriatal neuronal integrity, differentiating Parkinsonism with loss of dopaminergic terminals (presynaptic Parkinsonian syndrome [PS]) from Parkinsonism without nigrostriatal degeneration (non-PS). We assessed the diagnostic accuracy of 18F-FP-CIT PET in patients with clinically uncertain PS (CUPS) at the first visit. Among the 272 patients who underwent 18F-FP-CIT PET imaging at the first visit between September 2008 and July 2012, 111 had CUPS (age, 62.6 ± 10.5 y; male:female, 45:66; symptom duration, 13.1 ± 8.8 months). Uncertainty criteria included only one of the three cardinal signs of Parkinsonism, two signs without bradykinesia, or atypical signs. The baseline clinical and 18F-FP-CIT PET imaging diagnostic accuracy was compared with the accuracy of clinical diagnosis after > 2-year follow-up. Nuclear medicine physicians assessed the 18F-FP-CIT PET images visually. Focal dopamine transporter binding deficit in the posterior putamen was considered PS. Bilateral symmetric striatum without focal deficit, suggesting normal 18F-FP-CIT PET, and focal deficits elsewhere in the striatum suggesting vascular Parkinsonism were considered non-PS. Seventy-nine patients had PS, and 32 did not. Baseline clinical diagnosis included PS in 45 patients, non-PS in 24, and inconclusive in 42. Among patients in whom initial clinical diagnosis (PS or non-PS) was possible, the sensitivity, specificity, and accuracy of the baseline clinical and 18F-FP-CIT PET imaging diagnoses were 54.4, 50.0, and 53.2%, and 98.7, 100, and 99.1%, respectively. The respective positive and negative predictive values were 95.6 and 66.7%, and 100 and 97.0%. Among those with initially inconclusive diagnosis, 64.2% were eventually diagnosed with PS while 35.7% were diagnosed with non-PS. The final clinical diagnosis of these patients all matched those made by 18F-FP-CIT PET imaging, except in one patient with scan without evidence of dopaminergic deficit (SWEDD). 18F-FP-CIT PET diagnosis was more accurate than clinical diagnosis, reducing the false-negative and inconclusive clinical diagnosis rates at baseline in patients with CUPS.
Similar content being viewed by others
Introduction
Parkinsonian syndrome is a group of diseases characterized by signs of Parkinsonism, including bradykinesia, rigidity, tremor, and postural instability1. Idiopathic Parkinson’s disease (IPD) is the most common cause of Parkinsonism, but Parkinsonism has several other etiologies as well. It could be present in all alpha synucleinopathies, including Lewy body diseases, IPD, and multiple system atrophy2, and tauopathies, including corticobasal degeneration and progressive supranuclear palsy, which were defined as atypical Parkinsonisms3,4. The differential diagnoses of neurodegenerative Parkinsonian syndrome include essential tremor (ET), vascular Parkinsonism, drug-induced Parkinsonism, and psychogenic Parkinsonism5. ET mainly affects voluntary movements rather than rest. Resting tremor, cogwheel rigidity, and other Parkinsonian characteristics can be present in a subgroup of patients with ET, making the clinical diagnosis a challenge6.
Dopamine is a neurotransmitter with a vital role in movement regulation in the brain. Therefore, disruption of nigrostriatal pathway leads to a loss of dopamine and cause Parkinsonism. For example, IPD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta, leading to dopamine depletion in the striatum7. The dopamine transporter (DAT) controls the duration and intensity of dopaminergic neurotransmission by reuptake of dopamine into presynaptic terminals8 and is used in the imaging of presynaptic dopaminergic neuronal distribution for presynaptic Parkinsonian syndrome (PS) diagnosis.
Several DAT tracers for SPECT and PET, including 123I-FP-CIT, 123I-β-CIT, 99mTc-TRODAT-1, and 18F-FP-CIT, are commercially available in the USA, Europe, Japan, Taiwan, and Korea5,9. Due to its high resolution and favorable kinetics, 18F-FP-CIT PET could achieve high accuracy in diagnosing Parkinsonism and in Parkinsonism differential diagnosis10,11,12.
This accuracy could help diagnose IPD in patients with clinically uncertain Parkinsonian syndrome (CUPS) when the clinical diagnosis is challenging due to the unclear characteristic symptoms of IPD that lead to some inconclusive diagnoses. This study investigated the diagnostic accuracy of 18F-FP-CIT PET in patients with CUPS when clinical diagnosis proves challenging.
Materials and methods
Subjects
We retrospectively selected patients with CUPS who underwent 18F-FP-CIT PET imaging as initial work-up for Parkinsonism during their first visit between September 2008 and July 2012 at our medical center. Uncertainty criteria included only one of the three cardinal signs of Parkinsonism, two signs without bradykinesia, or atypical signs13. Symptom duration was under three years, and follow-up was over two years in all patients by movement specialist. Patients with known causes of tremors such as hyperthyroidism, or with significant cognitive impairment with a minimal mental status score of ≤ 24 were excluded. The Institutional Review Board (IRB) of Asan Medical Center approved this study and informed consent was waived because of the retrospective nature of this study (IRB no. 2014–0688).
Radiopharmaceutical synthesis
18F-FP-CIT was synthesized using a protic solvent (t-butanol or t-amyl alcohol) as a reaction solvent and N-[3′-(tosyloxy)propyl]-2β-carbomethoxy-3β-(4′-iodophenyl)nortropane as a precursor14. The decay-corrected radiochemical yield was 42.5% ± 10.9%; the radiochemical purity after purification with high-performance liquid chromatography was > 98%; the specific activity at the end of synthesis was 64.4 ± 4.5 GBq/μmol.
18F-FP-CIT PET/CT imaging
All 18F-FP-CIT PET images were acquired for ten minutes after three hours after intravenous administration of 185 MBq of 18F-FP-CIT using a Biograph Truepoint 40 PET/CT scanner (Siemens Medical Systems, USA). The medications that may influence DAT binding including benzatropine, D-amphetamine, methylphenidate were stopped before the 18F-FP-CIT PET/CT scanning. PET images were achieved in a 3-dimensional mode immediately after performing a brain computed tomographic scan for image fusion and attenuation correction. PET images were reconstructed with a TrueX algorithm and an all-pass filter using a 336 × 336 matrix.
Visual analysis of 18F-FP-CIT PET/CT
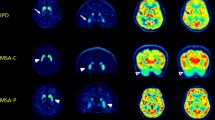
All PET images were visually assessed by two board-certified nuclear medicine physicians with 15 and two years of clinical experience in nuclear medicine who were blinded to all clinical and diagnostic information (masked for review) working in consensus. 18F-FP-CIT PET image visual assessment was classified as PS when showing DAT loss in unilateral posterior putamen, unilateral putamen, bilateral putamen, or putamen and caudate nuclei. It was defined as non-presynaptic PS (non-PS) when showing no significant DAT loss in the striatum or focal DAT loss in areas other than the posterior putamen (Fig. 1).
Visual interpretation criteria for (A) non-presynaptic Parkinsonian syndrome (non-PS) and (B) presynaptic Parkinsonian syndrome (PS). Patients with non-PS showed no significant DAT loss in the striatum or focal DAT loss in areas other than the posterior putamen. Patients with PS showed DAT loss in unilateral posterior putamen, unilateral putamen, bilateral putamen, or putamen and caudate nuclei.
Quantitative analysis of 18F-FP-CIT PET/CT
Image processing was performed with Statistical Parametric Mapping (Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, London, UK) in MATLAB R2013a for Windows (The MathWorks Inc.) and MRIcro, Version 1.40 (Chris Rorden, Columbia, SC; http://www.mccauslandcenter.sc.edu/crnl/). All reconstructed PET images were spatially normalized to Talairach space by a standard 18F-FP-CIT PET template10,15,16.
Quantitative analysis was performed as described previously12 based on 12 volume-of-interest (VOI) templates of bilateral striatal subregions (ventral striatum, anterior caudate, posterior caudate, anterior putamen, posterior putamen, and ventral putamen) and one template of the occipital subregion. It was adjusted manually using VOI editing software (ANTIQUE; Asan Medical Center, Seoul, Korea)16.
The activity level in each VOI was calculated and the specific to nonspecific binding ratio (SNBR) was defined as: [(mean standardized uptake value of the striatal subregional VOI − mean standardized uptake value of the occipital VOI)/mean standardized uptake value of the occipital VOI]. We used a normal database for comparison, as previously described10.
Statistical analysis
The accuracies of baseline clinical (without 18F-FP-CIT PET) and 18F-FP-CIT PET imaging diagnoses were compared, using the final clinical diagnosis made after over two years of follow-up by movement specialist as the reference standard. This specialist meticulously monitored each patient every 3–6 months for over two years for any symptom development that deviated from established diagnostic criteria.The baseline clinical diagnosis was classified as PS, non-PS, or inconclusive. The baseline 18F-FP-CIT PET and final clinical diagnoses were classified as PS or non-PS. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA).
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board (IRB) of Asan Medical Center and with the principles of the1964 Declaration of Helsinki and its later amendments.
Results
Demographic and clinical characteristics
Among the 272 patients who underwent 18F-FP-CIT PET imaging at the first visit, 111 had CUPS (age, 62.6 ± 10.5 y; 45 male and 66 female patients; symptom duration, 13.1 ± 8.8 months). Their demographic and clinical characteristics are presented in Table 1. The most common reasons for classifying as CUPS were showing only one of the three cardinal signs of Parkinsonism (n = 68, 61.2%), followed by atypical signs (n = 36, 32.4%) including postural tremor rather than rest tremor (n = 18, 16.2%) and mild rigidity (n = 15, 13.5%).
Initial clinical diagnosis and visual 18F-FP-CIT PET image interpretation
Among the 111 patients with CUPS, 45 (40.5%) were classified clinically as having PS, 24 (21.6%) as having non-PS, and 42 (37.8%) with inconclusive diagnosis (Table 2). Among the 45 patients classified clinically as having PS, 43 (95.6%) were classified as having PS via 18F-FP-CIT PET and two (4.4%) as having non-PS. The parallel values for the 24 patients clinically classified as having non-PS were 8 (33.3%) and 16 (66.7%) and were 27 (70.3%) and 15 (29.7%) for the 42 patients with inconclusive diagnosis at baseline.
Comparison of baseline clinical diagnosis and visual 18F-FP-CIT PET interpretation to the final clinical diagnosis
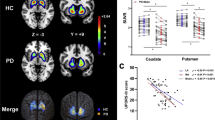
The final clinical diagnosis was PS for 43/45 patients classified as having PS based on the baseline clinical diagnosis, 8/24 classified as having non-PS, and 28/42 whose diagnosis was inconclusive. Among patients in whom initial clinical diagnosis (PS or non-PS) was possible, when compared to the final diagnosis, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value for baseline clinical diagnosis were 54.4, 50.0, 53.2, 95.6, and 66.7%, respectively (Fig. 2 and Table 3). Among those with initially inconclusive diagnosis, 64.2% were eventually diagnosed with PS while 35.7% were diagnosed with non-PS.
Comparison of the (A) initial clinical diagnosis and (B) visual 18F-FP-CIT PET interpretation to the final clinical diagnosis in patients with presynaptic Parkinsonian syndrome. Comparison of the initial clinical diagnosis to the visual 18F-FP-CIT PET interpretation (C). PS presynaptic Parkinsonian syndrome, Non-PS non-presynaptic Parkinsonian syndrome.
All patients classified as having non-PS via 18F-FP-CIT PET were also classified as having non-PS based on the final clinical diagnosis. Among 33 patients classified as having PS using the initial clinical diagnosis, one was classified as having PS and 32 as having non-PS based on the final clinical diagnosis. 18F-FP-CIT PET diagnosis had a sensitivity of 98.7%, a specificity of 100.0%, a positive predictive value of 100.0%, a negative predictive value of 97.0%, and an accuracy of 99.1%. One patient (0.9%) received a false-negative diagnosis, and none received a false-positive diagnosis. The scans of the 76-year-old male patient with a false-negative diagnosis showed no evidence of dopaminergic deficit. The patient started showing gait disturbance with mild rigidity and bradykinesia one year before the examination. The patient’s tremor was postural rather than at rest. Initially, the patient was considered to have vascular Parkinsonism due to a mild periventricular leukoaraiosis in the white matter observed using brain magnetic resonance imaging. While the 18F-FP-CIT PET imaging was normal, the patient’s Parkinsonism progressed during follow-up. Therefore, this patient was considered to present scans without evidence of dopaminergic deficit (SWEDD).
Quantitative analysis of 18F-FP-CIT PET/CT images
The putamen SNBR for healthy controls (6.91 ± 1.32) was larger than that for patients with PS (2.78 ± 1.20; p < 0.001) and non-PS (6.21 ± 1.09; p = 0.027) based on visual 18F-FP-CIT PET image interpretations. The posterior putamen SNBR for healthy controls (7.26 ± 1.31) was larger than that for patients with PS (2.06 ± 1.10; p < 0.001) and non-PS (6.00 ± 1.07; p < 0.001) (Fig. 3).
Discussion
This study investigated the diagnostic accuracy of baseline clinical 18F-FP-CIT PET image assessments in patients with CUPS, showing a higher accuracy for 18F-FP-CIT PET in differentiating PS from non-PS and some SWEDDs. Therefore, 18F-FP-CIT PET can be useful in reducing the rates of false-negative and inconclusive clinical diagnoses at baseline in patients with CUPS.
IPD diagnosis is still largely based on identifying its clinical features correctly. A correct diagnosis depends upon the clinical interpretation of the characteristic asymmetrical Parkinsonism responsive to anti-Parkinson therapy17. The clinical diagnosis of Parkinsonism is relatively straightforward in many patients18. However, diagnosis is challenging in cases with CUPS because the characteristic symptoms of IPD are unclear, resulting in a high portion of clinically inconclusive diagnoses13 and, according to a European multicenter study with repeat DAT SPECT, over-diagnoses17 at baseline. The diagnosis of approximately one-third of the patient with CUPS in this study was considered inconclusive at the initial clinical diagnosis stage. A prospective study supported some of the clinical difficulties in accurately diagnosing the underlying pathology in the early cases of Parkinsonian syndrome19. Recent clinicopathologic studies have shown that the diagnostic accuracy of IPD remains relatively low and one fourth of diagnoses are incorrect despite improvements in the diagnostic methods and the development of diagnostic clinical criteria20.
DAT SPECT is a widely used, cost-effective imaging technique that helps differentiate non-PS, such as ET, from PSs related to PD, multiple system atrophy, and progressive supranuclear palsy6,21,22. A previous study reported that the initial diagnosis was changed in 54% of patients with CUPS after DAT SPECT13. Among the initial clinical diagnoses in this study, two of the 45 patients initially classified as having PS were reclassified as having non-PS (4.4%), eight of the 24 patients classified as having non-PS were reclassified as having PS (33.3%), and the 42 patients classified as having inconclusive diagnosis were reclassified as having PS (n = 27, 64.2%) or non-PS (n = 15, 35.7%). Overall, the initial clinical diagnosis was changed after 18F-FP-CIT PET in 47% of the patients (n = 52). Both DAT SPECT and 18F-FP-CIT PET results had a higher agreement at the final diagnosis than at the baseline clinical diagnosis.
The higher proportion of SWEDDs in previous studies puts in doubt the diagnostic accuracy of DAT SPECT. The term SWEDD refers to the absence of an imaging abnormality in patients clinically presumed to have IPD23. The SWEDD frequency in some drug trials and imaging studies on IPD ranged between 3.6 and 19.6%24,25,26,27. A SWEDD rate of about 16% was reported in the Parkinson's Progression Markers Initiative (PPMI), showing similar visual and quantitative characteristics to those of healthy controls28. Researchers disagreed on whether SWEDD suggested different PD lookalike disorders or a benign subtype of PD; however, several longitudinal studies suggested that patients with SWEDD do not have early PD and show minimal clinical or imaging evidence of PD progression29,30,31.
Several longitudinal studies suggested a ceiling effect in DAT SPECT imaging, as the change rate shows little difference over time32. These patients initially showed low DAT levels in the nigrostriatal pathways, which further decreased over time, reaching a level at which Parkinsonism was detectable. Thus, statistical “floor” and “ceiling” DAT binding effects must be considered when employing imaging as an outcome measure in clinical trials on IPD. These results cast doubt on the sensitivity of DAT SPECT in detecting early-stage IPD.
Only 0.9% of the patients in this 18F-FP-CIT PET study presented with SWEDD after a 24-month follow-up, suggesting the suitability of 18F-FP-CIT PET as a biomarker for early IPD detection and disease monitoring33,34. A previous head-to-head comparative study of 18F-FP-CIT PET and 123I-FP-CIT SPECT found no difference between the methods in visual diagnostic accuracy35. Both showed high accuracy in differentiating between Parkinsonism and ET, with a sensitivity of 95–97% and 100% and a specificity of 93–100% and 97% in DAT SPECT and 18F-FP-CIT PET, respectively36,37. However, a semi-quantitative analysis indicated that 18F-FP-CIT PET had better contrast than 123I-FP-CIT SPECT35. This finding suggested that 18F-FP-CIT PET could help make more accurate decisions in equivocal cases than 123F-FP-CIT SPECT.
The lack of a definitive post-mortem diagnostic validation is a limitation of this study. Therefore, a follow-up period of 24 months had to be used to confirm the diagnosis. A quantitative analysis would be needed to support our results.
In conclusion, 18F-FP-CIT PET imaging was more accurate than a clinical diagnosis in distinguishing PS from non-PS, with a low false-negative rate. Therefore, 18F-FP-CIT PET imaging would be useful in reducing the false-negative and inconclusive clinical diagnosis rates at baseline in patients with CUPS.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 79, 368–376 (2008).
MartÌ, M. J., Tolosa, E. & Campdelacreu, J. Clinical overview of the synucleinopathies. Mov. Disord. 18, 21–27 (2003).
Armstrong, M. J. et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503 (2013).
Höglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 32, 853–864 (2017).
Park, E. A new era of clinical dopamine transporter imaging using 123I-FP-CIT. J. Nucl. Med. Technol. 40, 222–228 (2012).
Morbelli, S. et al. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur. J. Nucl. Med. Mol. Imaging 47, 1885–1912 (2020).
Dauer, W. & Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 39, 889–909 (2003).
Giros, B. & Caron, M. G. Molecular characterization of the dopamine transporter. Trends Pharmacol. Sci. 14, 43–49 (1993).
FDA approved drug products. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022454.
Oh, M. et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J. Nucl. Med. 53, 399–406 (2012).
Oh, M. et al. Diagnostic accuracy of dual-phase 18F-FP-CIT PET imaging for detection and differential diagnosis of Parkinsonism. Sci. Rep. 11, 1–8 (2021).
Han, S. et al. Subregional pattern of striatal dopamine transporter loss on 18F FP-CIT positron emission tomography in patients with pure akinesia with gait freezing. JAMA Neurol. 73, 1477–1484 (2016).
Catafau, A. M. & Tolosa, E. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov. Disord. 19, 1175–1182 (2004).
Lee, S. J. et al. One-step high-radiochemical-yield synthesis of [18F]FP-CIT using a protic solvent system. Nucl. Med. Biol. 34, 345–351 (2007).
Seo, M. et al. The effect of SSRIs on the binding of (18)F-FP-CIT in Parkinson patients: A retrospective case control study. Nucl. Med. Mol. Imaging 48, 287–294 (2014).
Kim, H. W. et al. Different loss of dopamine transporter according to subtype of multiple system atrophy. Eur. J. Nucl. Med. Mol. Imaging 43, 517–525 (2016).
Marshall, V. L. et al. Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: A 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov. Disord. 24, 500–508 (2009).
Berardelli, A. et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease. Eur. J. Neurol. 20, 16–34 (2013).
Rajput, A., Rozdilsky, B. & Rajput, A. Accuracy of clinical diagnosis in parkinsonism—A prospective study. Can. J. Neurol. Sci. 18, 275–278 (1991).
Joutsa, J., Gardberg, M., Roytta, M. & Kaasinen, V. Diagnostic accuracy of Parkinsonism syndromes by general neurologists. Mov. Disord. 29, S111–S111 (2014).
Van Laere, K. et al. The cost effectiveness of 123 I-FP-CIT SPECT imaging in patients with an uncertain clinical diagnosis of parkinsonism. Eur. J. Nucl. Med. Mol. Imaging 35, 1367–1376 (2008).
Antonini, A. et al. Cost-effectiveness of 123I-FP-CIT SPECT in the differential diagnosis of essential tremor and Parkinson’s disease in Italy. Mov. Disord. 23, 2202–2209 (2008).
Marek, K., Jennings, D. & Seibyl, J. Imaging the dopamine system to assess disease-modifying drugs: Studies comparing dopamine agonists and levodopa. Neurology 61, S43–S48 (2003).
Walton-Hadlock, J. L. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 352, 1386 (2005).
Jennings, D., Tabamo, R., Seibyl, J. & Marek, K. InSPECT: Investigating the effect of short-term treatment with pramipexole or levodopa on [1231] beta-CIT and SPECT imaging. In Movement Disorders S143–S144 (Wiley, 2006).
Group, P. S. & Group, P. S. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. Jama 287, 1653–1661 (2002).
Benamer, H. T. et al. Prospective study of presynaptic dopaminergic imaging in patients with mild parkinsonism and tremor disorders: part 1. Baseline and 3-month observations. Mov. Disord. 18, 977–984 (2003).
Marek, K. et al. The Parkinson’s progression markers initiative (PPMI)–establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol. 5, 1460–1477 (2018).
Lee, J. W., Song, Y. S., Kim, H., Ku, B. D. & Lee, W. W. Patients with scans without evidence of dopaminergic deficit (SWEDD) do not have early Parkinson’s disease: Analysis of the PPMI data. PLoS ONE 16, e0246881 (2021).
Marshall, V. L., Patterson, J., Hadley, D. M., Grosset, K. A. & Grosset, D. G. Two-year follow-up in 150 consecutive cases with normal dopamine transporter imaging. Nucl. Med. Commun. 27, 933–937 (2006).
Marek, K. et al. Longitudinal follow-up of SWEDD subjects in the PRECEPT study. Neurology 82, 1791–1797 (2014).
Kaasinen, V. & Vahlberg, T. Striatal dopamine in Parkinson disease: A meta-analysis of imaging studies. Ann. Neurol. 82, 873–882 (2017).
Sung, C. et al. Longitudinal decline of striatal subregional [18 F] FP-CIT uptake in Parkinson’s disease. Nucl. Med. Mol. Imaging 51, 304–313 (2017).
Son, H. J. et al. Test–retest reproducibility of dopamine transporter density measured with [18 F] FP-CIT PET in patients with essential tremor and Parkinson’s disease. Ann. Nucl. Med. 35, 299–306 (2021).
Lee, I. et al. Head-to-head comparison of 18F-FP-CIT and 123I-FP-CIT for dopamine transporter imaging in patients with Parkinson’s disease: A preliminary study. Synapse 72, e22032 (2018).
Benamer, H. T. et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov. Disord. 15, 503–510 (2000).
Efficacy and Safety of F-18 FPCIT PET in Parkinson's Disease and Essential Tremor Patients (FPCIT) https://clinicaltrials.gov/ct2/show/NCT00468078.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0016 and HU22C0031).
Author information
Authors and Affiliations
Contributions
M.O. and J.S.K. were involved in conception and design of the study. M.O., S.J.L., J.S.O. and S.J.O. collected and analyzed the data. S.J.C. provided clinical supervision and consultation. All authors reviewed and approved the final manuscript. J.S.K. was the principal investigator.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oh, M., Oh, S.J., Lee, S.J. et al. Diagnostic accuracy of 18F-FP-CIT PET for clinically uncertain Parkinsonian syndrome. Sci Rep 13, 15069 (2023). https://doi.org/10.1038/s41598-023-42135-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42135-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.