Abstract
Forty-six promising chitinolytic isolates were recovered during a screening for chitinolytic bacteria in the environment of Saudi Arabia. The top three isolates belonged to the genus Streptomyces. Streptomyces variabilis Am1 was able to excrete the highest amount of chitinases, reaching the maximum at 84 h with 0.5% yeast extract and nitrogen source and 2% galactose as a carbon source. Purification of chitinase by DEAE-Cellulose and Sephadex G75 improved the specific activity to 18.6-fold and the recovery to 23.8% and showed a mass at 56 kDa. The optimal catalysis of the purified chitinase was at 40 °C and pH 8 with high thermostability and pH stability as reflected by a midpoint temperature value of 66.6 °C and stability at pH 4–9. The protein reagents SDS, EDTA, and EGTA significantly inhibited the enzyme and the EDTA-chelated chitinase restored its activity after the addition of Fe2+ ions suggesting a metallo-chitinase type with ferric ions as cofactors. Chitinase exerted high antifungal activity against some phytopathogenic fungi. Interestingly, the tested Streptomyces were able to produce chitosan nanocubes along with chitosan from chitin degradation which may be an additional power in their antifungal activity in nature. This work also reveals the importance of unexplored environments as a pool of promising microorganisms with biotechnological applications.
Similar content being viewed by others
Introduction
One of the richest biopolymers found in the world is chitin. It consists of N-acetyl D-glucosamines (NAG) connected by β-1,4-glycosidic linkages. It supports the outer protective layer of many organisms including insects, fungi, and other crustaceans1. Additionally, eggshells of nematodes such as Globodera rostochiensis and Meloidogyne javanica are composed of 9 and 30% of chitin, respectively2. Chitin protects these pathogenic, parasitic, and disease-causing organisms, from extreme environmental conditions and the host's defensive mechanisms, therefore, facilitating parasitism and the spread of these organisms to other hosts and other localities. Regarding phytopathogenic fungi, crop losses could range up to 5–25% in developed countries whereas in undeveloped countries crop losses may reach 20–50%3.
Chitinases are those enzymes breaking down chitin by hydrolyzing β-1,4-glycosidic bonds converting it to chitooligosaccharide (COS), further action of chitobiases leading to NAG. Depending on their activity, chitinases can be categorized into exochitinases and endochitinases subgroups4. In general, chitinases have a molecular mass of 20–120 kDa5. They are within the glycoside hydrolase groups (GH) GH18 and GH19. Mammals, fungi, and bacteria are observed to accommodate chitinases in the GH18 family. Whereas some bacteria and higher plants contain chitinases in the GH19 family6.
Interestingly, chitinases are produced by all arthropods including pathogenic and non-pathogenic species to play a significant role in the ecdysis or molting process of the current cuticle and helps in the production of newer one. Additionally, they are present in the salivary glands of several insects to be used in the breakdown of the host cuticle7. Also, several fungi can produce chitinases to be utilized for their nutrition, morphogenesis, development, and as a defense mechanism against other organisms containing chitin. These species include Saccharomyces cerevisiae and some filamentous fungi such as Trichoderma sp., Penicillium sp., Lecanicillium sp., Aspergillus sp., Stachybotrys sp. and Agaricus sp. Moreover, some nematodes such as Caenorhabditis elegans produce chitinase as a form of defense against surrounding competitive species2.
Chemical insecticides, fungicides, and nematicides are the main tools for managing these pathogens. However, because of their detrimental impact on animals and humans, and the environment, novel techniques are being introduced. One of the most important strategies is to target these pathogens with chitinases produced by bacteria such as Bacillus, Pseudomonas, Enterobacter, Serratia, and Streptomyces8.
Genus Streptomyces is a common producer of several hydrolytic enzymes including chitinases. Chitinases were successfully produced from Streptomyces venezuelae9, Streptomyces halstedii10, Streptomyces viridificans11, Streptomyces aureofaciens12, Streptomyces sp. ANU627713, Streptomyces sp. M-2014, Streptomyces sp. DA1115, and Streptomyces sp. S-8416. Concerning Streptomyces mutabilis, only two strains were preliminarily checked before for chitinase productivity along with other metabolites while studying several actinobacterial isolates from the Algerian Sahara17 and endophytic actinobacteria from the native plant roots in France18. Furthermore, chitinase enzyme hasn’t been fully characterized in Streptomyces mutabilis once before.
The production of chitinases from these microorganisms is not only employed in the biological control of pests but also utilized for various purposes including biomedical, industrial, and environmental applications. Additionally, providing aid in cosmetics, and flavor enhancement. For example, chitinous wastes from the food industry such as shrimp and crab shells are degraded using chitinases to accomplish high-quality food and feed products such as COS19.
Chitinases as a biocontrol agent against phytopathogenic fungi, successfully inhibited many disease-causing fungi such as Pestalotia theae, Rhizoctonia solani, Botrytis cinerea, and Bipolaris oryzae, thereby displaying antimicrobial activity20,21. The current study covers the green production of chitinase from bacterial sources such as Streptomyces variabilis as a potential tool against crop-threatening phytopathogenic fungi. According to our information, this is the first research on enzyme characterization of Streptomyces variabilis chitinase.
Results
Screening of isolates for their chitinolytic activity
During a screening for chitinolytic microorganisms in the terrestrial environment of Saudi Arabia, we obtained more than forty-six promising isolates. The chitinolytic activity on chitin agar was confirmed in 21.2% of isolates. The highest number of chitinolytic isolates (15%) was recorded for samples collected from the rhizosphere region of natural plants growing in the Nafud desert, Saudi Arabia. The top three isolates Nof21, Am1, and Bat2 were recovered from the root region of wild plants growing in the Great Nafud desert, Dahna desert, and North Shaqra desert of Saudi Arabia, respectively. Collection of soil samples were done from September to December 2021.
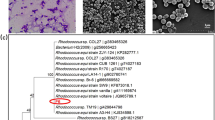
The tested species of Streptomyces were able to produce chitosan and chitosan nanocubes (NCs) from chitin degradation (Fig. 1a–e). Interestingly, during the assessment of enzymatic productivity among the positive isolates, characteristic beads (Fig. 1 upper left) appeared along with the fermentation broth. These beads were examined under SEM which revealed the formation of chitosan and the formation of chitosan nanocrystals with chitosan in the case of Bat2 and Am1 (Fig. 1c). The chitosan NCs of isolate Am1 were characterized by prominent invaginations at their centers. In terms of chitinase productivity, isolate designated as Nof21 was selected for the completion of the current study.
Characteristic beads observed inside the fermentation broth after 6 days of fermentation (upper left of panel a, b, and c). Examination of beads under SEM declared the formation of chitosan (white arrowhead) and chitosan nanocubes (red arrowhead) especially in case of strain Bat2 and strain Am1. Panel (d) represents the chitin loosening and degradation during the course of chitin degradation by isolate Am1 while, Panel (e) represents chitin before the enzymatic hydrolysis.
Regarding chitinase production, the most potent isolates (Fig. 2a) were characterized molecularly based on 16SrDNA gene fingerprint as Streptomyces mutabilis Nof21 (accession number: OP647097), Streptomyces variabilis Am1 (accession number: OP647095), and Streptomyces roietensis Bat2 (accession number: OP647096).
(a) Chitinolytic activity of some local isolates belonging to actinomycetes on chitin agar composed of colloidal chitin (10.0 g/l), NH4Cl (5 g/l), MgSO4.7H2O (0.5 g/l), KH2PO4 (2.4 g/l), K2HPO4 (0.6 g/l), and bacteriological agar (15 g/l). The medium was adjusted to pH 7.0 and incubation of cultures was done at 30 °C up to 6 days. Clear zones are indicative of chitinase production and hydrolysis of chitin. (b) Time course of enzyme production. (c) assay of chitinase in chitin agar petridish containing agar (1.5%, w/v) and colloidal chitin (1%, w/v) at pH 7.0 (b). Forty microliters of chitinase were applied to each well (7 mm diameter). By the end of incubation period a thin layer of Lugol’s iodine solution was applied on the surface of petridish for 10 min for visualization of the enzymatic reaction. Simple sugars resulted from chitin degradation don’t absorb the iodine solution while undegraded chitin absorb the dye and appears red.
Chitinase production
The enzyme was produced using the basal medium described in materials and methods at pH 6 and 35 °C for up to 6 days. Figure 2b displays the impact of time on enzyme production by Streptomyces variabilis Am1. Enzyme production enhanced with time during the logarithmic phase of bacterial growth achieving the highest value after 84 h (110.2 U/ml). While the best bacterial growth was reached on the 5th day of incubation (24 mg dry weight/1 ml broth).
The impact of carbon sources such as cellulose, lactose, glucose, galactose, fructose, maltose, mannitol, sucrose, and soluble starch was examined at a concentration of 2% (w/v). Figure 3a shows that, best chitinase productivity by Streptomyces variabilis Am1 was achieved by galactose where enzyme productivity was 3.1-fold greater than the blank test. On the other hand, both sucrose and mannitol have repressed the chitinase productivity compared with the control treatment.
Effect of carbon source (a) and nitrogen source (b) on chitinase production. The initial fermentation medium was consisting of colloidal chitin (10 g/l), (NH4)2SO4 (5 g/l), MgSO4.7H2O (0.5 g/l), KH2PO4 (2.4 g/l), K2HPO4 (0.6 g/l), NaCl (0.6 g/l), FeSO4.7H2O (0.001 g/l), ZnSO4. 7H2O (0.0001 g/l), and MnSO4. 7H2O (0.0001 g/l). During the nitrogen source experiment, (NH4)2SO4 was replaced with the tested material. The basal medium was adjusted to pH 6.0 and the fermentation was done at 35 °C for 6 days in a submerged mode with 150 rpm shaking speed.
The influence of organic nitrogen such as casein, gelatin, soybean meal, tryptone, yeast extract, and peptone in addition to the inorganics such as ammonium acetate, ammonium nitrate, ammonium sulfate, ammonium chloride, and NaNO3, on chitinase secretion, were tested at 0.5% (w/v) concentration (Fig. 3b). Maximum chitinase productivity was achieved by yeast extract with a 3.3-fold increase over that of control. None of the ammonium nitrate, ammonium acetate, and sodium nitrate could enhance chitinase productivity by the tested strain (Fig. 3b).
Properties of the purified chitinase
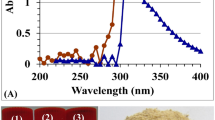
Optimization of enzyme productivity by the most potent isolate Streptomyces variabilis Am1 was done to proceed with enzyme purification. The chitinase was refined to homogeneity by anion exchange chromatography (Fig. 4a) and gel permeation chromatography (Fig. 4b), respectively after being precipitated by 80% ammonium sulfate. The recovery of enzyme reached 23.8% and the specific activity reached 18.6-fold (Table 1). SDS-PAGE presented one band at a molecular mass of approximately 56 kDa (Fig. 4c Lane 1). Zymogram analysis (Fig. 4c Lane 2) of the culture supernatant also showed one band of enzymatic activity.
Chromatogram of the chitinase produced by Streptomyces variabilis Am1 on a DEAE-Cellulose column (2 × 30 cm2) (a). The pooled active fractions from the DEAE-Cellulose column were further purified with a Sephadex G75 FF column (2.0 × 45 cm2) (b). The -■- line represents chitinase activity, while -○- line represents the amount of protein in terms of absorbance at wavelength 280 nm. The last recovered protein was subjected to SDS-PAGE (c) with 5% (w/v) stacking gel and 15% (w/v) resolving gel. M represents the standard proteins, lane 1 represents the purified enzyme on SDS-PAGE gel, while lane 2, represents the activity gel electrophoresis (zymogram). The native gel containing contained 1.0% colloidal chitin as substrate, the gel was immersed in 0.2 M Tris–HCl buffer, pH 8.0 at 40 °C for 16 h. Chitinase activity in the native gel was visualized by staining with Lugol’s iodine solution for 15 min and destaining with 1 N NaCl solution for 5 min at room temperature. Full-length gel is included in the Supplementary Information file (Fig. S1).
The optimum pH for chitinase was 8.0 and chitinase was stable at pH 4–9 for 60 min at 35 °C (Fig. 5a). The best temperature for chitinase was 40 °C (Fig. 5b) and the enzyme was stable for 30 min under 60 °C (Fig. 5c). It appeared that chitinase wasn’t altered by preincubating at 50 °C for 15 min. From the thermal inactivation profile (Fig. 5c), the Tm value of chitinase was at 66.61 °C using 50 mM phosphate buffer (pH 8.0) (Fig. 5d).
Effect of pH on enzyme activity (-●-) was done by adjusting the reacting mixtures to different pHs (a). While the pH stability (-○-) was examined by preincubation of enzyme at different pHs, then residual activity against colloidal chitin was determined (a). The effect of temperature on chitinase activity (b) was studied by incubation of the reacting mixtures at different temperatures. While the thermal stability (c) was determined by exposing chitinase to 50–80 °C for 15–60 min then assayed for residual activity against colloidal chitin. The midpoint temperature (Tm) at which the tested chitinase lost half of its activity was calculated from (d).
Impact of metals and protein reagents on chitinase activity
Results in Table 2 show the effect of metal ions such as Mg2+, Cu2+, Ca2+, Mn2+, Hg2+, Ag+, Fe2+, and Fe3+ in addition to β-mercaptoethanol, SDS, EGTA, and EDTA on chitinase activity at 5 mM concentration. In all treatments, a universal blank was made for comparisons. The enzyme was slightly inhibited by Cu2+ and Mn2+ ions while was stimulated by Fe2+ ions. The protein reagents SDS, EDTA, and EGTA significantly inhibited the tested chitinase. Interestingly the EDTA-chelated enzyme restored its activity upon the addition of Fe2+ ions at 5 mM concentration.
Antifungal activity
After 7 days of incubation of Eurotium amstelodami, Fusarium oxysporum, and Fusarium verticillioides on PDA plates with each well loaded with 40 µl of the enzyme preparation containing 15 U of chitinase, the antifungal potential of tested chitinases was calculated in millimeters (mm) utilizing a digital caliber and calculating the zones devoid of fungal growth around the hole. The best antifungal activity (Fig. 6) was reported for chitinase produced from Streptomyces variabilis Am1 where it exerted good inhibitory results against all tested fungi; Eurotium amstelodami (14.28 mm), Fusarium verticillioides (22.40 mm), and Fusarium oxysporum (16.18 mm). The chitinases of Streptomyces mutabilis Nof21 and Streptomyces roietensis Bat2 only inhibited the plant pathogenic fungus Fusarium verticillioides (14.20 and 14.98 mm, respectively). The heat-inactivated chitinase didn’t show activity against any of the tested phytopathogenic fungi.
The antifungal activity of three chitinases produced by local Streptomyces spp (Am1, Bat2 and Nof21) against Eurotium amstelodami (a), Fusarium verticillioides (b), and Fusarium oxysporum (c). Exactly 40 µl (15 U) of enzyme preparations were applied to each well. A heat-inactive enzyme preparation was used as a negative control. Fungal growth was observed periodically till 7 days of incubation at 30 °C.
Discussion
Chitinases are very useful in the degradation of chitin wastes and in the biological control of chitin containing pathogens such as fungi, insects and nematodes. Hence, screening for chitinolytic bacteria was performed in the terrestrial environments of Saudi Arabia. We obtained more than forty-six promising isolates. The most potent isolates were characterized based on 16SrDNA gene fingerprint as Streptomyces mutabilis, Streptomyces variabilis, and Streptomyces roietensis. Regarding previous studies, very few authors discussed the ability of these species to produce chitinases. The ability of Streptomyces variabilis to produce chitinase was only detected in two strains; strain LCP1822 and strain YH2123. To our knowledge, chitinase production from Streptomyces mutabilis and Streptomyces roietensis has never been reported.
In terms of enzyme productivity, Streptomyces variabilis was selected for completion of this study. Enzyme production enhanced with time during the logarithmic phase of bacterial growth achieving the highest value after 84 h (110.2 U/ml). While the best bacterial growth was reached on the 5th day of incubation (24 mg dry weight/1 ml broth). Fermentation was best by Streptomyces venezuelae P10 at 30 °C after 96 h9 while, Streptomyces sp. ANU6277 produced the maximum level of chitinase at 35 °C after 60 h of fermentation13.
The best productivity by Streptomyces variabilis Am1 was achieved in presence of galactose as a carbon source (3.1-fold greater than the blank test). According to other investigators, in the case of Streptomyces halstedii, chitinase production was highly potent in the presence of glucose10, arabinose in the case of Streptomyces viridificans11, and starch in the case of Streptomyces sp. ANU627713 and Streptomyces aureofaciens12.
Maximum chitinase productivity was achieved by yeast extract with a 3.3-fold increase over that of control. None of the ammonium nitrate, ammonium acetate, and sodium nitrate could enhance chitinase productivity by the tested strain. Yeast extract was also the optimal N-supply for chitinase productivity from Streptomyces sp. ANU627713.
When chitinase was refined to homogeneity by anion exchange chromatography and gel permeation chromatography, it showed a molecular mass of approximately 56 kDa by SDS-PAGE (Fig. 4c, lane 1) and activity zymographic gel electrophoresis (Fig. 4c, lane 2). In general, chitinases produced by microorganisms have a molecular mass ranging from 20 to 120 kDa. Fungal chitinases have a weight ranging from 35 to 45 kDa24 while most bacterial chitinases mostly weight around 20–60 kDa25. Chitinases of Streptomyces weight 20 kDa for Streptomyces sp. M-20 (Kim et al. 2003)14, 28 kDa, 35 kDa, and 45 kDa for Streptomyces sp. NK 1057 (Nawani and Kapadnis 2004)26, 34 kDa for Streptomyces sp. DA11 (Han et al. 2009)15, 43 kDa and 45 kDa for S. albovinaceus S-2227, 45 kDa for Streptomyces sp. ANU627713 and 49 kDa for S. griseus HUT 603728 and 66 kDa for Streptomyces venezuelae P109.
The optimum pH for chitinase was 8.0 and chitinase was stable at pH 4–9 for 60 min at 35 °C (Fig. 5a). The best temperature for chitinase was 40 °C (Fig. 5b) and the enzyme was stable for 30 min under 60 °C (Fig. 5c). Previously, it was found the optimum reaction temperature for chitinases from Streptomyces specifically at 40 °C29 and in the range of 30–55 °C in general30. Streptomyces sp. M-20 chitinase exerted its maximal activity at 30 °C and pH 5.0 in addition, it was stable at pH 4.0–8.0 and thermally stable up to 40 °C14. Streptomyces venezuelae P10 chitinase was maximally active at 35 °C and pH 6–89. While the chitinase of Streptomyces sp. DA11 was maximally active at 50 °C and pH 815.
We suggest a metallo-enzyme type with ferric ions as cofactors because the EDTA-chelated chitinase restored its activity upon the addition of Fe2+ ions. We don’t suggest chitinase with thiol groups as there was no increase in activity upon treatment with β-mercaptoethanol and Hg2+ ions. The chitinase inhibition by SDS was also reported by Groleau et al.31. The inhibition of enzyme by chelating agents EGTA and EDTA was also reported by Streptomyces sp. DA11 chitinase15 and was different with Streptomyces sp. M-20 chitinase14, and Streptomyces sp. S-84 chitinase16.
Streptomyces variabilis Am1 chitinase exerted good inhibitory results against all tested fungi; Eurotium amstelodami, Fusarium verticillioides, and Fusarium oxysporum. The dependence on the hydrolytic activity of microbial chitinolytic enzymes in the biocontrol of harmful fungi especially the phytopathogenic type is well-established in many studies. Chitinolytic enzymes from actinobacteria especially genus Streptomyces were found to have various mechanisms of antifungal activity such as inhibition of fungal spore germination, inhibition of germ tube elongation, and induction of spores and hyphal tips bursting12,32. The application of bacterial chitinases in antifungal applications was first reported from Streptomyces griseus HUT 603733. Streptomyces venezuelae P10 chitinase was found to inhibit Aspergillus niger maximally but didn’t inhibit Penicillium chrysogenum9. Streptomyces sp. DA11 chitinase was employed for the biocontrol of Aspergillus niger (11.0 mm) and Candida albicans (10.5 mm)15. Hoster et al.29 found chitinase activity from a strain of Streptomyces against Fusarium culmorum, Aspergillus nidulans, Gulgnardia bidwellii, Botrytis cinerea, and Sclerotia sclerotiorum. Narayana and Vijayalakshmi13 reported the chitinolytic activity of Streptomyces sp. ANU6277 against Fusarium udum. Kim et al.14 revealed the inhibition of the phytopathogen Botrytis cinerea by chitinase from Streptomyces sp. M-20.
The ability of tested species of Streptomyces to produce chitosan and chitosan NCs from chitin degradation (Fig. 1a–e) may be additional tools in their antifungal activity besides the hydrolysis activity of chitinase. Regarding other investigators, Chitosan was used by many researchers as an antibacterial and antifungal agent due to its polycationic nature that facilitates adsorption to the negatively charged cellular components on the surface of pathogenic microorganisms34.
Further degradation of chitosan into chitosan nanoparticles (NPs) and NCs enhances its antifungal potential owing to improved surface area and encapsulation proficiency35. In addition, exposing microbes including fungi to metal nanoparticles creates oxidative stress in the form of reactive oxygen species that disrupts cell selective permeability, and inhibits nucleic acids, and protein synthesis.
Chitosan NPs and NCs were prepared by chemical and physical methods and used by agro-researchers as antifungals to substitute the use of chemical fungicides. For example, Chouhan et al.36 have controlled the Fusarium rot of wheat by chemically prepared chitosan NPs. Biologically prepared NPs and NCs are eco-friendly, biocompatible, have superior permeability into the cell, lower toxicity, and lower cost37. Regarding other researchers, a conjugate of chitosan NPs and silver NPs was used as an antifungal composite against Fusarium species. Unfortunately, it was expensive and became unsuitable for field application in agriculture38. The value of this research is that it is the first time to produce chitosan NPs and NCs utilizing Streptomyces. The nanocrystals in particular have superior activity than other forms of nanoparticles.
As a conclusion, forty-six chitinolytic isolates were recovered from the rhizosphere of some desert plants in Saudi Arabia. The top three were recovered from the root region of wild plants growing in the Great Nafud desert, Dahna desert, and North Shaqra desert and then were identified as Streptomyces mutabilis Nof21, Streptomyces variabilis Am1, and Streptomyces roietensis Bat2, respectively. According to our knowledge, chitinases production from Streptomyces mutabilis and Streptomyces roietensis have never been reported, in addition, chitinase characterization from Streptomyces variabilis has never been done. Interestingly, SEM revealed the formation of chitosan NCs along with chitosan from the degradation of chitin. The ability of tested species of Streptomyces to produce chitosan and chitosan NCs from chitin degradation may be additional tools in their antifungal activity besides the hydrolysis activity of chitinase. Chitosan itself is an antimicrobial agent due to its ability to adsorb the cells of pathogenic microorganisms. Chitosan NCs enhance the antifungal potential owing to improved surface area and the creation of oxidative stress. The value of this research is that it is the first time to produce chitosan NCs using Streptomyces. In addition, the unique ability of this metallo-chitinase in chitin degradation, pH stability, and thermal resistance than many chitinolytic enzymes, it could be considered a more effective chitinolytic agent targeting phytopathogenic fungi in particular.
Materials and methods
Materials
Sephadex G75 FF (fast flow) and DEAE-Cellulose were obtained from Pharmacia Biotech (Sweden). Chitin, β-mercaptoethanol, SDS, EGTA, and EDTA were obtained from Sigma-Aldrich (USA). Additional reagents were of analytical degree and were purchased from regional providers.
Collection of shrimp shell wastes and preparation of the substrate
Wastes of shrimp shells were gathered from Dammam regional fish market in Saudi Arabia. To remove the moisture, they were then sundried till constant dry weight was obtained. They were then milled to a fine powder. Chitin was extracted from the whole shrimp powder through the demineralization step and deproteinization step39. Colloidal chitin was then prepared by the addition of 5 g from the previous material to 88 ml of concentrated HCl and allowed to stir on a magnetic stirrer for 3 h. Exactly one liter of chilled distilled water was added with stirring for another 3 h. The suspension was left in the refrigerator at 4 °C for 24 h. The dense white pellet was collected by Buchner filtration. The paste is the colloidal chitin which was suspended in distilled water and filtration is repeated to get rid of HCl and the unwanted soluble residues. The last paste is kept at 4 °C for the next uses.
Isolation, and maintenance of chitinolytic microorganisms
Several soil samples were obtained from different localities of Saudi Arabia. Initially, 1000 µl from appropriate dilutions were mixed with chitin agar plates consisting of colloidal chitin (10.0 g/l), NH4Cl (5 g/l), KH2PO4 (2.4 g/l), MgSO4.7H2O (0.5 g/l), K2HPO4 (0.6 g/l), and bacteriological agar (15 g/l). The medium was corrected to pH 7.0 and incubation was at 35 °C for up to 6 days. Cleared rings around colonies were taken as the first indication of chitin hydrolysis. Chitinolytic isolates were purified by quadrate streaking and single-cell colonies were preserved at − 80 °C into 20% (v/v) of sterilized glycerol.
Assessment of chitinase productivity
The initial fermentation medium used for evaluation of chitinase productivity among the recovered isolates from soil samples was consisting of colloidal chitin (10.0 g/l), (NH4)2SO4 (5.0 g/l), MgSO4.7H2O (0.5 g/l), KH2PO4 (2.4 g/l), K2HPO4 (0.6 g/l), NaCl (0.6 g/l), FeSO4.7H2O (10.0 mg/l), ZnSO4. 7H2O (1.0 mg/l), and MnSO4. 7H2O (1 mg/l). The basal medium was corrected to pH 6.0 and then autoclaved for 15 min at 121 °C. After being cooled to below 45 °C, each 250 ml Erlenmeyer flask contained 50 ml liquid and was inoculated with 2 ml (107 spores/ml) of a 7-day-old culture. Incubation was done at 35 °C for 6 days on a shaking incubator at 150 rpm speed. The microbial growth was withdrawn by filtration and then centrifugation at 5000 rpm for 20 min. Enzyme production among isolates was evaluated and the most potent isolate was selected. The nutritional and environmental parameters were then optimized to achieve the highest level of chitinase productivity. The final fermentation broth was used for enzyme purification.
Molecular characterization of chitinolytic microorganisms
The QIAamp DNA Mini Kit (Qiagen, Germany) was employed for the extraction of bacterial DNA following the provider's guidelines. The gene coding 16S rRNA was amplified partially by employing 10 µM of each universal primer: 27F (5′-AGAGTTTGATCATGGCTCAG-3′) as forward primer and 800R (5′TACCAGGGTATCTAATCC3′) as reverse primer. The PCR was then initiated at 95 °C for 10 min, followed by 35 denaturation cycle at the same temperature for 1 min, the annealing step at 56 °C for 1.15 min, and the extension step for 2 min at 72 °C, followed by final extension step at 72 °C for 5 min. The reaction product was eluted into agarose gel electrophoresis of 1.5% agarose. The gel was dyed with ethidium bromide solution then, gel bands were visualized under UV. The PCR amplicons were purified by ExoSAP-IT (Applied Biosystems, USA) and the purified amplicons were prepared for sequencing by Big Dye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) and DyeEx purification kit (Qiagen, Germany). The sequencing was conducted on a SeqStudio Genetic Analyzer (Applied Biosystems, USA). Consequently, the acquired 16S rRNA partial gene sequences were deposited in the GenBank database (www.ncbi. nlm.nih.gov/genbank/) after identifying species with highest similarity using National Center for Biotechnology Information (NCBI) through BLAST searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Assay of chitinolytic activity and protein determination
The enzyme–substrate reaction was initiated by mixing 1 ml of 10% (w/v) colloidal chitin suspension in 0.2 M sodium phosphate buffer (pH 7.0) to 1 ml of enzyme preparation. The reaction proceeded at 50 °C then was terminated after 1 h by 1 ml of 1% NaOH and incubation in a boiling water bath (100 °C) for 5 min. Centrifugation was done at 5000 rpm for 15 min to split the product from the non-reacting substrate. Then the total reducing sugars were measured by dinitrosalicylic acid (DNS) assay40. Exactly, 1 ml of the supernatant was combined with 1 ml of 1% DNS in 30% sodium potassium tartrate in 2 M NaOH. The mixture was vortexed and reincubated in a boiling water bath for 10 min. The shift of color towards red is a direct indication of the presence of reducing sugars and therefore proportional to the enzymatic activity. The absorption of the test sample was measured at A535 (UV5 Excellence UV/VIS Spectrophotometers, Mettler Toledo, Switzerland). One unit (U) of chitinase was calculated as the quantity of enzyme necessary to yield 1 µmol of NAG as reducing sugar per minute at 50 °C. Protein quantification was done at A280 using bovine serum albumin (BSA) as a standard protein.
In some experiments, chitinolytic activity was visualized by chitin agar well diffusion plates consisting of colloidal chitin (10 g/l) and agar (15 g/l) at pH 7.0. Exactly, 10 mm diameter wells were bunched off using a sterile cork porer. Fifty-μl enzyme preparation was added to each well. Blank was done by 50 μl of a thermally inactivated chitinase preparation. Cleared halos formation was checked after 24 h at 35 °C by the addition of a thin layer of Lugol’s iodine solution on the surface of the petridish for 10 min. Simple sugars resulting from chitin degradation don’t absorb the iodine solution while undegraded chitin absorbs the dye and appears red.
Enzyme purification
The procedure of chitinase separation from the culture broth of the most potent isolate was initiated by centrifugation at 5000 rpm for 15 min to separate the cells and debris from the bacterial products. The proteins and enzymes present in the supernatant were then precipitated by the addition of ammonium sulfate gradually with frequent stirring till 80% saturation level was achieved. After 12 h incubation at 4 °C, the agglomerated enzyme molecules were harvested by centrifugation at 7000 g for 10 min at 4 °C. The harvest was then suspended in 100 mM Tris–HCl buffer of pH 7.4 (buffer A) for dialysis against buffer A to get rid of ammonium sulfate and other salts. The resulting dialysate was then applied on the top of DEAE-Cellulose anion-exchanger column chromatography of 2.0 × 30 cm2 dimension. Elution was done with 100 mM borate buffer pH 9.4 (buffer B) with 0–1 M NaCl in a linear gradient manner. The active fractions from the anion-exchanger column were assembled and lyophilized for further purification with the gel permeation chromatography technique. For this, a Sephadex G75 FF column of dimension 2.0 × 45 cm2 was used. The elution through this column was done with buffer A at a 0.5 ml per minute flow rate. Again, the fractions representing chitinase activity were combined and lyophilized for characterization and application. Ultimately, SDS-PAGE was accomplished using 5% (w/v) stacking gel and 12% (w/v) separating gel to check the purity of chitinase and to verify the exact molecular mass of the tested chitinase. For zymogram analysis, the crude chitinase was mixed with the loading buffer without reducing agent and boiling step. After electrophoresis, in gel containing 1.0% colloidal chitin as substrate, the gel was immersed in 0.2 M Tris–HCl buffer, pH 8.0 at 40 °C for 16 h. Chitinase activity in the native gel was visualized by staining with Lugol’s iodine solution for 15 min and destaining with 1 N NaCl solution for 5 min at room temperature.
Biochemical properties
Impact of pH on chitinase activity and stability
Chitinase-substrate reaction mixtures were corrected to various pHs. The pH range of 2.0–6.0 was achieved by the citrate–phosphate buffer, the pH range of 7.0–8.0 was achieved by Tris–HCl buffer, and pHs 9.0–11.0 were achieved by the glycine–NaOH buffer. The assay of chitinase activity was done at 35 °C using the substrate colloidal chitin. In addition, the pH stability was assessed by preincubation of chitinase alone for 60 min at 35 °C at the same pH range, then the remaining activity against colloidal chitin was assessed at pH 8.0 with 50 mM Tris–HCl buffer.
Impact of temperature on chitinase activity and stability
The impact of temperature on the enzyme was evaluated at 20 °C to 50 °C. The substrate colloidal chitin was used at a concentration of 1.5% (w/v). The colloidal chitin was adjusted to pH 8.0 in 50 mM Tris–HCl buffer. The heat stability of chitinase was assessed by preincubation of chitinase preparations without substrate in the same buffer at high temperatures of 50–80 °C for 15–60 min. At time intervals, the remaining activity was assessed. The temperature at which chitinase lost half of its original activity after 60 min exposure was defined as the midpoint temperature (Tm).
Impact of metal ions, activators, and inhibitors
The purified chitinase was preincubated at 35 °C for 60 min in the existence of a metal such as Mg2+, Cu2+, Ca2+, Mn2+, Hg2+, Ag+, Fe2+, and Fe3+ in addition to common reagents such as β-mercaptoethanol, SDS, EGTA, and EDTA. They were tested at a strength of 5 mM in 50 mM phosphate buffer (pH 8.0). The remaining activity was then assessed. The activity of chitinase in the absence of metals, activators, or inhibitors was considered 100%.
Antifungal activity
The antifungal potential of the three purified chitinases Am1, Bat2, and Nof21 was studied by the method of agar well diffusion according to the National Committee for Clinical Laboratory Standards (NCCLS)41. Five days-old spore suspensions (3 × 107/ml) at the concentration of 1 ml of Eurotium amstelodami (Aspergillus amstelodami), Fusarium oxysporum, and Fusarium verticillioides were uniformly and spread individually on plates of Potato Dextrose Agar with pH adjusted at 5.5. The cultures were left at room temperature for 10 min to allow absorption of the inoculum. Eight-mm wells were punched off using a sterile cork borer and forty-µl of chitinase preparation was applied to each well (15 U). A heat-inactive enzyme preparation was utilized as a negative test. The growth of fungi was observed periodically at 30 °C for 7 days. Tests were conducted in three replicates to assure reliability.
Statistical analysis
Tests were achieved in triplicates and results were given as means ± standard deviations.
Data availability
All data generated and analyzed during this study are included in this published article. The datasets generated during the current study are available in GenBank database under accession number OP647097 for Streptomyces mutabilis Nof21, accession number OP647095 for Streptomyces variabilis Am1, and accession number OP647096 for Streptomyces roietensis Bat2.
Abbreviations
- NCCLS:
-
National Committee for Clinical Laboratory Standards
- DEAE-Cellulose:
-
Diethylaminoethyl cellulose
- DNS:
-
Dinitrosalicylic acid
- EDTA:
-
Ethylenediamine tetraacetic acid
- EGTA:
-
Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- NAG:
-
N-acetyl-D-glucoseamine
- PDA:
-
Potato dextrose agar
- SDS:
-
Sodium dodecyl sulfate
- SDS-PAGE:
-
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis
- T m value:
-
Midpoint temperature value
References
Chen, W. & Yang, Q. Development of novel pesticides targeting insect chitinases: A minireview and perspective. J. Agric. Food Chem. 68(16), 4559–4565. https://doi.org/10.1021/acs.jafc.0c00888 (2020).
Yang, Q. & Fukamizo, T. Advances in Experimental Medicine and Biology (Springer, 2019).
Delfini, C. D., Villegas, L. B., Martínez, M. A. & Baigorí, M. D. Extracellular antifungal activity of chitinase-producing bacteria isolated from guano of insectivorous bats. Curr. Microbiol. 78(7), 2787–2798. https://doi.org/10.1007/s00284-021-02555-0 (2021).
Le, B. & Yang, S. H. Microbial chitinases: Properties, current state and biotechnological applications. World J. Microbiol. Biotechnol. 35(9), 144. https://doi.org/10.1007/s11274-019-2721-y (2019).
Kumar, M. et al. Chitinases-potential candidates for enhanced plant resistance towards fungal pathogens. Agriculture 8(7), 88. https://doi.org/10.3390/agriculture8070088 (2018).
He, X. et al. Two highly similar chitinases from marine Vibrio species have different enzymatic properties. Mar. Drugs 18(3), 139. https://doi.org/10.3390/md18030139 (2020).
Sunny, N. E., Kumar, S. R. & Kumar, S. V. A review on chitinase synthesis from varied sources and its applications towards environment. Res. J. Pharm. Technol. 11(9), 4200. https://doi.org/10.5958/0974-360x.2018.00770.9 (2018).
Singh, R. V., Sambyal, K., Negi, A., Sonwani, S. & Mahajan, R. Chitinases production: A robust enzyme and its industrial applications. Biocatal. Biotransformation 39(3), 161–189. https://doi.org/10.1080/10242422.2021.1883004 (2021).
Mukherjee, G. & Sen, S. K. Purification, characterization, and antifungal activity of chitinase from Streptomyces venezuelae P10. Curr. Microbiol. 53(4), 9–265. https://doi.org/10.1007/s00284-005-0412-4 (2006).
Joo, J. G. Purification and characterization of an extracellular chitinase from the antifungal biocontrol agent Streptomyces halstedii. Biotechnol. Lett. 27, 1483–1486. https://doi.org/10.1007/s10529-005-1315-y (2005).
Gupta, R., Saxena, R. K., Chaturvedi, P. & Virdi, J. S. Chitinase production by Streptomyces viridificans: Its potential in fungal cell wall lysis. J. Appl. Bacteriol. 78(4), 83–378. https://doi.org/10.1111/j.1365-2672.1995.tb03421.x (1995).
Taechowisan, T., Peberdy, J. F. & Lumyong, S. Chitinase production by endophytic Streptomyces aureofaciens CMU Ac 130 and its antagonism against phytopathogenic fungi. Ann. Microbiol. 53, 447–461 (2003).
Narayana, K. J. & Vijayalakshmi, M. Chitinase production by Streptomyces sp. ANU 6277. Braz. J. Microbiol. 40(4), 33–725. https://doi.org/10.1590/S1517-83822009000400002 (2009).
Kim, K. J., Yang, Y. J. & Kim, J. G. Purification and characterization of chitinase from Streptomyces sp. M-20. J. Biochem. Mol. Biol. 36(2), 9–185. https://doi.org/10.5483/bmbrep.2003.36.2.185 (2003).
Han, Y., Yang, B., Zhang, F., Miao, X. & Li, Z. Characterization of antifungal chitinase from marine Streptomyces sp. DA11 associated with south china sea sponge Craniella australiensis. Mar. Biotechnol. 11(1), 40–132. https://doi.org/10.1007/s10126-008-9126-5 (2009).
Ueno, H., Miyashita, K., Swada, Y. & Oba, Y. Purification and some properties of extracellular chitinase from Streptomyces sp. S-84. J. Gen. Appl. Microbiol. 36, 377–392 (1990).
Boukaya, N. et al. Biocontrol and plant-growth-promoting capacities of actinobacterial strains from the algerian sahara and characterisation of Streptosporangium becharense SG1 as a promising biocontrol agent. Biocontrol Sci. Technol. 28(9), 858–873. https://doi.org/10.1080/09583157.2018.1501466 (2018).
Goudjal, Y., Zamoum, M., Sabaou, N., Mathieu, F. & Zitouni, A. Potential of endophytic Streptomyces spp. for biocontrol of Fusarium root rot disease and growth promotion of tomato seedlings. Biocontrol Sci. Technol. 26(12), 1691–1705. https://doi.org/10.1080/09583157.2016.1234584 (2016).
Veliz, E. A., Martínez-Hidalgo, P. & Hirsch, A. M. Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol. 3(3), 689–705. https://doi.org/10.3934/microbiol.2017.3.689 (2017).
Nayak, S., Nayak, S., Mohanty, S., Sundaray, J. & Mishra, B. Microbiology Microbial chitinases and their applications: An overview. In Environmental and Agricultural 1st edn 313–340 (Wiley-Scrivener, 2021).
Zhang, W., Ma, J., Yan, Q., Jiang, Z. & Yang, S. Biochemical characterization of a novel acidic chitinase with antifungal activity from Paenibacillus xylanexedens Z2–4. Int. J. Biol. Maromol. 182, 1528–1536. https://doi.org/10.1016/j.ijbiomac.2021.05.111 (2021).
Quach, N. T. et al. Plant-derived bioactive compounds produced by Streptomyces variabilis LCP18 associated with Litsea cubeba (Lour.) pers as potential target to combat human pathogenic bacteria and human cancer cell lines. Braz. J. Microbiol. 52(3), 1215–1224. https://doi.org/10.1007/s42770-021-00510-6 (2021).
Gherbawy, Y., Elhariry, H., Altalhi, A., El-Deeb, B. & Khiralla, G. Molecular screening of Streptomyces isolates for antifungal activity and family 19 chitinase enzymes. J. Microbiol. 50(3), 68–459. https://doi.org/10.1007/s12275-012-2095-4 (2012).
Roberts, R. L. & Cabib, E. Serratia marcescens chitinase: One-step purification and use for the determination of chitin. Anal. Biochem. 127(2), 12–402. https://doi.org/10.1016/0003-2697(82)90194-4 (1982).
Bhattacharya, D., Nagpure, A. & Gupta, R. K. Bacterial chitinases: Properties and potential. Crit. Rev. Biotechnol. 27, 21–28. https://doi.org/10.1080/07388550601168223 (2007).
Nawani, N. N. & Kapadnis, B. P. Production dynamics and characterization of chitinolytic system of Streptomyces sp. NK 1057, a well equipped chitin degrader. World J. Microbiol. Biotechnol. 20, 487–494 (2004).
El-Sayed, E. S. A., Ezzat, S. M., Ghaly, M. F., Mansour, M. & El-Bohey, M. A. Purification and characterization of two chitinases from Streptomyces albovinaceus S-22. World J. Microbiol. Biotechnol. 16, 87–89. https://doi.org/10.1023/A:1008926214392 (2000).
Tanabe, T., Kawase, T., Watanabe, T., Uchida, Y. & Mitsutomi, M. Purification and characterization of a 49 kDa chitinase from Streptomyces griseus HUT 6037. J. Biosci. Bioeng. 89, 27–32 (2000).
Hoster, F., Schmitz, J. E. & Daniel, R. Enrichment of chitinolytic microorganisms: Isolation and characterization of a chitinase exhibiting antifungal activity against phytopathogenic fungi from a novel Streptomyces strain. Appl. Microbiol. Biotechnol. 66(4), 42–434. https://doi.org/10.1007/s00253-004-1664-9 (2005).
Shaikh, S. A. & Deshpande, M. V. Chitinolytic enzymes: Their contribution to basic and applied research. World J. Microbiol. Biotechnol. 9(4), 75–468. https://doi.org/10.1007/BF00328035 (1993).
Groleau, P. E., Morin, P., Gauthier, S. F. & Pouliot, Y. Effect of physicochemical conditions on peptide-peptide interactions in a tryptic hydrolysate of beta-lactoglobulin and identification of aggregating peptides. J. Agric. Food Chem. 51(15), 4370. https://doi.org/10.1021/jf0259720 (2003).
Gomes, R. C. et al. Purification of a thermostable endochitinase from Streptomyces RC 1071 isolated from a cerrado soil and its antagonism against phytopathogenic fungi. J. Appl. Microbiol. 90, 653–661. https://doi.org/10.1046/j.1365-2672.2001.01294.x (2001).
Watanabe, T. et al. Family 19 chitinases of Streptomyces species: characterization and distribution. Microbiology 145, 3353–3363. https://doi.org/10.1099/00221287-145-12-3353 (1999).
Sathiyabama, M. & Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 151, 321–325 (2016).
Kong, M., Chen, X. G., Xing, K. & Park, H. J. Antimicrobial properties of chitosan and mode of action: A state-of-the-art review. Int. J. Food Microbiol. 144(1), 51–63. https://doi.org/10.1016/j.ijfoodmicro.2010.09.012 (2010).
Chouhan, D., Dutta, A., Kumar, A., Mandal, P. & Choudhuri, C. Application of nickel chitosan nanoconjugate as an antifungal agent for combating Fusarium rot of wheat. Sci. Rep. 12, 14518. https://doi.org/10.1038/s41598-022-18670-2 (2022).
Hans, M. L. & Lowman, A. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 6(4), 319–327. https://doi.org/10.1016/S1359-0286(02)00117-1 (2002).
Dananjaya, S. H. S. et al. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 105(1), 478–488. https://doi.org/10.1016/j.ijbiomac.2017.07.056 (2017).
Zhao, D. et al. Two-step separation of chitin from shrimp shells using citric acid and deep eutectic solvents with the assistance of microwave. Polymers 11(3), 409. https://doi.org/10.3390/polym11030409 (2019).
Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 31(3), 426. https://doi.org/10.1021/ac60147a030 (1959).
CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests CLSI Standard M02 13th edn. (Clinical and Laboratory Standards Institute, 2018).
Acknowledgements
The authors would like to thank professor Medhat A. ElNaggar from the Agricultural Research Center, Plant Pathology Research Institute, Egypt, and Research Central Lab., SAGO, Saudi Arabia for providing us with the phytopathogenic fungi.
Funding
The authors express gratitude to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number IF-2020-028-BASRC at Imam Abdulrahman bin Faisal University (IAU)/ Basic and Applied Scientific Research Center (BASRC).
Author information
Authors and Affiliations
Contributions
E.K. and A.H.A. performed enzyme assays and characterization. A.I.A. and I.M.A. assessed the antifungal activity of recovered chitinases and the SEM study. S.A.A. and A.M.A. have performed the molecular characterization of isolates. S.K.A., S.M.A., and B.M.A. collected the soil samples, isolated the microorganisms, and performed the initial screening for chitinase activity. A.A.A. performed the statistics, graphing, and presentation of data. All authors have equally contributed to writing, editing, and drafting the manuscript and approved the final form.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kotb, E., Alabdalall, A.H., Alghamdi, A.I. et al. Screening for chitin degrading bacteria in the environment of Saudi Arabia and characterization of the most potent chitinase from Streptomyces variabilis Am1. Sci Rep 13, 11723 (2023). https://doi.org/10.1038/s41598-023-38876-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38876-2
This article is cited by
-
Xanthomonas chitinilytica sp. nov., a novel chitinolytic bacterium isolated from a microbial fermentation bed material
Antonie van Leeuwenhoek (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.