Abstract
Rapid and accurate bioburden detection has become increasingly necessary for food, health, pharmaceutical and environmental applications. To detect bioburden accurately, and in a highly sensitive manner, we have fabricated a novel microfluidic device with an integrated filter to trap the cells. Bioburden is detected on the filter paper in situ using the redox reaction of fluorescent label resorufin and a portable multichannel fluorometer is used for fluorescence measurement. The microfluidic device was fabricated in a facile, low-cost, and rapid way with microwave-induced thermally assisted bonding. To characterize the bonding quality of the microfluidic cassettes, different tests were performed, and the filter paper material and size were optimized. Primary Bacillus subtilis culture bacterial samples were filtered through the device to validate and investigate the performance parameters. Our results show that a limit of detection (LOD) of 0.037 CFU/mL can be achieved through this microfluidic device whereas the LOD in a normal microfluidic cassette in the fluorometer and the golden standard spectrophotometer are 0.378 and 0.128 CFU/mL respectively. The results depict that three to ten times LOD improvement is possible through this microfluidic cassette and more sensitive detection is possible depending on the volume filtered within a rapid 3 min. This novel microfluidic device along with the fluorometer can be used as a rapid portable tool for highly sensitive, accurate and high-throughput bacterial detection for different applications.
Similar content being viewed by others
Introduction
Millions of people become ill and die from food, water, or medicine contamination every year1. A global disease burden of 45% is attributed to bioburden, according to the World Health Organization2. In the manufacturing of biopharmaceuticals, contamination associated with bioburden is also a major concern3. As a result, diagnostic devices have become increasingly important for identifying bacteria and their antibiotic susceptibilities4,5. For the detection of bioburdens, there are many analytical methods available—adenosine triphosphate bioluminescence6, flow cytometry7, nucleic acid amplification8, respiration7, impedance methods8, and antibody detection7, to mention just a few. Most detection techniques require a relatively long time to detect (3 h to 7 days)9. Furthermore, when detection rates are sufficiently fast, cost and sensitivity become limitations10,11,12. Currently, cell viability assays are often used in drug development to study growth factors, cytokines, and cytotoxic agents that are capable of detecting viable cells and bacteria rapidly. In recent years, resazurin has been used in cell viability measurements to measure the oxidation–reduction of a given sample. To detect bioburden rapidly and in a sensitive manner, our previous study13 demonstrated the use of a USB-powered portable fluorometer for detecting viable cells in a given sample based on the detection of high quantum yield resorufin from resazurin. In this low-cost system, fluorescence intensity slope served as a criterion for detecting bioburden. Viable cells were monitored using the redox indicator dye resazurin. The sample under investigation was spiked with resazurin and loaded in a special-design microfluidic cassette and the rate of change of resazurin to resorufin was observed via the fluorometer. To detect bioburden with higher sensitivity, a novel microfluidic device with an integrated filter for automated filtration and detection is introduced in this study with the reported fluorometer and assay.
Microfluidics has become a powerful technology in the last few decades and has found applications in several frontier research areas that include analytical chemistry14, pharmaceuticals15, synthesis of chemicals16 and clinical application17,18. Microfluidic technologies have also been applied to microorganism studies recently. Microfluidic devices with micro-sized scale and large-scale integration offer many special advantages including low cost, higher throughput, and higher efficiency in microorganism analysis19,20,21,22,23 and antibiotic persistence24. Recent developments in microfluidics also utilize filter paper as a means of trapping and detecting bacteria and are becoming popular owing to the low-cost, ease of access, processing, modification and disposal25,26. However, the reported paper-based microfluidic devices have a very high limit of detection (LOD) ranging from 57 to 500 CFU/mL, and low accuracy27,28,29,30. The devices often require complex modifications i.e., antibody functionalization, immobilization of nanoparticles and UV-curing for patterns on the filter paper for detection27,28,29,30. Moreover, the devices are often specific to a certain strain of bacterial detection due to certain antibody functionalization27,28,29,30. These drawbacks hinder the rapid, facile, low-cost filter paper-based microfluidic devices to be widely used in bacterial detection, especially in field applications.
In this study, we have developed and validated a microfluidic device integrated with filter paper to trap viable bacterial cells initially and detect them finally. An easy, low-cost, rapid and environment-friendly microwave-induced thermally assisted solvent bonding was employed to bond the microfluidic device31. Bacterial trapping is done on the filter paper and detection is done in situ. After trapping the cells onto the filter paper, a resazurin-based assay is used as previously reported, and the fluorescence change due to the trapped cells (if any) is monitored using the previously developed multichannel fluorometer13. To the best of our knowledge, in this study, we have shown microwave-induced bonding to develop a microfluidic device with integrated filter papers for the first time for bioburden detection.
Different microfluidic layers were designed and fabricated for the accommodation and support of the filter paper inside the device. We have explored different materials and sizes of filter papers and optimized the parameters for higher fluorescence signal attainment and sensitive detection of bioburden. The Bacillus subtilis strain was tested to validate the device. Bacillus is a widespread, gram-positive bacterium that is harmful to humans, plants, or other organisms and causes severe infectious diseases32,33,34. Different bacterial concentrated samples were prepared and passed through the device at the same amount along with the negative control. Our results show that the integrated filter-based microfluidic cassette demonstrated much higher sensitivity and lower LOD than the gold standard fluorescence measurement device spectrophotometer and a normal microfluidic cassette in the multichannel fluorometer. Also, the LOD can be improved with filtering of higher volume. Sensitivity enhanced by the microfluidic device can lead to higher detection accuracy and detection can be done within a rapid 3 min in the fluorometer after 6 h of incubation of samples. Each microfluidic device costs about $1.6 and the 8-channel fluorometer costs approximately $1300 which can be further reduced by scaling up the production and are cost-effective compared to the conventional instruments. The facile, easily bonded microfluidic device in combination with the fluorometer can be used for rapid, highly sensitive and accurate detection of bioburden in health, pharmaceutical and environmental applications overcoming the limitations of the previously reported microfluidic devices.
Methods and materials
Device fabrication and bonding method
The Polymethyl methacrylate (PMMA) sheets used in this study were laser cut and bonded as described in our previous publication31. Briefly, microfluidic channels were cut into substrates according to the requirements. Ethanol was taken in a syringe or micropipette and sprayed on the surface of the sheet to cover the whole surface of the PMMA to be bonded. The PMMA sheets to be bonded were then hand-pressed and then inserted into a Polyetheretherketone (PEEK) vise and the whole setup was placed in the microwave for 1.5 min. The absolute ethanol and microwave heating used for bonding the device are effective for both the sterilization of the filter and the device as well.
The microfluidic device consisted of six different layers of PMMA of different thicknesses of dimensions 50 mm × 22 mm. Figure 1a shows the schematic diagram of different layers used for the fabrication of microfluidic devices with the layer numbers next to it. The top 0.2 mm layer had an opening for the inlet and outlet. Polycarbonate luer locks were used for injecting and taking out the samples. The second layer consisted of a channel to guide the sample out of the device. The third- and fourth layers acted as support for the filter paper with openings or passages for the sample to filtrate and pass through. The third layer also consists of several diagonal structures that support the filter paper and prevent deformation during filtration. The fifth layer had a channel for guiding the sample through the filter paper and the bottom layer formed a seal for the whole device. The filter diameter was 2 mm larger than that of the circular orifice to cover the whole filtration region. Additionally, the filter paper diameter was chosen such that it has an overlap with the inlet and outlet channel to ensure the entirety of the sample is filtered. As the photodetector is located on the bottom side of the device, the cassettes were fabricated and bonded in such a way, that the sample goes through the inlet and passes through the bottom side of the filter first and then goes out of the outlet. In this way, the filter paper does not shade the emission from the fluorescence. For the bonding, each of the layers was wet with ethanol and the filter paper was placed in between the layers as shown in Fig. 1a. Figure 1b shows an actual photograph of the microfluidic cassette with different sections of the cassette labeled. To detect bioburden in the developed microfluidic device, the filter paper does not need any surface modifications making the overall process easier and no deformation was observed in the filter paper after the bonding.
Filter paper selection
Initially five different filter papers were tested in this study. They are polyethersulfone (PES), polyacrylonitrile (PAN), gray polycarbonate (PC), nucleopore track etched (NTE) and nylon filters. These different filter papers were chosen because of their extensive use for bacterial trapping and detection35,36,37,38,39. The different filter papers were tested for autofluorescence in the fluorometer. The filter papers were cut from the filter membrane sheets using a hole punch. Microfluidic devices were bonded using the microwave-induced technique for all the filter papers and deformations and distortions i.e., bending, cracking were noted. For bioburden detection, sterility is an important concern. As the filter papers are not pre-sterilized, they may be susceptible to contamination depending on the storage condition. Therefore, the filter papers were tested for sterility and the best filter paper in terms of sterility and autofluorescence was selected for further experiments.
For sterility testing, 10 mL of negative control Lysogeny broth (LB) was filtered with different non-sterile filters in a manual filter housing. The cells were recovered afterward in 1 mL fresh LB and 200µL of the sample were incubated on the agar plate at 37°c for 24 h and colonies were counted. Additionally, to determine the effectiveness of alcohol sterilization, 5 mL of 70% ethanol was poured into a sterile petri dish. Filters were soaked in 70% ethanol for one minute. Filters were then put in filter holders and washed two times with 10 mL sterile DI water. Afterward, 10 mL of LB was filtered through sterile filters, and cells were recovered in 1 mL fresh LB media and the above procedure was repeated to count the colony forming units.
Multichannel fluorometer and sample preparation
The details of the multichannel fluorometer are described in the supporting information.
Microfluidic device tests
The microfluidic devices were tested for leakage and burst upon selecting the filter paper material and diameter. For the burst tests, the inlet of the device was connected to a pressurized airflow that can go up to 60 psi and the outlet was locked. The device was placed under water and the flow was increased by one psi at a time until air bubbles were seen in the water coming from the bonded device and the filter paper was observed for any ruptures and distortions. The same configurations were applied to evaluate the leakage test as well, only the pressure was slightly increased from zero and the cassette was inspected for air bubbles originating from the bonded microfluidic devices. A statistically significant value of the burst pressure and percent of defective devices were obtained by performing the leakage and burst tests ten times each.
Additionally, the pressure buildup profile of the microfluidic device was tested to ensure the filter paper and microfluidic device assembly had the bonding strength to handle the pressure without any deformations during the sample injection and filtration. The pressure drop in the device was evaluated with a negative control LB medium and a highly concentrated (100 CFU/mL) bacterial solution after 10 h of incubation. The pressure was measured using a syringe pump and a pressure sensor assembly. The pressure sensor (PendoTECH PMAT) was placed in between the inlet of the microfluidic device and a syringe pump. The flow of the syringe pump was set at a rate of 5 mL/min and 20 mL of each sample was filtered. The pressure drop was calculated for both samples and compared with the burst test to ensure the proper operation of the filter paper and the device.
Sample preparation, filtration and fluorescence measurement procedure
The preparation of primary and secondary culture cells is done in the same way as our previous publications13. Briefly, a 50 mL primary culture was prepared using 200 µL of Bacillus cells, which was grown at 37 °C in a shaker at 150 rpm (Lab-line Instruments, Melrose Park, IL) overnight. The optical density of the primary culture was measured to be 2.5 at 600 nm (SpectraMax M5). The primary seed culture (1%) was used to inoculate into 200 mL secondary culture and was grown at 37 °C in a shaker at 150 rpm to reach an optical density of 0.4 at 600 nm (~ 3 × 108 CFU/mL). This was centrifuged at 5000 RPM (Avanti J-25 I centrifuge, Beckman Coulter, Inc., Brea, CA) for 10 min. The cell pellet was washed with phosphate-buffered saline (PBS, pH 7.2) and the washed cells were re-suspended in PBS, pH 7.2. This sample is used for making serial dilutions (in 50 mL tubes) from 1:10 to 1:105. The 1:105 diluted sample is used to make further dilutions to make a final concentration of viable cells which is calibrated against a standard plate count. For this, the diluted samples from 1000 CFU/mL to 1 CFU/mL were prepared. After preparing different samples, they were incubated in shake flasks at 37 °C for 6 h as optimized in our previous publication13. In parallel, 200 µL of each sample was spread on an LB agar plate, and the plates were placed in an incubator at 37 °C overnight. After about 24 h, the number of colonies showing up on the plates was counted. 5 µM resazurin is mainly used in our study for the reduction study. The mechanism of using resazurin for the viability test is given in the supplementary information.
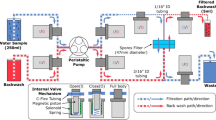
After the incubation, the samples were passed through the inlet of the microfluidic device. A particular volume (initially 1 mL) of the sample was passed slowly through the microfluidic device. Two syringes were used for injecting and backflushing the samples. As there were cavities under and above the filter paper due to the structure, any remaining samples from these regions were flushed out with the syringes. After filtering all the samples, 5 µM resazurin was taken and pushed inside the cassette with a syringe in just the amount (~ 150µL) to be needed to fill the lower side of the filter paper where the cells are trapped. After the resazurin was injected, the cassette was immediately placed in a cassette holder in the multichannel fluorometer, and fluorescence was measured. In parallel, the samples were also tested in the normal microfluidic cassette (Fig. S2) in the fluorometer6 and 96-well plates in the spectrophotometer. Bacillus subtilis were tested to compare the LOD for the filter-integrated microfluidic device with the normal microfluidic cassettes in the multichannel fluorometer and 96 well plates in the spectrophotometer. Bacterial samples were prepared and tested in two different types of microfluidic cassettes and the spectrophotometer in duplicates. Each test was performed with the negative control LB media. The overall experimental protocol is illustrated in Fig. 2.
Analytical methods
LOD was calculated as the cell concentration that can induce a signal equivalent to 3 times of noise.
The sensitivity is the average fluorescence slope calculated from the different bacterial samples with negative control LB, assuming LB media containing 0 CFU/mL. The noise is defined as the standard deviation of the intercepts for different response plots of the same experiment performed as the intercept is the fluorometer slope at negative control. The statistical difference between any two samples was calculated with a two-tailed statistical t-test. A p-value of ≤ 0.05 means there is a significant difference between the samples in the test. All the analysis was calculated on 3 min of data for both the spectrophotometer and multichannel fluorometer.
Results and analysis
Autofluorescence of the filter paper
As autofluorescence can significantly interfere with the fluorescence measurement, the filter paper with the lowest autofluorescence should be selected for this application. The autofluorescence of different filter papers was evaluated in the multichannel fluorometer with the microfluidic device. Table 1 shows the autofluorescence in the multichannel fluorometer for microfluidic devices with different filter materials. From the table, it can be seen that the gray PC filter has the least autofluorescence and the nylon filter has the highest. The other four materials have quite similar autofluorescence.
Though having the lowest autofluorescence, the gray PC filter was susceptible to deformation during microwave-induced bonding. Therefore, this filter paper was discarded from the further sterilization test.
Sterility of different filter papers
The results of the sterility of different filter papers are illustrated in the supplementary information. Based on the results, considering the pre-sterility, autofluorescence and extensive applications, the PES filter was chosen for the microfluidic device.
Diameter of the filter paper
For the filter paper to cover the entire filtration zone and hold in place, the filter diameter was consistently 2 mm greater than the different diameter circular orifices as mentioned earlier. Three different diameters i.e., 8.2 mm, 10.2 mm and 11.2 mm of the circular opening were tested. The diameter of 8.2 mm was tested as it matches the photodiode opening in the cassette holder. The maximum diameter was set to 11.2 mm as exceeding it would make the filter paper touch the edges of the cassette and result in leaking. The same volume of 0.1 µM resorufin solution was passed through and fluorometer response was recorded. Figure 3 shows fluorescence intensity for varying diameters from the fluorometer. From the figure, the larger diameter opening has higher fluorescence intensity due to the larger area of emission. So, the filter diameter of 13.2 mm was selected for further experiments.
Leakage and burst test results
The leakage and burst pressure of the whole assembly were tested as previously mentioned. Out of 10 tested microfluidic devices, only 1 out of 10 devices was observed to fail. This is evidence of the reliability of the bonding mechanism and the bonded microfluidic devices. The burst pressure was calculated with and without the filter assembly. Table S2 in the supplementary information shows the average burst pressure of a total of 10 devices.
Additionally, the pressure build-up profile of the whole microfluidic assembly with LB media and a 1000 CFU/mL bacterial sample is shown in supplementary Fig. S3. As can be seen, the maximum pressure drop during filtration of the 20 mL sample is ~ 6 psi which is much less than the average burst pressure (29.2 psi) of the whole assembly and the filter paper. Additionally, for all the burst tests performed, no deformations were seen on the filter paper. So, the microfluidic device bonded using this method is reliable and facilitates the filtration of large volumes without leaking and deformation of the filter paper.
Results in the fluorometer and LOD comparison
Samples were tested in two different microfluidic cassettes in the fluorometer and 96 well in the spectrophotometer to determine the LOD as previously mentioned. Figure 4a shows the response of different bacterial samples with the negative control in the microfluidic device with the integrated filter. The measurements were taken every 1.5 s and the inset shows a part of the 5 CFU/mL sample response. Although all the samples were tested in duplicate, only one sample data is presented graphically. Figure 4b shows the response of 5 CFU/mL bacterial samples in the normal microfluidic cassette(C), microfluidic cassette with filter (CF) in the fluorometer and 24 well plates in the spectrophotometer.
Figure 4a shows that with increasing bacterial load, there is a higher slope observed than that in the LB medium which exhibits very little or no slope. Figure S4 shows the detection region after filtration of LB and bacterial sample and injection of resazurin. For the bacterial sample, the blue dye resazurin is converted to pink resorufin as evident from the figure. For 20 and 100 CFU/mL, a decreasing slope is observed after some time which is due to the reduction of resorufin to colorless dihydroresorufin40. The decaying occurs faster and at a lower time for higher concentrations. From Fig. 4b, the fluorescence slope is significantly higher for samples in CF cassettes than from the C cassettes and the spectrophotometer. The slope values are respectively 3.28, 0.57 and 0.33 a.u./s in CF, C in the fluorometer and spectrophotometer which shows a significant increase in the sensitivity with the cassettes integrated with the filter. The average slope values were used to calculate the LOD in different cassettes in the fluorometer and spectrophotometer.
Figure 5a shows the fluorescence slope comparison of two different microfluidic cassettes in the fluorometer and the spectrophotometer for different bacterial concentrated samples. Figure 5b Shows the LOD and sensitivity comparison of these samples. The data analysis along with the fluorescence slope for the calculation of sensitivity and LOD are shown in Table 2. The first four rows represent the slope values for different bacterial concentrations along with the negative control.
From Fig. 5a, the slope values of LB media for all the samples are significantly smaller than the other bacterial concentrated samples. This is also shown by the p-values of the statistical t-test in Table 2. Also, for increasing bacterial concentrations the slope values are higher for all the samples except for the 100 CFU/mL sample in CF which is due to dihydroresorufin conversion. Nevertheless, the slope values for samples in CF are significantly higher than the other two samples for all cases. So, the CF microfluidic cassettes are significantly more sensitive than the normal microfluidic cassettes and the spectrophotometer. Figure 5b illustrates the results of the sensitivity and LOD for all the samples calculated for the linear region. From the results, the cassettes with integrated filter (CF) in the fluorometer are much more sensitive than the other two types of samples resulting in much lower LOD. The LOD is improved ~ 3.5 times than the spectrophotometer and 10 times than the C in the fluorometer. The p-value of the t-test between the sensitivity values of CF and C is 0.0097 and CF and spectrophotometer is 0.000079 which shows that the sensitivity is statistically significantly higher for the CF. Consequently, the samples in CF are around 14 and 19 times more sensitive than C in the fluorometer and spectrophotometer respectively. The exact values are depicted in Table 2. The sensitivity was calculated using the linear range (first 3 min) of responses using 0 to 20 CFU/mL data as the 100 CFU/mL exhibited non-linear response.
Change of sensitivity with higher filtrate volume
Different volume of the same concentrated bacterial sample (5 CFU/mL) was passed through the cassette to compare the LOD with varying volumes of 1, 3 and 5 mL. The sample response for different filtered volumes is shown in Fig. 6a. The sensitivity comparison for varying volumes is shown in Fig. 6b.
From the figure, it is observed that with increasing volume filtered through the microfluidic device, the fluorescence intensity and slope increase significantly. The increase in turn enhances the sensitivity of the microfluidic device with the fluorometer as shown in Fig. 6b. With more volume filtered, more cells were trapped on the filter paper. This caused resazurin to be reduced to resorufin more rapidly. For higher volumes filtered, in this case, for 5 mL, the sensitivity or average fluorescence slope is decreased. This occurs as a result of decreasing slope after some time which is due to the reduction of resorufin to colorless dihydroresorufin which is evident from Fig. 6a. A similar change of response and sensitivity augmentation with increasing volume for 20 CFU/mL is shown in Figure S5. Nevertheless, the fluorescence intensity and sensitivity are significantly higher than negative control LB media which confirms the presence of bioburden. It is possible to filter more volumes by this microfluidic device to detect very low bioburden concentrations when applicable.
Our device and method can detect bacteria within 6 h after incubation with laboratory cultured samples, reducing detection time by at least four times than the golden standard culture method. The comparatively rapid methods like PCR, ELISA, and other biosensing methods have the disadvantage of low sensitivity, false positive detection, technical complicated procedure and high cost. Following incubation, sample injection takes only one minute depending on volume and can be done easily. Whereas other bacterial detection procedures i.e., PCR, ELISA, and bacterial culture methods require certain procedures, technical preparation and extremely clean operating areas which are more complicated and require trained personnel to complete. This is where our microfluidic device with the fluorometer outperforms other conventional methods for detecting ultra low-level bioburdens. The optimized assay can be used with this device to detect other strains with higher sensitivity as well.
Conclusion
There is an inherent need for rapid and sensitive bioburden detection in a variety of applications, including public health. To detect bioburden with higher sensitivity and accuracy, a novel microfluidic device has been fabricated and bonded in a facile, environmentally friendly way in this study. Microfluidic cassette parameters have been optimized in conjunction with the multichannel fluorometer for ultra-sensitive detection. The microfluidic device has proven effective for detecting bioburden with a much higher sensitivity than a normal microfluidic device reported before and the spectrophotometer. Our future studies will include testing the microfluidic device with other bacterial strains, real-world assays, filtration efficiency of filter paper and implementing different biodegradable materials for fabrication and bonding. The microfluidic device can have a far-reaching impact on detecting bioburden, especially in a point-of-care setting.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Change history
15 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-40387-z
References
Greb, E. An overview of rapid microbial-detection methods. Pharm. Tech. 34, 25 (2010).
WHO. The World Health Organization Report 2004 Changing History. World Health. 95 (1) (2004).
Centers for Disease Control and Antibiotic Resistance Threats in the United States. U.S Dept. of Health and Human serv. (2019).
Hoehl, M. M., Lu, P. J., Sims, P. A. & Slocum, A. H. Rapid and robust detection methods for poison and microbial contamination. J. Agri. Food Chem. 60(25), 6349–6358 (2012).
Hobson, N. S., Tothill, I. & Turner, A. P. F. Microbial detection. Biosen. Bioelectron. 11(5), 455–477 (1996).
Kim, S. U. et al. Adenosine triphosphate bioluminescence-based bacteria detection using targeted photothermal lysis by gold nanorods. Anal. Chem. 90(17), 10171–10178 (2018).
Hentz, P. N. G. Pharmaceutical Bioburden Testing. In Encycl. of Industrial Biotech. Wiley (2013).
Jami Al-Ahmadi, G. & Zahmatkesh Roodsari, R. Fast and specific detection of pseudomonas aeruginosa from other pseudomonas species by PCR. Ann. Burns Fire Disasters 29(4), 264–267 (2016).
Nemati, M., Hamidi, A., Dizaj, S. M., Javaherzadeh, V. & Lotfipour, F. An overview on novel microbial determination methods in pharmaceutical and food quality control. Adv. Pharm. Bull. 2, 54 (2016).
Pettit, A. C. et al. The index case for the fungal meningitis outbreak in the United States. New England J. Med. 367(22), 2119–2125 (2012).
Gurramkonda, C., Mupparapu, K., Abouzeid, R., Kostov, Y. & Rao, G. Fluorescence-based method and a device for rapid detection of microbial contamination. PDA J. Pharm. Sci. Technol. 68(2), 164–171 (2014).
Estes, C. et al. Reagentless detection of microorganisms by intrinsic fluorescence. Biosen. Bioelectron. 18(5–6), 511–519 (2003).
Hasan, M. S. et al. Rapid ultrasensitive and high-throughput bioburden detection: Microfluidics and instrumentation. Anal. Chem. 94(24), 8683–8692 (2022).
Njoroge, S. K. et al. Integrated continuous flow polymerase chain reaction and micro-capillary electrophoresis system with bioaffinity preconcentration. Electrophoresis 32(22), 3221–3232 (2011).
Wong Hawkes, S. Y., Chapela, M. J. & Montembault, M. Leveraging the advantages offered by microfluidics to enhance the drug discovery process. QSAR Combin. Sci. 24, 712–721 (2005).
Elvira, K. S., Solvas, I. X. C., Wootton, R. C. & Demello, A. J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 5, 905 (2013).
Sackmann, E. K., Fulton, A. L. & Beebe, D. J. The present and future role of microfluidics in biomedical research. Nature 507, 181–189 (2014).
Mao, X. & Huang, T. J. Microfluidic diagnostics for the developing world. Lab Chip 12, 1412–1416 (2012).
Zhou, W. et al. Recent advances in microfluidic devices for bacteria and fungus research. TrAC Trends Anal. Chem. 112, 175–195 (2019).
Enders, J. R. et al. Towards monitoring real-time cellular response using an integrated microfluidics-MALDI/NESI-ion mobility-mass spectrometry platform. IET Syst. Biol. 4(6), 416 (2010).
Hu, Y. et al. Microfluidic enrichment of small proteins from complex biological mixture on nanoporous silica chip. Biomicrofluidics 5(1), 013410 (2011).
Baker, C. A. & Roper, M. G. A continuous-flow, microfluidic fraction collection device. J. Chromatogr. A 1217(28), 4743–4748 (2010).
Hol, F. J. & Dekker, C. Zooming in to see the bigger picture: Microfluidic and nanofabrication tools to study bacteria. Science 346(6208), 1251821 (2014).
Golchin, S. A., Stratford, J., Curry, R. J. & McFadden, J. A microfluidic system for long-term time-lapse microscopy studies of mycobacteria. Tuberculosis 92(6), 489–496 (2012).
Martinez, A. W., Phillips, S. T., Butte, M. J. & Whitesides, G. M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. 119(8), 1340–1342 (2007).
Jokerst, J. C. et al. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal. Chem. 84(6), 2900–2907 (2012).
Li, C. Z. et al. Paper based point-of-care testing disc for multiplex whole cell bacteria analysis. Biosens. Bioelectron. 26(11), 4342–4348 (2011).
Fronczek, C. F., Park, T. S., Harshman, D. K., Nicolini, A. M. & Yoon, J.-Y. Paper microfluidic extraction and direct smartphone-based identification of pathogenic nucleic acids from field and clinical samples. RSC Adv. 4, 11103 (2014).
Ma, S., Tang, Y., Liu, J. & Wu, J. Visible paper chip immunoassay for rapid determination of bacteria in water distribution system. Talanta 120, 135–140 (2014).
Lin, D. et al. Low-cost fabrication of microfluidic paper-based analytical devices with water-based polyurethane acrylate and their application for bacterial detection. Sens. Actuat. B Chem. 303, 127213 (2020).
Hasan, M. S. et al. Microwave induced thermally assisted solvent-based bonding of biodegradable thermoplastics: An eco-friendly rapid approach for fabrication of microfluidic devices and analyte detection. Sci. Rep. 12(1), 1–12 (2022).
Irenge, L. M. & Gala, J. L. Rapid detection methods for Bacillus anthracis in environmental samples: A review. Appl. Microbiol. Biotechnol. 93(4), 1411–1422 (2012).
Cao, J. et al. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods. 64, 103643 (2020).
Zhang, Y. J. et al. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 16(4), 7493–7519 (2015).
Chien, H. W., Tsai, M. Y., Kuo, C. J. & Lin, C. L. Well-dispersed silver nanoparticles on cellulose filter paper for bacterial removal. Nanomaterials 11(3), 595 (2021).
Heydarifard, S., Pan, Y., Xiao, H., Nazhad, M. M. & Shipin, O. Water-resistant cellulosic filter containing non-leaching antimicrobial starch for water purification and disinfection. Carbohyd. Polym. 163, 146–152 (2017).
Ferris, F. G., Schultze, S., Witten, T. C., Fyfe, W. S. & Beveridge, T. J. Metal interactions with microbial biofilms in acidic and neutral pH environments. Appl. Environ. Microbiol. 55(5), 1249–1257 (1989).
Zweifel, U. L. & Hagstrom, A. Total counts of marine bacteria include a large fraction of non-nucleoid-containing bacteria (ghosts). Appl. Environ. Microbiol. 61(6), 2180–2185 (1995).
Kamińska, A. et al. Rapid detection and identification of bacterial meningitis pathogens in ex vivo clinical samples by SERS method and principal component analysis. Anal. Methods 8(22), 4521–4529 (2016).
Ali-Vehmas, T., Louhi, M. & Sandholm, M. Automation of the resazurin reduction test using fluorometry of microtitration trays. J. Vet. Med. Ser. B 38(1–10), 358–372 (1991).
Acknowledgements
This work was supported by the U.S. Food and Drug Administration under contract no. BAA 75F40119C10132.
Author information
Authors and Affiliations
Contributions
M.S.H., C.S. and M.T. performed the experiments. Y.K. and G.R. conceived the project. X.G planned the experiments and analyzed the data. M.S.H. wrote the manuscript. X.G., A.A., Y.K. and G.R. supervised the research. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.R. and Y.K. are listed inventors on US Patent 10,948,414 B2 “Methods and Apparatus for Rapid Detection of Bacterial Contamination”.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Abhay Andar which was incorrectly given as Abhay Andara. In addition, the zip code in affiliations 1, 2 and 3 was incorrect.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hasan, M., Sundberg, C., Tolosa, M. et al. A novel, low-cost microfluidic device with an integrated filter for rapid, ultrasensitive, and high-throughput bioburden detection. Sci Rep 13, 12084 (2023). https://doi.org/10.1038/s41598-023-38770-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38770-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.