Abstract
This study evaluates to what extent symptoms are present before, during, and after a positive SARS-CoV-2 polymerase chain reaction (PCR) test, and to evaluate how the symptom burden and quality of Life (QoL) compares to those with a negative PCR test. Participants from the Dutch Lifelines COVID-19 Cohort Study filled-out as of March 2020 weekly, later bi-weekly and monthly, questions about demographics, COVID-19 diagnosis and severity, QoL, and symptoms. The study population included those with one positive or negative PCR test who filled out two questionnaires before and after the test, resulting in 996 SARS-CoV-2 PCR positive and 3978 negative participants. Nearly all symptoms were more often reported after a positive test versus the period before the test (p < 0.05), except fever. A higher symptom prevalence after versus before a test was also found for nearly all symptoms in negatives (p < 0.05). Before the test, symptoms were already partly present and reporting of nearly all symptoms before did not differ between positives and negatives (p > 0.05). QoL decreased around the test for positives and negatives, with a larger deterioration for positives. Not all symptoms after a positive SARS-CoV-2 PCR test might be attributable to the infection and symptoms were also common in negatives.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), manifests during the acute infection as heterogeneous with a wide spectrum of respiratory and non-respiratory related symptoms1,2. The severity of these symptoms can range from mild to severe or even fatal3,4. Fortunately, most patients fully recover. Nevertheless, it is becoming increasingly evident that a substantial proportion of people continues to experience symptoms for several weeks or months following the initial infection5,6,7,8. This is often referred to as long-COVID or post-COVID-19 condition, amongst others9,10. Commonly reported symptoms include fatigue, dyspnoea, cough, headache, brain fog, and this list is still growing5,6,7,8. These long-lasting symptoms negatively impact everyday functioning and quality of life (QoL)7,11,12.
The latest clinical case definition of the post COVID-19 condition proposed by the World Health Organization (WHO, dated 6th of October 2021) touches upon the nature of these persisting symptoms by stating that “…symptoms may be new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms may also fluctuate or relapse over time”10. Nevertheless, to date it remains to be determined whether symptoms were already present prior to a SARS-CoV-2 infection, as prospective data are scarce13. In addition, few studies have investigated how the symptom burden differs between subjects with a positive and negative test before, during, and after a SARS-CoV-2 polymerase chain reaction (PCR) test14,15. Some of the persisting symptoms, such as headache, are not disease-specific, are common in the general population and can be aggravated due to another viral infection or underlying chronic disease, ageing, and/or the effect of the pandemic itself (e.g. lockdowns)16. We aimed to study to what extent symptoms are present before, during, and after a positive SARS-CoV-2 polymerase chain reaction (PCR) test, and to evaluate how the symptom burden and QoL compares to those with a negative PCR test.
Results
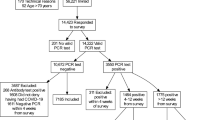
167,729 people are enrolled in the Lifelines Cohort Study, of whom 139,375 were invited as of March 2020 for the first questionnaire of the Lifelines COVID-19 sub-study. Including only the eligible respondents for analyses, see Online Supplement Fig. 1, the study population comprised 996 SARS-CoV-2 PCR positive and 3978 negative participants. Participants with a SARS-CoV-2 PCR positive test were on average three years younger, slightly more often female, and had a higher BMI, compared to SARS-CoV-2 PCR negative participants. Education level was comparable between the SARS-CoV-2 positive and negative participants. Of the SARS-CoV-2 PCR positive tested participants 29 were hospitalized (3%). Of these, 23 participants used antibiotics, 25 participants used supplemental oxygen, and 11 participants were admitted to the ICU. Further details regarding demographical characteristics can be found in Table 1.
Symptom prevalence
In the weeks or months before the test, the symptom prevalence did not significantly differ between SARS-CoV-2 PCR positive and negative participants (p > 0.05), except for fever and pain in the upper back (p < 0.05) (Fig. 1). During the test, prevalence rates were significantly higher for all symptoms in positive compared to negative tested participants (for all: p < 0.05). In the weeks or months after the test, symptoms with significantly higher prevalence in positives versus negatives were, loss of sense of smell or taste, shortness of breath, a feeling of heaviness in your arms or legs, part of your body feeling limp or heavy, headache, difficulty breathing, heart or chest pain, dizziness, a lump in your throat, and pain with breathing (all p < 0.05, Fig. 1, see Online Supplement Table 1 for the symptom severity before, during, and after a SARS-CoV-2 positive and negative test).
Change in symptom prevalence
Online Supplement Fig. 2 visually depicts the change in prevalence of symptoms over the time-periods by test-result. Before the test, symptoms were already partly present. Nearly all symptoms were significantly more often reported after a positive test compared to the period before the test (p < 0.05), except for fever (p > 0.05). The increase in symptom prevalence after compared to before a positive test ranges from 1% for sore throat (3% before versus 4% after the test, p < 0.05) to 13% for loss of sense of smell or taste (1% before versus 14% after, p < 0.05) and muscle pain or aches (18% before versus 31% after, p < 0·05). A higher symptom prevalence after compared to before a test was also found for nearly all symptoms in negative tested participants (p < 0.05), besides loss of sense of smell or taste (1% before versus 1% after, p > 0.05) and fever (< 1% before versus < 1% after, p < 0.05) which remained equal before and after a negative SARS-CoV-2 PCR test.
Symptom burden
Participants with a positive SARS-CoV-2 PCR test reported a median number of 1[0–2] symptom before, 12[7–16] symptoms during, and 2[0–5] symptoms after the test (p < 0·05). Participants with a negative SARS-CoV-2 PCR test experienced 1[0–2] symptom before, 5[3–9] symptoms during, and 2[0–4] symptoms after the test (p < 0.05). In the weeks to months following the testing, the total number of symptoms remained equal or decreased in 44% of positive and 52% of negative tested participants compared to pre-test levels (Fig. 2).
Fatigue
The prevalence of severe fatigue was significantly increased after a positive test compared to the period before (4% versus 18% severe fatigue; p < 0.05). A higher prevalence of severe fatigue after compared to before the test was also found in negative tested participants, though to a lesser degree (5% versus 8%; p < 0.05) (Fig. 3).
Quality of life
On average the experienced QoL was 8[7-8] points before, 6[5–7] points during, and 7[7-8] points after a positive SARS-CoV-2-PCR test (p < 0.05). For negative tested persons the QoL was lower during the test (though higher than in positives, i.e. before: 8[7-8], during: 7[6–8], after: 7[7-8], p < 0.05) (Fig. 4).
Discussion
The study design of the Lifelines COVID-19 Cohort allows us to get a first detailed insight in the symptom burden and QoL before, during, and after a positive and negative SARS-CoV-2 PCR test. In the weeks or months following a SARS-CoV-2 infection, nearly all symptoms were more frequently present compared to before. A higher symptom prevalence after compared to before a test was also found for nearly all symptoms in negative tested participants. Before the test, symptoms were already partly present and reporting of nearly all symptoms before did not differ between positives and negatives. QoL decreased around the SARS-CoV-2 PCR test for both positive and negative tested participants, with a larger deterioration for positives.
The current study re-confirms that SARS-CoV-2 presents itself as a heterogeneous disease1,2. Fortunately, many positive tested participants fully recover. Nevertheless, a substantial proportion of participants continues to experience symptoms in the weeks or months after a positive SARS-CoV-2 PCR test. This is in accordance with previous studies performed in patients with mild to severe COVID-195,6,7,8. Thus far, it was unknown whether and to what extent positive tested participants were free of symptoms before the actual infection, as data about the pre COVID-19 status in longitudinal studies were scarce. The current study showed that symptoms were already partly present before the infection. This implies that these persisting symptoms may not solely be attributed to the SARS-CoV-2 infection. This is in accordance with Wu and colleagues, who found that 44% of a sample from the U.S. community experienced at least one symptom already before the infection13. Then again, a part of the ongoing symptoms could not be explained by the pre-infection status and are, therefore, most probably new-onset or persisting symptoms following a SARS-CoV-2 infection.
Another difficulty when characterizing Long-COVID is the lack of data from participants with a negative SARS-CoV-2 test, as many symptoms are non-specific, common in the general population, and may be related to another infection, an underlying chronic disease, ageing, or changes in behaviour due to the pandemic16. Indeed, an increase in symptom burden was also found for participants after a negative SARS-CoV-2 PCR test compared to before, though not for the symptoms loss of sense of smell or taste and fever which remained equally prevalent. This is in accordance with Søraas and colleagues, who studied the persistence of symptoms 3 to 8 months after a SARS-CoV-2 positive or negative test and found that especially the symptom loss of sense of smell or taste was significantly more frequently reported by positive tested persons compared to negative 132 days after testing15. In addition, a French population-based cohort, comparing groups according to both European Center for Diseases Control (ECDC) criteria for COVID-19 (ECDC + or ECDC–) and serological SARS-CoV-2 tests results (sero + or sero–), found that individuals in all groups had a similar risk of having at least one symptom lasting more than 2 months17. Of note, these results should be interpreted with caution as some individuals do not show sero-conversion or because sero-reconversion occurs over time, leading to serological misclassification. Nevertheless, all these findings together suggest that persistent symptoms are also common in the general population and may, therefore, not all be directly related to a SARS-CoV-2 infection.
With regards to QoL, it was expected that participants with a positive SARS-CoV-2 PCR test would experience lower levels of QoL after the infection, compared to negative tested participants. The current findings, however, indicated that the QoL levels for both positive and negative tested participants nearly returned to pre-test values on group level. This while Huang et al. showed that community-dwelling COVID-19 survivors still had lower health status one year after the infection than non-COVID-19 controls14. Nevertheless, the included participants in the study from Huang and colleagues were all previously hospitalized patients, whereas the proportion of hospitalized participants in the current study was small, which could explain the difference in experienced health status in the weeks or months following the infection.
Some limitations have to be considered when interpreting the current findings. First, SARS-CoV-2 diagnosis and severity is based on self-report without the use of medical records. In addition, the precise date of the COVID-19 diagnosis was unknown. Therefore, the precise duration of the symptoms post-COVID-19 was also unknown. Second, the results cannot be generalized to all people with COVID-19, as adolescents (< 18 years of age) were not included in the current analyses. Moreover, cases with severe SARS-CoV-2 were less likely to participate in the current study. Hence, the small proportion of hospitalized participants. Third, the participants with a negative SARS-CoV-2 PCR test should not be considered as healthy controls, since these participants were eligible for PCR testing. Nevertheless, the reason for testing (e.g. testing due to COVID-19 like symptoms, contact with persons with COVID-19 or travels) was unknown. In addition, SARS-CoV-2 serology tests for the retrospective diagnosis of COVID-19 were not performed in the current study and, therefore, an undetected infection during the first months of the COVID-19 pandemic cannot be completely ruled out. Fourth, only participants with one positive or negative SARS-CoV-2 PCR test were taken into account. Hence, results cannot be generalized to people with multiple periods with symptoms that could be attributed to infections. Fifth, data concerning pre-existing comorbidities are lacking and the effect of pre-existing comorbidities on the symptom burden could not be determined. Sixth, BMI was assessed during the first visit of the general Lifelines Cohort Study (e.g. recruitment period 2006–2013). Therefore, the effect of a change in BMI on symptom severity (i.e. back pain, shortness of breath) could not be evaluated. Seventh, no information was available on whether the participants received a vaccination during the course of the study. Then again, the protective effect of vaccinations against Long-COVID is unknown yet18,19. Eight, the onset of new COVID-19 variants was not considered. Nevertheless, the current data are from approximately September 2020 to July 2021, while Omicron presented itself in Europe in late 2021 and early 2022. At last, the list of symptoms might not be all-inclusive, as people with a SARS-CoV-2 infection may experience up to 203 different symptoms, including brain fog, concentration problems, and fluctuating symptoms like post-exertional malaise7,20.
Conclusions
This study contributes to the rapidly developing knowledge on COVID-19 and its long-term consequences. The findings indicate that not all symptoms after a positive SARS-CoV-2 test might be attributable to the infection and symptoms were also common in participants with a negative SARS-CoV-2 PCR test. Nonetheless, a part of the ongoing symptoms could not be explained by the pre-infection status. These new-onset or persisting symptoms after a SARS-CoV-2 infection are a major public health concern and warrant attention.
Methods
Study design and participants
The current study used data from the Lifelines COVID-19 Cohort Study, an add-on study to the Lifelines Cohort Study21,22. The Lifelines Cohort Study is an ongoing multi-disciplinary prospective population-based cohort study examining the health and health-related behaviours of 167,729 persons living in the North of The Netherlands in a unique three-generation design. It employs a broad range of investigative procedures in assessing the biomedical, socio-demographic, behavioural, physical and psychological factors which contribute to the health and disease of the general population, with a special focus on multi-morbidity and complex genetics. The participants of the Lifelines Cohort Study were recruited between 2006 and 2013, through general practitioners and self-enrollment. Participants who were unable to understand the Dutch language, were not able to fill in questionnaires, not able to visit the general practitioner, had severe mental illness (i.e. not fully capable to make rational decisions), or who had limited life expectancy (< 5 years) due to severe illness were not considered eligible21,22,23.
For the add-on Lifelines COVID-19 Cohort Study digital questionnaires were sent out to all adult participants with a known e-mail address of the Lifelines Cohort Study. At the beginning questionnaires were sent out on a weekly basis starting March 2020, later bi-weekly starting June 2020, and monthly starting August 2020. In the current study data from 24 questionnaires were used, collected over a period from approximately March 2020 through July 2021. A description of the cohort and study specifications has been published previously24.
Of note, the current analyses only take into account participants who reported one positive or negative SARS-CoV-2 PCR test as of COVID-19 Questionnaire 13 which was sent out starting September 2020 during the second wave of the pandemic when PCR tests were more readily available in The Netherlands. In addition, only participants who filled out at least two questionnaires before and after a positive or negative SARS-CoV-2 PCR test were included in the population of analyses.
The Lifelines Cohort Study, including the Lifelines COVID-19 Cohort Study, was conducted according to the principles of the Declaration of Helsinki and approved by the Medical Ethics Committee of the University Medical Center Groningen, the Netherlands (number 2007/152). No additional ethical approval is needed to request data collected within the regular protocol of Lifelines. All participants signed an informed consent.
Measures
Demographic characteristics
Sex, age, educational level (low, medium, and high25), and self-reported health status (poor, mediocre, good, very good, and excellent) were assessed during the first COVID-19 questionnaire (COVID-19 Questionnaire 1). Body Mass Index (BMI) was assessed during the first visit of the general Lifelines Cohort Study by a research assistant using a standardized protocol.
COVID-19 related characteristics
Participants were asked to self-report whether they were tested for COVID-19 and if the result was positive or negative, since the last time they filled out a questionnaire. As of November 2020, a question was added to evaluate if the test was performed by the Municipal Public Health Service in The Netherlands. This was to ensure that only participants with a positive or negative SARS-CoV-2 PCR test, not self-administered test (antigen), were taken into account in the current analyses. Before November 2020 antigen tests were rarely available so most participants are likely to have had a PCR test. In case of a reported positive SARS-CoV-2 test, the severity of the infection was assessed (e.g. hospitalization, the use of antibiotics/supplemental oxygen during hospitalization, and intensive care unit (ICU) admission).
Self-reported symptoms
Self-report questions were used to evaluate the extent to which the participants experienced 28 symptoms (symptom severity) in the last 7 days using a five-point Likert-scale (1 = not at all; 2 = a little bit; 3 = somewhat; 4 = quite a lot; 5 = very much) (See Online Supplement for the list of symptoms). When questionnaires were sent out bi-weekly and monthly instead of weekly, the reference period of the questions was adapted to the last 14 and 28 days, respectively. A cut-off score of ≥ 2 points (‘a little bit’) was used to determine the prevalence of the symptom at the time of questionnaire administration.
Fatigue severity was measured using the Shortened Fatigue Questionnaire (SFQ). The SFQ consists of four items scored on a seven-point Likert scale (“I feel tired”, “I tire easily”, “I feel fit”, and “I feel physically exhausted”). The score ranges from 4 to 28 points, with higher scores indicating more severe fatigue. Severe fatigue is indicated by a SFQ score of ≥ 18 points26.
Quality of life
QoL was assessed with the following question “how would you rate your quality of life over the past 7, respectively 14 and 28, days”, using a 10 point-Likert scale (1 = terrible, 10 = excellent).
Defining the symptom severity, -prevalence, -burden and QoL before, during, after a positive or negative test
To determine the symptom severity and QoL during a positive and negative SARS-CoV-2 PCR test, a so-called time-point zero was defined. This was the first administered questionnaire in which the respondent reported a positive or negative PCR test since the last time they filled in a questionnaire. To calculate the symptom severity and QoL in the weeks/months prior and after a positive or negative SARS-CoV-2 PCR test, the average was calculated of the available answers on the Likert-scales from COVID-19 Questionnaire 1 to 24. In these calculations, information on symptoms and QoL from the questionnaire that was filled out directly before the test was not taken into account to prevent that an emerging infection influenced the symptom burden and QoL in the pre-test period. Based on the symptom severity in the weeks/months before, during, and after a PCR test, the prevalence of each symptom was determined using the cut-off score of ≥ 2 points (‘a little bit’). The total number of symptoms present before, during, or after a PCR test is referred to as the symptom burden.
Statistical analyses
Statistical analyses and visualization were conducted using SPSS (V.25.0 for Windows, Chicago, IL, USA) and SankeyMATIC (http://sankeymatic.com/build/). Descriptive statistics on group level were reported as mean and standard deviation, median and interquartile range, or frequency and percentage, as appropriate. Chi-square was conducted to compare symptoms and QoL by test-result (e.g. positive versus negative tested participants) for each time-period separately. One-way repeated measured ANOVA and McNemar test were used to determine if there were differences in symptoms and QoL between time-periods (before, during, and after a SARS-CoV-2 test) for positives and negatives separately. In case of significant differences between time-periods, a post-hoc analysis with Bonferroni adjustment was carried out. The level of significance was set at < 0.05.
Data availability
We are not permitted to share individual data from the Dutch Lifelines study. Information on applying for access to the Dutch Lifelines data is available at https://www.lifelines.nl/researcher/how-to-apply.
Change history
10 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-58848-4
References
Docherty, A. B. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 369, m1985 (2020).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020).
Verity, R. et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 20, 669–677 (2020).
WHO. Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 20, e192–e197 (2020).
Carfì, A., Bernabei, R. & Landi, F. Persistent symptoms in patients after acute COVID-19. JAMA 324, 603–605 (2020).
Vaes, A. W. et al. Recovery from COVID-19: A sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 7, 00141–02021 (2021).
Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38, 101019 (2021).
Goërtz, Y. M. J. et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome?. ERJ Open Res. 6, 00542–02020 (2020).
Callard, F. & Perego, E. How and why patients made long Covid. Soc. Sci. Med. 268, 113426 (2021).
WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. https://apps.who.int/iris/rest/bitstreams/1376291/retrieve (Accessed 6 October 2021).
Meys, R. et al. Generic and respiratory-specific quality of life in non-hospitalized patients with COVID-19. J. Clin. Med. 9, 3993 (2020).
Delbressine, J. M. et al. The impact of post-COVID-19 syndrome on self-reported physical activity. Int. J. Environ. Res. Public Health 18, 6017 (2021).
Wu, Q., Ailshire, J. & Crimmins, E. Long COVID and symptom trajectory in a representative sample of Americans. Sci. Rep. 12, 11647 (2022).
Huang, L. et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 398, 747–758 (2021).
Søraas, A. et al. Persisting symptoms three to eight months after non-hospitalized COVID-19, a prospective cohort study. PLoS One 16, e0256142 (2021).
Amin-Chowdhury, Z. & Ladhani, S. N. Causation or confounding: Why controls are critical for characterizing long COVID. Nat. Med. 27, 1129–1130 (2021).
Robineau, O. et al. Persistent symptoms after the first wave of COVID-19 in relation to SARS-CoV-2 serology and experience of acute symptoms: A nested survey in a population-based cohort. Lancet Reg. Health Eur. 17, 100363 (2022).
Strain, W. D. et al. The impact of COVID vaccination on symptoms of long COVID: An international survey of people with lived experience of long COVID. Vaccines 10, 652 (2022).
Taquet, M., Dercon, Q. & Harrison, P. J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 103, 154–162 (2022).
Brown, D. A. & O’Brien, K. K. Conceptualising long COVID as an episodic health condition. BMJ Glob. Health 6, e007004 (2021).
Stolk, R. P. et al. Universal risk factors for multifactorial diseases: LifeLines: A three-generation population-based study. Eur. J. Epidemiol. 23, 67–74 (2008).
Scholtens, S. et al. Cohort profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 44, 1172–1180 (2015).
Klijs, B. et al. Representativeness of the LifeLines Cohort Study. PLoS One 10, e0137203 (2015).
Mc Intyre, K. et al. Lifelines COVID-19 cohort: Investigating COVID-19 infection and its health and societal impacts in a Dutch population-based cohort. BMJ Open 11, e044474 (2021).
International Standard Classification of Education ISCED. http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf (Accessed 6 October 2011).
Penson, A. et al. Short fatigue questionnaire: Screening for severe fatigue. J. Psychosom. Res. 137, 110229 (2020).
Funding
The Lifelines initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG), Groningen University and the Provinces in the North of the Netherlands (Drenthe, Friesland, Groningen). Y.M.J.G. is financially supported by Lung Foundation Netherlands grant 4.1.16.085. The funding organization had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Y.M.J.G., M.A.S., M.V.H., C.B. and D.J.A.J. formulated the research questions and applied for the use of Lifelines data. Y.M.J.G. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. Y.M.J.G., M.A.S., and D.J.A.J interpreted the findings and drafted the manuscript. All authors critically revised and reviewed the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Corresponding author
Ethics declarations
Competing interests
Y.M.J.G., M.V.H., N.D., C.J.H.K., and C.B., have nothing to disclose. M.A.S. reports grants from Lung Foundation Netherlands, Stichting Astma Bestrijding, Boehringer Ingelheim, AstraZeneca, Chiesi and TEVA, outside the submitted work. D.J.A.J. reports grants from the Netherlands Organisation for Health Research and Development (ZonMw), Stichting Astmabestrijding, and the Netherlands Respiratory Society, fees from Boehringer Ingelheim, Chiesi and Abbott, outside the submitted work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Consortium list ‘Lifelines Corona Research Initiative’, in which Judith G. M. Rosmalen was incorrectly listed as an author.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goërtz, Y.M.J., Spruit, M.A., Van Herck, M. et al. Symptoms and quality of life before, during, and after a SARS-CoV-2 PCR positive or negative test: data from Lifelines. Sci Rep 13, 11713 (2023). https://doi.org/10.1038/s41598-023-38223-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38223-5
This article is cited by
-
The biomarkers’ landscape of post-COVID-19 patients can suggest selective clinical interventions
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.