Abstract
Chronic inflammation and dyslipidemia are important risk factors in developing atherosclerotic cardiovascular disease, such as coronary heart disease. Acute coronary syndrome (ACS) is one of the most dangerous syndromes in coronary heart disease. Type 2 diabetes mellitus (T2DM) is considered equal to coronary heart disease owing to the high cardiac risk induced by chronic inflammation and dyslipidemia. The neutrophil to high-density lipoprotein cholesterol ratio (NHR) is a novel and straightforward marker that reflects inflammation and lipid metabolic disorder. However, few studies have been on the role of NHR in assessing the risk of ACS in T2DM patients. Here we analyzed NHR level in ACS patients with T2DM, exploring its predictive and diagnostic values. 211 hospitalized ACS patients with T2DM were recruited as the case group, and 168 hospitalized T2DM patients as the control group (all patients collected from 6/2020 to 12/2021 in Xiangya Hospital). Biochemical test results and echocardiograms, as well as demographic information such as age, BMI, diabetes mellitus, smoking, drinking, and history of hypertension, were recorded. Frequencies, percentages, means, and standard deviations were used to describe the data. The shapiro–Wilk test was used to assess the normality of the data. Normally distributed data were compared using the independent sample T-test, and non-normally distributed data were compared using Mann–Whitney U test. Correlation analysis was performed using the Spearman rank correlation test, and receiver operating characteristic (ROC) curve analysis and multivariable logistic regression analysis were performed by SPSS version 24.0 (SPSS Inc) and GraphPad Prism 9.0 (GraphPad Software Inc). p < 0.05 was considered significant. In the study population, NHR was higher in patients with T2DM combined with ACS than in T2DM patients without ACS (p < 0.001). After adjusting for BMI, alcohol consumption, and history of hypertension, multifactorial logistic regression analysis revealed that NHR is a risk factor for T2DM patients combined with ACS (OR 1.221, p = 0.0126). Correlation analysis on all ACS patients with T2DM showed that NHR level was positively correlated with cTnI (r = 0.437, p < 0.001), CK (r = 0.258, p = 0.001), CK-Mb (r = 0.447, p < 0.001), LDH (r = 384, p < 0.001), Mb (r = 0.320, p < 0.001), LA (r = 0.168, p = 0.042) and LV levels (r = 0.283, p = 0.001). And meanwhile, NHR level was negatively correlated with EF (r = − 0.327, p < 0.001) and FS levels (r = − 0.347, p < 0.001). ROC curve analysis showed that NHR ≧ 4.32 had a sensitivity of 65.45% and a specificity of 66.19% for predicting ACS in T2DM patients [area under the curve (AUC) = 0.722, p < 0.001]. Furthermore, in all ACS patients with T2DM, the diagnostic power of NHR was stronger in ST-segment elevated ACS patients (STE-ACS) than that in non-ST-segment elevated ACS patients (NSTE-ACS) (p < 0.001). With its convenience and effective character, NHR could be a potential and new marker for predicting the presence, progression, and severity of ACS in T2DM population.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is becoming a significant public health problem and a hot spot for clinical research with the rising incidence of it. The prevalence of diabetes in the Chinese adult population is about 11.6%, and the prevalence of prediabetes is around 50.1%1. As comorbidity of T2DM, cardiovascular disease (CVD) is also increasing nowadays2,3. A study has shown that CVD is the leading cause of death in patients with T2DM, accounting for approximately half of all deaths during the study period4. Acute coronary syndrome (ACS), one of the most severe types of coronary artery disease (CAD), is the most common CVD and is widely interrelated with T2DM5. ACS is triggered by the rupture of unstable atheromatous plaque in the coronary artery, resulting in thrombosis and coronary artery obstruction. Varying degrees of coronary stenosis bring varying degrees of myocardial ischemia6 and different symptoms. Non-ST-segment elevation ACS (NSTE-ACS) is characterized by the absence of significant ST-segment elevation on ECG. It is caused by partial or intermittent coronary arterial occlusion, which accounts for approximately 70% of ACS. ST-segment elevation ACS (STE-ACS) caused by complete coronary artery occlusion accounts for approximately 30% of ACS7. Commonly, many T2DM patients have ACS, which significantly increases the risk of major adverse cardiac events (MACE)4,8. Finding a relevant biomarker and identifying the ACS patients in T2DM might be of great benefit in preventing disease aggravation.
Studies have shown that inflammation and dyslipidemia play a key role in plaque formation and atherosclerosis progress, which leads to ACS9,10. Neutrophils, one of the markers that reflect inflammation, also participate in plaque instability and in the early formation of atherosclerosis11,12. In contrast, high-density lipoprotein cholesterol (HDL-C) is considered a protective factor against atherosclerosis due to its role in reversing cholesterol transport and its antioxidant ability13. Furthermore, HDL has been shown to regulate neutrophil activation and reduce neutrophil proliferation and migration14. HDL has been shown to regulate neutrophil activation and reduce neutrophil proliferation and migration15. The neutrophil to HDL-cholesterol ratio (NHR) is a composite marker reflecting inflammation and lipid metabolism. NHR is a strong predictor of cardiovascular disease in many studies16,17,18. Even though these two hematological parameters are inexpensive and readily available, the critical value of NHR in ACS patients has yet to be noticed or emphasized. This research aimed to assess the predictive and diagnostic value of NHR on the risk of developing ACS in T2DM patients.
Materials and methods
Study participants
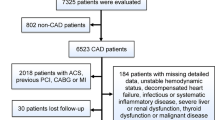
Hospitalized patients diagnosed with T2DM at Xiangya Hospital of Central South University from June 2020 to December 2021 were enrolled. T2DM patients combined with ACS, including STE-ACS and NSTE-ACS study, were treated as the study population, and T2DM patients without ACS were treated as a control population. This cross-sectional study was performed per the ethical guidelines of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University. Written informed consent was obtained from all subjects before enrollment. ACS was defined as (1) clinical manifestations, (2) elevated cTnI, (3) elevated cardiac enzymes19, and (4) electrocardiographic changes. Exclusion criteria included: chronic kidney disease with serum creatinine > 2.5 mg/dL, hepatic sclerosis, congestive heart failure, chronic lung disease, symptomatic peripheral vascular disease, tumors, and chronic infections; those with combined hematologic, autoimmune, and inflammatory diseases; those with combined multi-organ insufficiency; and those taking glucocorticoids or non-steroidal anti-inflammatory drugs (Fig. 1).
Clinical and biochemical parameters
Information such as age, sex, body mass index (BMI), smoking history, drinking history, history of hypertension, and parameters such as RBC, Hb, PLT, Glu, TG, TC, HDL-C, LDL-C, UA, Urea, Cre, CK, CK Mb, LDH, Mb, and NHR were all collected. The levels of hemoglobin, red blood cells, and platelets were measured based on the Kurt principle by the German BeckmanDxH800 blood analyzer. Glu, TG, TC, HDL-C, LDL-C, UA, Urea, Cre, CK, CK Mb, LDH, and Mb were measured in the spectrophotometric determination method by Germany BeckmanAU680 biochemical analyzer. The dimensions of the Left Ventricle (LV) and Left Atrial (LA) on the end-diastolic phase, the ejection fraction (EF), and the fractional shortening (FS) were performed by echocardiogram.
Statistical analysis
SPSS 24.0 statistical software and GraphPad Prism 9.0 were used to analyze the data. Data are expressed as the mean ± standard deviation (SD) for continuous variables and as percentages for discreet variables. The normality of the data was assessed using the Shapiro–Wilk test. Comparison of normally distributed continuous variables between groups was performed by independent sample T-test. Data that did not have a normal distribution were expressed as medians (interquartile range). Mann–Whitney U test was used for comparison of variables that did not have a normal distribution. The data for categorical variables were analyzed by the χ2 test. Correlation between variables was determined by Spearman’s correlation test. Different logistic regression models were implemented to interrogate the association of ACS in patients with T2DM. In model 1, no covariates were adjusted; in model 2, BMI, smoking and history of hypertension were adjusted. The model was assessed using the Hosmer–Lemeshow test. And ROC curve analysis was used to evaluate the diagnostic value and to define the diagnostic cut-off value of NHR concentrations in the T2DM patients combined with ACS. A two-sided p value < 0.05 was considered to indicate statistical significance.
Ethics approval and consent to participate
The study protocol was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University and all methods were performed in accordance with the relevant guidelines and regulations.
Results
Baseline clinical characteristics and biochemical measurements
A total of 379 study subjects were included, including 211 cases in the T2DM combined with the ACS group and 168 cases in the T2DM group. Demographic, clinical, and biochemical data for the cases (T2DM with ACS) and controls (T2DM) were shown in Table 1. In comparing the general data of the 2 groups, the differences in age and history of hypertension were statistically significant (p < 0.05). Cases had statistically significantly higher NHR, CK, CK-Mb, LDH, Urea, and Cre levels than controls. Cases had statistically significantly lower serum LDL-C and TC levels than controls. The differences in sex, BMI, smoking history, drinking history, RBC, Hb, PLT, Glu, TG, HDL-C, and UA were not statistically significant (p > 0.05, Table 1).
Multi‑logistic regression analysis to determine the relationship between the NHR and the presence or absence of ACS in T2DM patients
In this cross-sectional study, the variables we included in the multivariable logistic regression analysis, including NHR, age, TC, LDL-C, UA, Urea, CK, CK-Mb, and LDH, which were statistically significant (p < 0.05) in the univariate logistic regression analysis. In the unadjusted model, higher NHR and age were independent risk factors for developing ACS in patients with T2DM (Model 1, Hosmer–Lemeshow test p = 0.519). Further, multivariable logistic regression analysis conducted in the 379 patients after adjustments for BMI, drinking, and history of hypertension suggested that higher NHR and age were independent risk factors of ACS in T2DM patients (Model 2, Hosmer–Lemeshow test p = 0.793) (Fig. 2).
Correlation between NHR level and clinical parameters in T2DM patients with ACS
Correlation analyses undertaken on all T2DM patients with ACS showed that NHR level was positively correlated with cTnI (r = 0.437, p < 0.001), CK (r = 0.258, p < 0.001), CK-Mb (r = 0.447, p < 0.001), LDH (r = 384, p < 0.001), Mb (r = 0.320, p < 0.001), LA (r = 0.168, p = 0.042) and LV levels (r = 0.283, p = 0.001) and negatively correlated with EF (r = − 0.327, p < 0.001) and FS levels (r = − 0.347, p < 0.001, Table 2, Fig. 3(*p < 0.05, **p < 0.01 and ***p < 0.001)).
NHR levels in groups
NHR level was statistically significantly higher in study cases as compared to controls [5.00 (3.65–6.88) vs 3.62 (2.69–5.13), p < 0.001]. Utilizing the ROC curve for the value of NHR and risk of ACS in patients with T2DM, the greatest increase in the ACS risk was seen at NHR levels of more than 4.32 [Fig. 4, AUC = 0.696, sensitivity of 65.45% (95% CI 57.61% to 72.57%) and specificity of 66.19% (95% CI 57.61% to 73.85%), Youden’s index = 0.316, p < 0.001].
The study included 211 T2DM patients combined with ACS, including 57 patients with STE-ACS. Of those 57 patients, 52 patients (91.23%) were identified by the above cutoff value (cutoff value = 4.32). In addition, there were 154 patients with NSTE-ACS and 100 NSTE-ACS patients (64.94%) were identified by it, which suggested that the NHR level has a significant difference between the above two sub groups in ACS patients combined with T2DM (Table 3, p < 0.001).
Discussion
In this study, we demonstrated that NHR is an independent risk factor for the development of ACS in the T2DM population. Our result showed that NHR had a significant positive correlation with some biochemical markers of myocardial ischemia, necrosis, and remodeling in T2DM patients combined with ACS. It has strongly suggested the potential importance of NHR in the progression of ACS in T2DM patients. Furthermore, our study showed that NHR≧4.32 has good discriminatory power in diagnosing ACS patients in the T2DM population, although the accuracy may need to be demonstrated by more patients and data. NHR may help predict ACS patients in T2DM patients.
With further analysis, we found the correlation of NHR with biochemical marks of myocardial ischemia, necrosis, and remodeling (cardiac ultrasound indices) in T2DM patients combined with ACS. The results revealed that NHR levels were significantly and positively correlated with cTnI, CK, CK-Mb, LDH, and MB, which are indicators of myocardial ischemia and injury. In addition, NHR levels also showed a significant positive correlation with left atria (LA) and left ventricle (LV) levels, suggesting a relationship between NHR and cardiac remodeling aspects. NHR levels were significantly and negatively correlated with EF and FS, indicators of cardiac function. It has been shown that neutrophils are a critical contributor to LV infarct wall thinning due to cardiac remodeling and that higher neutrophils correspond to lower EF and FS20. The correlation of these markers may explain their diagnostic value in T2DM patients combined with ACS.
To evaluate the diagnostic and predictive value of NHR level for ACS in T2DM patients, we performed ROC analysis to determine the cut-off value, sensitivity, specificity, AUC, and Youden’s index of NHR for diagnosing acute coronary syndrome in T2DM patients. Further, we examined the discriminatory ability of the cut-off value of NHR in T2DM patients combined with STE-ACS and NSTE-ACS. Our results showed that NHR had higher discriminatory accuracy and diagnostic value in T2DM patients combined with STE-ACS, which is of higher severity than NSTE-ACS. This suggests that NHR may correlate with the severity of ACS in T2DM patients. Traditionally, the diagnosis of ACS has been made by clinical symptoms, ECG changes, cardiac biomarkers, and, in some cases, cardiac imaging. However, the search for new biomarkers is increasing, which will help to improve the diagnosis and prognosis of patients with ACS. Our study suggests that NHR levels could be potentially used as a new complementary marker, with the traditional cardiac markers together, for diagnosing ACS patients in the T2DM population.
Recently, an increasing number of studies have used composite predictors of hematological parameters as novel potential risk markers. The ratios of different parameters can provide more comprehensive information than traditional single parameters and have considerable diagnostic and predictive power16,17,18,21,22,23. For example, neutrophil to HDL-C ratio (NHR)16,17,18,23, platelet to lymphocyte ratio (PLR)24, monocyte to HDL-C ratio (MHR)21,22, lymphocyte to HDL-C ratio (LHR)25, platelet to HDL-C ratio (PHR)26, and triglyceride to HDL-C ratio (THR)27. These markers from routine blood tests have a good future due to their affordability and accessibility.
Many previous studies have found that NHR has a better application in the above-mentioned indices due to its unique advantages16,17,18,23. Firstly, T2DM and ACS involve complex pathological processes of inflammatory response and abnormal lipid metabolism9,10,28. NHR can not only present both inflammatory state and lipid metabolism, but also indicate the interaction between neutrophils and HDL-C. Advanced atherosclerotic plaques have a high number of neutrophils and their counts are positively correlated with the histopathological features of rupture-prone atherosclerotic lesions29. It has also been demonstrated that low HDL-C is an important factor in accelerating atherosclerosis in diabetic patients30. Neutrophils and HDL-C play a mutually regulating role in ACS. Activated neutrophils can also mediate HDL oxidation and impair cholesterol efflux by possessing enzymes that produce oxidants31. In contrast, HDL-C can inhibit neutrophil activation, adhesion, proliferation and migration15. Secondly, neutrophils make up the major part of leukocytes and therefore they can better reflect cardiovascular risk than monocytes and lymphocytes11,12,32,33. On the other hand, neutrophils are considered to be the primary players in the acute inflammatory response and play an important role in the subsequent activation of monocytes and lymphocytes34,35,36. These key roles of neutrophils allow NHR to provide better diagnostic and predictive value for cardiovascular disease.
However, our study still has some limitations that should be noted. First, the NHR was measured only once at baseline, which may not reflect the time-dependent association of dynamic changes in NHR with clinical performance. Therefore, our study can only demonstrate that NHR is helpful in the diagnosis and prediction of ACS in patients with T2DM, and more data and more diverse studies are needed to support our view. Second, although the study process was adjusted for covariates as much as possible, we cannot exclude possible residual confounding effects of unmeasured or unincluded variables, such as the different disease duration and different treatments for each T2DM patient. Third, variables such as lifestyle factors and medical history were self-reported, which could lead to recall bias. Finally, this was a cross-sectional study, so a causal relationship between NHR and the development of ACS in T2DM patients could not be demonstrated. Further prospective studies are needed to analyze whether lowering NHR reduces the occurrence and progression of ACS.
Conclusion
In conclusion, this study suggests that elevated NHR has great potential to be a convenient and effective measure to predict the presence and progression of ACS in T2DM patients. Although more investigations, especially longitudinal ones, are still needed to further validate this.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Xu, Y. et al. Prevalence and control of diabetes in Chinese adults. JAMA 310(9), 948–959 (2013).
Ma, R. C. W. Epidemiology of diabetes and diabetic complications in China. Diabetologia 61(6), 1249–1260 (2018).
Einarson, T. R., Acs, A., Ludwig, C. & Panton, U. H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 17(1), 83 (2018).
Zhou, M. et al. Prevalence and in-hospital outcomes of diabetes among patients with acute coronary syndrome in China: Findings from the improving care for cardiovascular disease in china-acute coronary syndrome project. Cardiovasc. Diabetol. 17(1), 147 (2018).
De Caterina, R., Madonna, R., Sourij, H. & Wascher, T. Glycaemic control in acute coronary syndromes: Prognostic value and therapeutic options. Eur. Heart J. 31(13), 1557–1564 (2010).
Timmis, A. Acute coronary syndromes. BMJ 351, h5153 (2015).
Bhatt, D. L., Lopes, R. D. & Harrington, R. A. Diagnosis and treatment of acute coronary syndromes: A review. JAMA 327(7), 662–675 (2022).
Farhan, S. et al. Impact of pre-diabetes on coronary plaque composition and clinical outcome in patients with acute coronary syndromes: An analysis from the PROSPECT study. JACC Cardiovasc. Imaging 12(4), 733–741 (2019).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352(16), 1685–1695 (2005).
Rymer, J. A. & Newby, L. K. Failure to launch: Targeting inflammation in acute coronary syndromes. JACC Basic Transl. Sci. 2(4), 484–497 (2017).
Soehnlein, O. Multiple roles for neutrophils in atherosclerosis. Circ. Res. 110(6), 875–888 (2012).
Pende, A., Artom, N., Bertolotto, M., Montecucco, F. & Dallegri, F. Role of neutrophils in atherogenesis: An update. Eur. J. Clin. Invest. 46(3), 252–263 (2016).
Assmann, G. & Gotto, A. M. Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation 109(23 Suppl 1), 8–14 (2004).
Gomaraschi, M. et al. Inflammation impairs eNOS activation by HDL in patients with acute coronary syndrome. Cardiovasc. Res. 100(1), 36–43 (2013).
Murphy, A. J. et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler Thromb. Vasc. Biol. 31(6), 1333–1341 (2011).
Jiang, M. et al. Prognostic role of neutrophil to high-density lipoprotein cholesterol ratio for all-cause and cardiovascular mortality in the general population. Front. Cardiovasc. Med. 9, 807339 (2022).
Huang, J. B. et al. Neutrophil to high-density lipoprotein ratio has a superior prognostic value in elderly patients with acute myocardial infarction: A comparison study. Lipids Health Dis. 19(1), 59 (2020).
Başyiğit, F. & Çöteli, C. Relationship between the neutrophil to HDL-C ratio and anatomical significance of coronary artery stenosis in patients with documented myocardial ischemia. Eur. Rev. Med. Pharmacol. Sci. 26(9), 3179–3184 (2022).
Amsterdam, E. A. et al. 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 64(24), e139–e228 (2014).
Ma, Y. et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 110(1), 51–61 (2016).
Chen, J. W. et al. The role of monocyte to high-density lipoprotein cholesterol ratio in prediction of carotid intima-media thickness in patients with type 2 diabetes. Front. Endocrinol. (Lausanne) 10, 191 (2019).
Li, C. et al. The monocyte to high-density lipoprotein cholesterol ratio and outcomes in type 2 diabetes mellitus patients with non-ST-segment elevation acute coronary syndrome. Ann. Transl. Med. 9(21), 1627 (2021).
Wang, Y. et al. Prognostic value of leucocyte to high-density lipoprotein-cholesterol ratios in COVID-19 Patients and the diabetes subgroup. Front. Endocrinol. (Lausanne) 12, 727419 (2021).
Dong, G., Huang, A. & Liu, L. Platelet-to-lymphocyte ratio and prognosis in STEMI: A meta-analysis. Eur. J. Clin. Invest. 51(3), e13386 (2021).
Chen, H. et al. Lymphocyte to high-density lipoprotein ratio as a new indicator of inflammation and metabolic syndrome. Diabetes Metab. Syndr. Obes. 12, 2117–2123 (2019).
Jialal, I., Jialal, G. & Adams-Huet, B. The platelet to high density lipoprotein-cholesterol ratio is a valid biomarker of nascent metabolic syndrome. Diabetes Metab. Res. Rev. 37(6), e3403 (2021).
Chang, T. I. et al. Association of serum triglyceride to HDL cholesterol ratio with all-cause and cardiovascular mortality in incident hemodialysis patients. Clin. J. Am. Soc. Nephrol. 12(4), 591–602 (2017).
Gomez-Delgado, F., Katsiki, N., Lopez-Miranda, J. & Perez-Martinez, P. Dietary habits, lipoprotein metabolism and cardiovascular disease: From individual foods to dietary patterns. Crit. Rev. Food Sci. Nutr. 61(10), 1651–1669 (2021).
Ionita, M. G. et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb. Vasc. Biol. 30(9), 1842–1848 (2010).
Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Jama. 285(19), 2486–2497 (2001).
Smith, C. K. et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: An additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. (Hoboken, NJ) 66(9), 2532–2544 (2014).
Horne, B. D. et al. Which white blood cell subtypes predict increased cardiovascular risk?. J. Am. Coll. Cardiol. 45(10), 1638–1643 (2005).
ó Hartaigh, B. et al. Which leukocyte subsets predict cardiovascular mortality? From the LUdwigshafen RIsk and Cardiovascular Health (LURIC) Study. Atherosclerosis 224(1), 161–169 (2012).
Mantovani, A., Cassatella, M. A., Costantini, C. & Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11(8), 519–531 (2011).
Silvestre-Roig, C., Hidalgo, A. & Soehnlein, O. Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood 127(18), 2173–2181 (2016).
Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc Biol. 108(1), 377–396 (2020).
Funding
The major special project of science and technology for the construction of innovative provinces in Hunan Province, 2021SK1020. Scientific Research Project of Hunan Province Health Commission, 202203014687. China international medical foundation 2021 Cardiovascular Multidisciplinary Integrated Thinking Research Fund Project, z-2016-23-2101-20, and z-2019-42-1908-3. Suzhou Industrial Park Xinxin Cardiovascular Health Foundation Project, 2020-CCA-ACCESS-117.
Author information
Authors and Affiliations
Contributions
H.R. and B.Z. contributed the central idea. H.R. and B.Z wrote the main manuscript text. Y.L. and Z.W. prepared Figs. 1, 2, 3 and 4. X.Z. and Z.Z. prepared Tables 1, 2 and 3. The remaining authors contributed to refining the ideas and finalizing this paper. K.X. proofread the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ren, H., Zhu, B., Zhao, Z. et al. Neutrophil to high-density lipoprotein cholesterol ratio as the risk mark in patients with type 2 diabetes combined with acute coronary syndrome: a cross-sectional study. Sci Rep 13, 7836 (2023). https://doi.org/10.1038/s41598-023-35050-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35050-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.