Abstract
Latent autoimmune diabetes in adults (LADA) has clinical and metabolic features of type 1 and type 2 diabetes. LADA does not have specific features for its diagnosis apart from autoantibody detection; however, these tests are not affordable in clinical settings. In this cross-sectional study, we analyzed clinical criteria, metabolic control, pharmacological treatment, and diabetic complications in two groups of patients with diabetes -LADA and T2D- in order to identify specific characteristic of these clinical entities. Finally, we evaluated if the estimated glucose disposal rate (eGDR) and age at diagnosis of diabetes could be used as a diagnostic criterion for LADA. Demographic, biochemical, clinical and treatment were measured in 377 individuals with diabetes. The diagnostics of LADA were determined using Glutamic acid decarboxylase autoantibodies levels. Chi-square test or t-Student test were used to establish differences between groups. To identify factors associated with LADA, a logistic regression analysis was used. Finally, a ROC curve was plotted to assess the possible variables as diagnostic criteria for LADA. The 377 patients with diabetes were separated into 59 patients with LADA and 318 patients with T2D. Patients with LADA showed lower fasting glucose values, fewer diabetic complications, younger age at diagnosis of diabetes, higher insulin use, and higher eGDR in comparison to patients with T2D. Both groups had a mean BMI classified as overweight. The ROC evaluated the sensitivity and specificity, this analysis indicated that an age younger than 40.5 years and an eGDR value higher than 9.75 mg/kg/min correlated better with LADA. These parameters could be useful to identify patients suspected to have LADA at the first level of medical care in the population of southeastern Mexico and refer them to a second level of care.
Similar content being viewed by others
Introduction
Individuals with latent autoimmune diabetes in adults (LADA) present autoantibodies that indicate autoimmune pathogenesis. The LADA autoimmune process is milder than type 1 diabetes (T1D); therefore, LADA has a slower progression of beta-cell failure1. The majority of individuals with LADA are positive for single islet autoantibodies, while the appearance of multiple islet autoantibodies is a characteristic of T1D2. Other differences between LADA and T1D are a later mean age of onset in LADA, a slower rate of beta-cell loss, and a more extended period of insulin independence3. In comparison to type 2 diabetes (T2D), individuals with LADA have lower insulin secretion and a faster progression to insulin dependency; furthermore, islet autoantibodies are biomarkers of autoimmune beta-cell destruction that distinguish LADA from T2D1.
Various studies have found LADA features halfway T1D and T2D. Individuals with LADA are younger at diagnosis than T2D, but older than those with T1D4,5. Individuals with LADA have a lower risk of complications than T2D, however, the risk becomes higher in LADA when there is poor glycemic control6,7. Insulin secretion is higher in LADA than in T1D, but lower than in T2D8. A hallmark of T2D is insulin resistance; nonetheless, insulin resistance is also present in LADA but is much lower9,10. Studies comparing insulin resistance in LADA and T2D have used the HOMA-IR index. As serum insulin collection is not always affordable in clinical contexts, the estimated glucose disposal rate (eGDR) is used instead as a validated score to assess insulin resistance11. The parameters of eGDR include easily accessible clinical measures: waist circumference, hypertension, and glycated hemoglobin (HbA1c)12. Hence, calculating eGDR in patients with LADA could offer an accessible measure of insulin resistance and the possibility to differentiate them from patients with T2D.
Nowadays, the diagnostic criteria for LADA include: age > 30 years at diagnosis, absence of insulin requirement for at least six months after diagnosis, and the presence of islet autoantibodies6. Although LADA has clinical and metabolic features similar to T1D and T2D, it has no categorically defined features6. Glutamic acid decarboxylase autoantibodies (GADA) are the most prevalent islet autoantibodies in individuals with LADA4,13,14. Therefore, GADA detection is considered sufficient to differentiate between patients with LADA and those with T2D1. Nevertheless, autoantibody detection is not affordable in clinical settings, meaning that many clinicians cannot make a confident LADA diagnosis6. This uncertainty affects screening strategies and therapeutic approaches; hence, patients with LADA are often misdiagnosed and show worse glycemic control than those with T2D3.
In this study we determined the presence of GADA in the serum of individuals diagnosed with T2D who attend a hospital in southeast Mexico. Afterwards, we identified the clinical criteria, metabolic control, drug treatment, and diabetic complications; then, they were separated into patients with LADA and patients withT2DM. Finally, we evaluated whether the estimated glucose clearance rate (eGDR), and the age of diabetes diagnoses could be used as diagnostic criteria for LADA.
Results
We first selected 566 individuals. We included 377 patients that fulfilled the inclusion criteria. The mean age in our sample was 57.1 ± 10.4 years; 261 individuals were females (69.2%) and 116 were males (30.8%). We found GADA positivity in 15.6% (n = 59) of individuals; these patients were considered as LADA, given they had already accomplished the other two diagnosis criteria. GADA-negative patients were 84.4% (n = 318) and were classified as T2D. We found differences in demographic features between our two groups; for instance, individuals with LADA were younger (53.9 ± 9.3 years) than those with T2D (57.7 ± 10.5). Patients with LADA had 8.6 ± 3.9 years of education, and subjects with T2D had fewer years of education (6.3 ± 4.1) (Table 1).
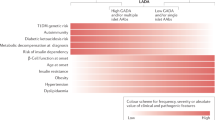
Some clinical features were also different between LADA and T2D: at diagnosis of diabetes, individuals with LADA were younger than those with T2D (40.0 ± 7.6 vs. 44.2 ± 9.5 years). Regarding diabetes complications, we found lower frequencies of complications in patients with LADA. NAFLD, NASH, DKD, neuropathy, and cardiovascular complications were most common in patients with T2D. Other clinical features such as alcohol and tobacco use were equally frequent in LADA and T2D (Table 1).
Both groups, LADA and T2D had similar anthropometric characteristics. The mean BMI was classified as overweight (29.4 and 29.2 kg/m2). Individuals with T2D showed higher levels of fasting glucose. Other biochemical parameters were similar in LADA and T2D groups (Table 1). For instance, eGDR was greater in patients with LADA (8.5 ± 4.6) than those with T2D; furthermore, the mean eGDR in patients with T2D (5.9 ± 2.5) was below the insulin resistance threshold (< 8.0) GADA levels in patients with LADA were 18.9 ± 16.8 IU/mL (Table 1). The majority of patients with LADA (n = 46) presented GADA levels between 5 and 20 IU/mL.
The pharmacologic treatment presented some differences between patients with LADA and T2D. Individuals with LADA used insulin more frequently than those with T2D (79.7% vs. 62.6%, respectively). Short-acting insulin use was less frequent in those with LADA (25.5% vs. 44.7%), while long-acting insulin use was more frequent (70.2% vs. 51.3%). Regarding antidiabetic drugs, metformin was the most frequently used in both groups. Metformin was predominant in LADA (96.6% vs. 84.6%). On the other hand, a large number of individuals with T2D have being used linagliptin (27.7% vs. 11.9%). Doses of pharmacological treatment were similar between patients with LADA and T2D. Nevertheless, short-acting insulin doses were lower in patients with LADA (15.0 ± 6.2 vs. 20.2 ± 14.6 mg) (Table 2).
Logistic regression included all significant variables associated with the univariable analysis. We found that being younger (OR: 1.1, 95% CI: 1.0–1.1), having more years of education (OR: 1.2, 95% CI: 1.1–1.3), using insulin (OR: 4.9, 95% CI: 1.2–20.1), and having higher eGDR (OR: 1.6, 95% CI: 1.3–1.9) were more frequent in individuals with LADA. Conversely, older age at diagnosis of diabetes (OR: 0.9, 95% CI: 0.9–1.0), DKD (OR: 0.2, 95% CI: 0 -0.6), neuropathy (OR: 0.3, 95% CI: 0.1–0.8), and linagliptin use (OR: 0.1, 95% CI: 0.0–0.3) were more frequent in patients with T2D (Table 3).
We performed a ROC curve analysis to evaluate the sensitivity and specificity of being in the LADA group in our sample; we used the significant variables from the logistic regression (Fig. 1). The area under the curve (AUC) for age at diagnosis of diabetes and eGDR were 0.6 (95% CI: 0.6–0.7, p: 0.001) and 0.7 (95% CI: 0.6–0.8, p: 0.004) respectively. In Table 4, we show the Youden index, which indicates the age at diagnosis younger than 41 years and eGDR greater than 9.75 correlated better with our group with LADA. Besides, positive and negative predictive values showed that both criteria are better for discarding than for diagnosis confirmation (Table 4).
Discussion
In our sample, patients with LADA had similar features to patients with T2D; nonetheless, they had fewer diabetic complications, they were younger, had a higher use of insulin and higher eGDR when compared to individuals with T2D. We suggest that this discrepancy can be partially attributed to the epidemiological characteristics of the population of southeast Mexico. Additionally, the clinical characteristics of individuals with T2D indicated poor glycemic control, obesity (BMI) and hypertension.
In a multicenter ADOPT study, it was found that participants with LADA and T2D were similar in terms of overweight/obesity; nevertheless, those with LADA were more insulin resistant and tended to be obese, but leaner than individuals with T2D9. Brahmkshatriya et al.8 used a different age criterion (> 25 years) for LADA and found that these individuals had a slim phenotype; interestingly, their study suggested that a younger age of LADA onset is associated with a slim phenotype. In contrast, we observed that individuals with LADA had a phenotype similar to T2D. On the other hand, a Swedish study found that patients with LADA and a healthy lifestyle showed higher levels of GADA and worse beta-cell function (HOMA-B) than those with a poor lifestyle; however, they presented less insulin resistance (HOMA-IR). One explanation could be that people with a poor lifestyle are overweight/obese and insulin resistant: two factors that increase the demand on the beta cells, which could unmask an autoimmune process at an earlier stage10.
Regarding diabetes complications, our patients with LADA showed lower frequencies of NAFLD/NASH, DKD, neuropathy and cardiovascular complications. Similarly, other researchers have found that a poor glycemic control increases the risk of microvascular complications in individuals with LADA3. Likewise, other studies have demonstrated the role of poor glycemic control in complications of prolonged diabetes1,15.
In our population, a mean eGDR of 8.51 ± 4.55 mg/Kg/min was found in patients with LADA, which was higher than in individuals with T2D. This difference may be because this parameter depends on HbA1c, waist circumference, and hypertension values, for its determination. It is known that these factors depend on the interaction of environmental, behavioral (diet, lifestyle) and genetic factors inherent to our Mexican population (presence of genetic variants that predispose to metabolic disorders, hypertension)16. Similarly, Zinman et al.17 found in patients with LADA a lower degree of insulin resistance and a lower probability of metabolic syndrome. Some authors propose that insulin resistance participates in the promotion of autoimmune diabetes due to the increase in insulin demand, and this can accelerate the appearance of the disease in people with an autoimmune process. In mild autoimmunity, the participation of factors related to insulin resistance in the progression to diabetes is considered9.
Low eGDR is a marker associated with insulin resistance. In this sense, a study of patients with T1D, showed that those who presented an eGDR lower than 6.6 mg/Kg/min had a higher probability of presenting acanthosis nigricans18. Some reports suggest that the prevention of insulin resistance would reduce atherosclerotic disease up to 40%. Zabala, et al.19 found that a low eGDR is associated with cerebrovascular events and a higher probability of death19. Therefore, eGDR can be used as a marker to assess insulin resistance and the risk of cardiovascular disease20.
Nowadays, the consideration of specific criteria is suggested when suspecting LADA, including: age > 30 years at diagnosis and the absence of insulin requirement for at least 6 months after diagnosis6. Additionally, clinical characteristics and the presence of autoantibodies are frequently used in studies to define LADA cases. Carlsson for instance, considered that for research purposes, it is enough to measure GADA to distinguish LADA from T2D1. Studies carried out in Chinese and European populations showed that GADA were the most frequent autoantibodies in patients with LADA4,14. Various authors suggest that other criteria (BMI, patient age, fasting blood glucose, glycosylated hemoglobin) should also be used for the diagnosis of LADA, and be adjusted according to the population studied6,21. However, GADA positivity rates vary widely among reports depending on the population sampled and the screening methodology. We found 18.55% of GADA-positive patients in our sample, a high detection rate when compared to studies performed in other populations (1.85–14%)22,23. On the other hand, these data are similar to that reported in a Spanish population (27%)24; likewise, Cardoso et al.25 study had a relatively high antibody detection rate (32.26%) in Mexican patients.
It has been reported that GADA levels can fluctuate or even become negative during the disease course, but patients with higher levels of GADA tend to remain positive and have an accelerated decline of the beta-cell function26,27. Due to the varied antibody titers reported in different populations, the criteria for the diagnosis of LADA are under constant discussion28.
Our findings indicated that individuals with LADA belong to a subtype of patients with a T2D phenotype (a high BMI; overweight), but with lower fasting glucose levels and a higher eGDR than those observed in T2D. The Youden index indicated that a mean age at diagnosis of diabetes of 41 years and a mean eGDR value of 9.75 mg/Kg/min correlated better with LADA. Nonetheless, positive and negative predictive values showed that both features would be better for discarding LADA than for its diagnosis. In this sense, these parameters could be used to identify patients suspected of having LADA at the first level of medical care. Due to the phenotypic heterogeneity observed in LADA between populations, it is necessary to have parameters that allow the identification of patients with a high suspicion of LADA, so that they are appropriately referred to the second level of clinical care.
The present study has limitations to be considered. We only evaluated patients from a single referral center in Southeast Mexico; therefore, other Mexican populations can differ from our data. Also, our sample was taken from a Diabetes Clinic where all patients are on intensive therapies; they were referred from other centers because they did not achieve a glycemic control. Hence, our studied population cannot be considered a typical T2D population. Lastly, we could not establish causal relationships because of the cross-sectional nature of this study.
Conclusions
We found that patients with clinical criteria for LADA were GADA positive in a population from southeastern Mexico. Patients diagnosed with LADA in our population presented low fasting glucose, fewer complications from the disease, younger age and younger age at onset, higher insulin use and higher eGDR than patients with T2D. Our results indicate that a mean age at diagnosis of 40.5 years and a mean eGDR value of 9.75 mg/kg/min correlate better with LADA. Since GADA screening is unavailable in several clinical centers, these parameters may be useful to identify patients with a high suspicion of LADA at the first level of care in populations of southeastern Mexico, in order to refer them to a second level of care. It is necessary to perform further research on LADA features in the Mexican population since it could differ from the population characteristics in other countries.
Materials and methods
Study population
We performed a cross-sectional study. The participants were recruited at the Diabetes Clinic of the High Specialization Regional Hospital “Dr. Gustavo A. Rovirosa Pérez” from January 2020 to May 2021. This Hospital cares for individuals from the Chiapas, Veracruz and Tabasco states (Southeast Mexico). We invited individuals with previous T2D diagnoses to participate in this study. A diabetologist evaluated and diagnosed these patients based on the World Health Organization (WHO) criteria29. The inclusion criteria were (1) diagnosis of T2D, (2) older than 30 years of age at diagnosis of diabetes, (4) at least 6 months after diagnosis without insulin therapy requirement, (5) agreeing to participate in the study, and signed an informed consent before the interview. We excluded patients with (1) a diagnosis of T1D, (2) less than a year of diagnosis of T2D, and (3) subjects without a blood sample. A sample of 377 patients completed the inclusion criteria. We determined the GADA levels of all individuals in the sample; we classified them as LADA subjects with present antibodies and T2D patients with absent antibody. The final sample was divided into two groups: 59 patients with LADA and 318 patients with T2D (Fig. 2).
Measurements sociodemographic and clinical features
We applied a structured questionnaire to obtain sociodemographic data (age, gender, occupation, education, marital status, and socioeconomic status). From medical records, we collected: age at diagnosis, diabetes complications, comorbidities, pharmacotherapy, alcohol consumption and tobacco consumption. The time of living with diabetes was calculated in years, from the time of diagnosis to the interview day. Diabetes complications included non-alcoholic fatty liver, disease/non-alcoholic steatohepatitis (NAFLD/NASH), diabetic kidney disease (DKD), neuropathy, and cardiovascular complications (cardiovascular complications enclosed coronary heart disease, cardiomyopathy, stroke, and diabetic foot syndrome). The comorbidities evaluated were hypertension and dyslipidemia. In terms of insulin use, we classified insulin as short-acting (Lispro), intermediate-acting (NPH, Lispro protamine), and long-acting (glargine, detemir).
Anthropometric parameters
We measured weight, height, waist circumference, and hip circumference after the interview. We calculated BMI as weight in kilograms divided by the square of height in meters (kg/m2). Blood pressure measurements followed the protocol recommended by the Official Mexican Standard and the American Heart Association.
Biochemical parameters
We drew blood samples from every participant and collected them in tubes with EDTA and no other anticoagulants. Fasting glucose, total cholesterol, HDL cholesterol, LDL cholesterol, and triacylglycerols were determined in blood serum using a Clinical Chemistry System from Random Access Diagnostics. We determined glycated hemoglobin and GADA concentrations by an enzymatic immunoassay method (Human Glycated Hemoglobin A1c ELISA Kit, Human Anti-Glutamic Acid Decarboxylase ELISA Kit; MyBioSource).
Estimated glucose disposal rate (eGDR)
We calculated the eGDR using the formula: eGDR = 21.158−(0.09 × waist [cm])−(3.407 × hypertension [yes = 1, no = 0])−(0.551 × HbA1c [%])12.
Diabetes classification
We diagnosed patients with LADA based on the following criteria: adult-onset of diabetes (> 30 years of age at diagnosis), absence of insulin requirement for at least six months after diagnosis, and presence of GADA (levels of ≥ 5 IU/mL)30. GADA positivity was the main classification factor, as we used age at diagnosis of diabetes and absence of insulin requirement as inclusion criteria for patients with T2D.
Statistical analysis
Means ± standard deviations describe numerical variables, and frequencies and percentages express categorical data. We compared two groups, one with LADA and another with T2D. We used the Chi-squared test for categorical variables and the t-Student test for numerical data. Significant variables in previous analyses were included as covariables in a binary logistic regression; results were expressed as OR and 95% CI for being in the LADA group. The receiver operating (ROC) curve evaluated the sensitivity and specificity of significant numerical variables in logistic regression. We calculated the Youden index to estimate the best cut-off point for sensitivity and specificity. A p-value < 0.05 was considered significant. All data were analyzed using SPSS v. 26 and GraphPad Prism 9.
Ethical considerations
We followed the Official Mexican Standard NOM-012-SSA3-2012 guidelines and the ethical principles of the Declaration of Helsinki. The Ethics Committee of High Specialization Regional Hospital “Dr. Gustavo A. Rovirosa Pérez” approved the study (00228/19; December 3rd, 2019). Every patient participated voluntarily, without receiving any remuneration and signed an informed consent. Additionally, they received verbal and written information about the objectives of this study. Participation in this study was not treatment dependent and did not change the medical care that the hospital provides. We registered this study on clinicaltrials.gov on February 11th, 2022, as NCT05235672.
Statement and ethical considerations of experiments involving human individuals
The Ethics Committee of The High Specialization Regional Hospital “Dr. Gustavo A. Rovirosa Pérez” approved the study (00228/19; December 3rd, 2019).
Statement of methods
All methods were performed according to the Official Mexican Standard NOM-007-SSA3-2011 for clinical laboratory determinations and the Official Mexican Standard NOM-012-SSA3-2012 for research in human individuals.
Statement of informed consent
The present study was performed according to the ethical principles of the Declaration of Helsinki. All patients participated voluntarily without receiving any remuneration and signed an informed consent. Additionally, they received verbal and written information about the objectives of this study. The participation in this study was not treatment dependent and did not change the medical care that the hospital provides.
Data availability
The datasets used and analyzed during this study are included in the published article and its supplementary information files.
References
Carlsson, S. Etiology and pathogenesis of latent autoimmune diabetes in adults (LADA) compared to type 2 diabetes. Front. Physiol. 10, 320 (2019).
Regnell, S. E. & Lernmark, Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 60, 1370–1381. https://doi.org/10.1007/s00125-017-4308-1 (2017).
Maddaloni, E., Moretti, C., Mignogna, C. & Buzzetti, R. Adult-onset autoimmune diabetes in 2020: An update. Maturitas 137, 37–44. https://doi.org/10.1016/j.maturitas.2020.04.014 (2020).
Hawa, M. I. et al. Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype. Diabetes Care 36, 908. https://doi.org/10.2337/dc12-0931 (2013).
Hawa, M. I. et al. LADA and CARDS: A prospective study of clinical outcome in established adult-onset autoimmune diabetes. Diabetes Care 37, 1643. https://doi.org/10.2337/dc13-2383 (2014).
Buzzetti, R. et al. Management of latent autoimmune diabetes in adults: A consensus statement from an international expert panel. Diabetes 69, 2037. https://doi.org/10.2337/dbi20-0017 (2020).
Maddaloni, E., Coleman, R. L., Agbaje, O., Buzzetti, R. & Holman, R. R. Time-varying risk of microvascular complications in latent autoimmune diabetes of adulthood compared with type 2 diabetes in adults: A post-hoc analysis of the UK Prospective Diabetes Study 30-year follow-up data (UKPDS 86). Lancet Diabetes Endocrinol. 8, 206–215. https://doi.org/10.1016/S2213-8587(20)30003-6 (2020).
Brahmkshatriya, P. P., Mehta, A. A., Saboo, B. D. & Goyal, R. K. Characteristics and prevalence of latent autoimmune diabetes in adults (LADA). ISRN Pharmacol. 2012, 580202–580202. https://doi.org/10.5402/2012/580202 (2012).
Hjort, R. et al. Overweight, obesity and the risk of LADA: Results from a Swedish case–control study and the Norwegian HUNT Study. Diabetologia 61, 1333–1343. https://doi.org/10.1007/s00125-018-4596-0 (2018).
Herzog, K. et al. Combined lifestyle factors and the risk of LADA and type 2 diabetes—results from a Swedish population-based case-control study. Diabetes Res. Clin. Pract. 174, 108760. https://doi.org/10.1016/j.diabres.2021.108760 (2021).
Nyström, T., Holzmann, M. J., Eliasson, B., Svensson, A.-M. & Sartipy, U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes. Metab. 20, 556–563. https://doi.org/10.1111/dom.13110 (2018).
Williams, K. V., Erbey, J. R., Becker, D., Arslanian, S. & Orchard, T. J. Can clinical factors estimate insulin resistance in type 1 diabetes?. Diabetes 49, 626–632. https://doi.org/10.2337/diabetes.49.4.626 (2000).
Fadiga, L. et al. Adult-onset autoimmune diabetes: Comparative analysis of classical and latent presentation. Diabetol. Metab. Syndr. 12, 107. https://doi.org/10.1186/s13098-020-00616-1 (2020).
Xiang, Y. et al. Glutamic acid decarboxylase autoantibodies are dominant but insufficient to identify most Chinese with adult-onset non-insulin requiring autoimmune diabetes: LADA China study 5. Acta Diabetol. 52, 1121–1127. https://doi.org/10.1007/s00592-015-0799-8 (2015).
Cabrera-Rode, E. et al. Slowly progressing type 1 diabetes: Persistence of islet cell autoantibodies is related to glibenclamide treatment. Autoimmunity 35, 469–474. https://doi.org/10.1080/0891693021000050574 (2002).
Canto-Osorio, F., Denova-Gutierrez, E., Sánchez-Romero, L. M., Salmerón, J. & Barrientos-Gutierrez, T. Dietary Inflammatory Index and metabolic syndrome in Mexican adult population. Am. J. Clin. Nutr. 112, 373–380. https://doi.org/10.1093/ajcn/nqaa135 (2020).
Zinman, B. et al. Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes 53, 3193–3200. https://doi.org/10.2337/diabetes.53.12.3193 (2004).
Teixeira, M. M. et al. Insulin resistance and associated factors in patients with Type 1 diabetes. Diabetol. Metab. Syndr. 6, 131. https://doi.org/10.1186/1758-5996-6-131 (2014).
Zabala, A. et al. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: A nationwide cohort study. Cardiovasc. Diabetol. 20, 202. https://doi.org/10.1186/s12933-021-01394-4 (2021).
Helmink, M. A. G. et al. Insulin resistance and risk of vascular events, interventions and mortality in type 1 diabetes. Eur. J. Endocrinol. 185, 831–840. https://doi.org/10.1530/EJE-21-0636 (2021).
Hjort, R. et al. Family history of type 1 and type 2 diabetes and risk of latent autoimmune diabetes in adults (LADA). Diabetes Metab. 43, 536–542. https://doi.org/10.1016/j.diabet.2017.05.010 (2017).
Adeleye, O. O. et al. Latent autoimmune diabetes mellitus in adults (LADA) and it’s characteristics in a subset of Nigerians initially managed for type 2 diabetes. Int. Arch. Med. 5, 23–23. https://doi.org/10.1186/1755-7682-5-23 (2012).
Tam, A. A. et al. Low rate of latent autoimmune diabetes in adults (LADA) in patients followed for type 2 diabetes: A single center’s experience in Turkey. Arch. Endocrinol. Metab. 64, 584–590. https://doi.org/10.20945/2359-3997000000268 (2021).
Hernández, M. et al. Preclinical carotid atherosclerosis in patients with latent autoimmune diabetes in adults (LADA), type 2 diabetes and classical type 1 diabetes. Cardiovasc. Diabetol. 16, 94. https://doi.org/10.1186/s12933-017-0576-9 (2017).
Cardoso-Sánchez, L. I., Gómez-Díaz, R. A. & Wacher, N. H. Vitamin D intake associates with insulin resistance in type 2 diabetes, but not in latent autoimmune diabetes in adults. Nutr. Res. 35, 689–699. https://doi.org/10.1016/j.nutres.2015.05.019 (2015).
Huang, G. et al. Persistence of glutamic acid decarboxylase antibody (GADA) is associated with clinical characteristics of latent autoimmune diabetes in adults: A prospective study with 3-year follow-up. Diabetes Metab. Res. Rev. 32, 615–622. https://doi.org/10.1002/dmrr.2779 (2016).
Zampetti, S. et al. High GADA titer increases the risk of insulin requirement in LADA patients: A 7-year follow-up (NIRAD study 7). Eur. J. Endocrinol. 171, 697–704. https://doi.org/10.1530/EJE-14-0342 (2014).
Grill, V. LADA: A type of diabetes in its own right?. Curr. Diabetes Rev. 15, 174–177. https://doi.org/10.2174/1573399814666180716150905 (2019).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998).
Oikawa, Y., Kondo, T., Shimada, A., Seino, Y. & Kitaoka, M. Actual condition survey regarding mismatch of measurements between radioimmunoassay and enzyme-linked immunosorbent assay tests for anti-glutamic acid decarboxylase antibody in real-world clinical practice. J. Diabetes Investig. 10, 685–689. https://doi.org/10.1111/jdi.12955 (2019).
Acknowledgements
The authors also thank CONACYT for supporting Germán Alberto Nolasco-Rosales with the scholarship No. 813279.
Author information
Authors and Affiliations
Contributions
G.A.N.R. data curation, writing reviewing. D.R.G., investigation. T.B.G.C. investigation, supervision. A.A.F. supervision, fund acquisition. C.A.T.Z. writing-reviewing; methodology, G.V. methodology, investigation, prepared figures 1–3. E.R. conceptualization, supervision. A.D.G.M. writing-reviewing; prepared figures 1–3, prepared table 1–5. J.L.B.C. preparation; prepared table 1–5. I.E.J.R. conceptualization, supervision, fund acquisition, writing-reviewing, original draft; A.M.M., conceptualization, methodology, fund acquisition, original draft. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nolasco-Rosales, G.A., Ramírez-González, D., Rodríguez-Sánchez, E. et al. Identification and phenotypic characterization of patients with LADA in a population of southeast Mexico. Sci Rep 13, 7029 (2023). https://doi.org/10.1038/s41598-023-34171-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34171-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.