Abstract
High levels of dietary fiber could restrict the inclusion of olive (Olea europea L.) pomace (OP) as a source of energy and bioactive compounds in Jumbo quail (Coturnix sp.) diets. In this study, the optimum inclusion level of dietary OP on growth and carcass performance, serum biochemistry, and meat quality parameters in Jumbo quail was investigated. One-week-old Jumbo quail (350; 28.9 ± 1.29 g live-weight) were reared on a standard mash grower diet with 0 (OP0), 100 (OP10), 150 (OP15), 200 (OP20), and 250 g/kg (OP25) OP for five weeks. The experimental diets were randomly allocated to 35 pens (experimental units) with seven replicates each. Overall body weight gain in Jumbo quail fed with diets OP20 and OP25 was lower (p < 0.001) than those fed diets OP0 and OP10. Including dietary OP had no effect on the overall gain-to-feed ratio, serum biochemistry, and internal organs but linearly reduced carcass yields. Diet OP25 promoted a higher (p < 0.022) meat hue angle value than the other diets. The inclusion of OP beyond 150 g/kg compromised growth and carcass performance, and altered some meat color attributes, but had no influence on serum biochemistry, and internal organs of the Jumbo quail.
Similar content being viewed by others
Introduction
The inclusion of agro-industrial by-products such as olive (Olea europea L.) pomace (OP) in animal feeds, which is rich in bioactive compounds and has a high energy value could be an environmentally friendly strategy to deliver sustainable Jumbo quail (Coturnix sp.) production1. This approach could also contribute positively towards food and nutrition security for the world’s rapidly growing human population while protecting the environment from wanton disposal of OP in landfills2. The OP’s high nutritional value could be of great importance to Jumbo quail production, which are birds that are known for their fast maturity, high product (meat and egg) quality, high reproductive efficiency, strong resistance to poultry diseases, high meat-to-bone ratio, and low feed and space demands3. Indeed, high energy and protein levels, as well as highly digestible nutrient sources are required to realize optimal quail production. However, modern poultry production systems rely on the use of maize grains and soybeans as major sources of dietary energy and protein4. Unfortunately, the over-reliance on these two conventional ingredients could in the future, render poultry intensification unsustainable due to price volatility, currency fluctuations, rising demands, and climatic constraints that have elevated their market prices5. As a result, alternatives that completely or partially replace maize or soybeans are required to reduce feeding costs, promote environmental and socio-economic sustainability, and develop profitable quail businesses6.
The recent increase in the demand for olive oil by the food and biofuel sectors has resulted in the generation of large quantities of OP, which is discarded in barren lands near olive processing industries. According to Adams et al.3, about 20% of the olive fruit is refined into extra virgin olive oil, while the remaining 80% is disposed of as olive waste. From an environmental perspective, the wanton disposal of olive waste contributes to land, soil, air, and water pollution3,5. Moreover, the presence of secondary metabolites such as polyphenols inhibits plant and bacterial growth, resulting in reduced biodiversity in surface water and soil5. Thus, the incorporation of OP in Jumbo quail feeds could be a long-term disposal management strategy, that could be adopted to enhance the sustainability of the olive oil sector6. Furthermore, quail producers could benefit from the OP’s low-cost complementary energy value due to high residual oil and the presence of oleuropein and hydroxytyrosol antioxidants7, which can potentially improve the quality of quail products. The OP contains 4.5% crude protein, 19.8% crude fat, 35.6% fiber, and 5.2% phenolics on dry matter bases8,9. However, the nutritional value of OP, like many other waste by-products, varies depending on the extraction method, residual oil, stage of maturity, harvesting site, and variety10. For this reason, it is important to investigate its feed value in Jumbo quail diets to monitor its impact on performance, and physiological and meat quality responses.
To this end, many research studies have focused on its impact as a supplemental energy source in broiler chicken diets, with conflicting results being reported. For example, dietary OP inclusion rates of up to 100 g/kg in broiler diets had no effect on weight gain11,12. However, the dietary inclusion of OP between 50 and 100 g/kg in the diets of broiler chicks was reported to reduce growth performance, which was linked to the high fiber content of OP13. According to Fadel and El-Ghonemy14 and Fathy et al.15, OP has a crude fiber content that ranges between 33 and 42.60%, which could be a major problem for simple non-ruminants such as the Jumbo quail. This is because their digestive systems cannot efficiently utilize fibrous diets, especially at an early age3. Thus, it is important to investigate the optimum dietary inclusion level of OP for the Jumbo quail. To date, no studies have established the maximum tolerance level of OP in the diets of Jumbo quail, which could be because it is a recently developed quail breed3. Thus, the aim of this study was to evaluate the effect of incremental levels of dietary OP on growth and carcass performance, serum biochemistry, visceral organs developments, and meat quality traits in Jumbo quail. It was hypothesized that the inclusion of dietary OP would improve productive performance, physiology, and meat quality traits in Jumbo quail.
Methods
Ethics approval
The rearing and slaughter procedure of the quail was approved by the Animal Research Ethics Committee of the North-West University (Approval no. NWU-00817-21-A5) according to established guidelines for the use and care of research animals.
Diet formulation and chemical analyses
Fresh and wet olive pomace was acquired from Stoneberg Farm Holding (PTY) LTD (Western Cape, South Africa) and was oven-dried (60 °C) to constant weight before milling (2 mm) using a cutting mill (SM 100, Retsch GmbH, Haan, Germany). The OP chemical composition is presented in Table 1.
The other feed ingredients were purchased from Simplegrow Agric Services (Pty) Ltd and Nutroteq (Centurion, South Africa). The five isocaloric and isonitrogenous dietary treatments were formulated to satisfy the daily nutrient requirement of grower quail following guidelines from the NRC16. The diets were formulated (Table 2) as follows: a standard grower diet without OP (OP0) and a standard grower diet with 100 (OP10), 150 (OP15), 200 (OP20), and 250 g/kg (OP25) OP (w/w). The experimental diets were analyzed for dry matter, ash, organic matter, crude protein, ether extract, and amino acids (arginine, histidine, isoleucine, lysine, methionine, threonine, tryptophan, and valine) by methods described by the AOAC17. Crude fiber, nitrogen detergent fiber (NDF), and acid detergent fiber (ADF) were analyzed using the ANKOMDELTA automated fiber analyzer (ANKOM Technologies, NY, USA). To evaluate the acid detergent lignin (ADL), the cellulose in the ADF residue bags was soaked in 72% H2SO4 for 3 h. The ether extract was determined using an ANKOMXT15 extractor (ANKOM Technologies, NY, USA). Minerals (calcium, chloride, phosphorus, and sodium) were analyzed using AgriLASA18. The metabolizable energy was calculated using the equations described by Egbu et al.19.
Experimental design and feeding trial
The feeding experiment was carried out in December 2021 at the North-West University Research Farm (25°86′00″ S; 25°64′32″ E), South Africa. A total of 350, 4-day-old Jumbo quail chicks were purchased from Golden Quail Farm (Randfontein, South Africa). In a completely randomized design, the chicks were evenly allocated into 35 replicate pens, to which the five experimental diets were randomly distributed producing 7 replicates per dietary treatment. The pens (100 cm Length × 60 cm Width × 30 cm Height) were considered as the experimental units, each holding 10 birds. The chicks were orally dosed with vitamins and electrolytes for 3 days to offset stress and adapted to the pens and experimental diets until day 7 of age. Measurements commenced from 7 to 42 days of age, and rearing was conducted under natural lighting (12 h of day length). The diets and clean water were offered ad libitum during the feeding trial. Initial live weights (28.9 ± 1.29 g) were recorded and subsequently weighed weekly per pen to determine average weekly body weight gain. Feed intake was determined from the amount of feed offered the previous day and the refusals received the following morning. Gain-to-feed ratio (G:F) was determined by dividing weight gain by feed consumption data. The mortality rates were considered in the calculation of the growth performance data.
Slaughter procedure and blood collection
On day 42 of age, all the birds per pen (experimental unit) were weighed to determine their final body weight. The birds were then transported to a nearby poultry abattoir where they were all stunned and then slaughtered after resting for 2 h. About 2 mL of fresh blood was randomly collected from two quail per experimental unit whose jugular veins had been sliced with a sharp knife. The blood was immediately transferred into red-top tubes and left standing at room temperature to allow the recovery of serum from clotted blood. The blood samples were then centrifuged for 10 min at 3500 rpm using a Centrifuge (model Z 206 A, Hermle Labortechnik GmbH, Germany) supplied by Lasec SA (Pty) Ltd (Midrand, South Africa). The following serum biochemical parameters: alanine transaminase (ALT), albumin, amylase, calcium, cholesterol, creatine, glucose, symmetric dimethylarginine (SDMA), total bilirubin, total protein, and urea were analyzed using an automated Vet Test Chemistry Analyzer from IDEXX Laboratories (Midrand, South Africa).
Carcass, visceral organs, and meat quality
The carcasses were weighed immediately after dressing and evisceration to obtain hot carcass weight (HCW) and then chilled at 16 °C for 24 h to determine cold carcass weight (CCW). The carcasses were then cut to measure (Explorer® EX224, OHAUS Corp, US) the wings, drumstick, thigh, and breast weights. Carcass yield was calculated as a proportion of HCW to final body weight. The weights of the gizzard, liver, spleen, proventriculus, small intestines, and ceca with their contents were measured using the digital scale described above. A measuring tape was used to determine the small intestines, ceca, and colon lengths. Breast pH was measured 1 h and 24 h post-mortem using a Corning Model 4 pH-temperature meter (Corning Glass Works, Medfield, MA, USA), which was calibrated using standard pH solutions after measuring each experimental unit20. Breast color coordinates (L* = brightness, a* = redness, and b* = yellowness) were determined using a color spectrophotometer (CM 2500c, Konika Minolta, Osaka, Japan) 1 h and 24 h post-mortem20. The filter-paper press technique by Whiting and Jenkins21 was used to determine breast meat water holding capacity (WHC). Honikel22 techniques were used to measure breast cooking loss.
Statistical analysis
Weekly measured data (feed intake, weight gain, and gain-to-feed ratio) were analyzed using the repeated measures analysis in the procedure of general linear model (PROC GLM) in SAS23 to assess the interaction effects of diet and time (quail age in weeks). The linear and quadratic coefficients for all the measured parameters were evaluated using polynomial contrasts by means of response surface regression (PROC RSREG) in SAS23. Dietary differences among the parameters were also analyzed using one-way ANOVA by means of PROC GLM23. The level of significance was set at p < 0.05 and the probability of difference option in SAS was used to separate the treatment means.
Animal welfare statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The authors confirm that they have followed EU standards for the protection of animals used for scientific purposes.
The authors confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Research involving plants
The collection of Olive (Olea europea) pomace complies with relevant institutional, national, and international guidelines and legislation as recommended by the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
The fresh and wet Olive (Olea europea) pomace used in the study was bought from Stoneberg Farm Holding (PTY) LTD (Western Cape, South Africa).
Research involving animals
The study was reported in accordance with ARRIVE guidelines.
Results
Growth performance
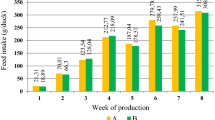
Repeated measures analysis revealed a significant week × diet interaction effect on average weekly feed intake (AWFI, p = 0.003) but no interaction effects were observed on body weight gain (p = 0.335) and G:F (p = 0.063). Table 3 shows that the inclusion of incremental levels of dietary OP resulted in linear decreases in feed intake in week 3 [y = 67.2 (± 2.44)–0.547 (± 0.709) x; R2 = 0.558, p = 0.021] and week 4 [y = 75.1 (± 2.58)–0.003 (± 0.752) x; R2 = 0.723, p = 0.002]. The GLM results showed that there were significant dietary effects from week 2 to week 6. Two-week-old quail reared on diet OP25 had the lowest feed intake followed by those reared on diet OP20 and the highest was from those on the OP0, OP10, and OP15 groups, whose feed intake did not differ (p > 0.05). Three-week-old quail fed with diets OP20 and OP25 had lower feed intake than those reared on diets OP0, OP10, and OP15, which were statistically similar (p > 0.05). In week 4, diet OP20 caused lower feed intake than diets OP0 and OP10, which statistically did not vary from diets OP15 and OP25. In week 5, diet OP25 promoted lower feed intake than diets OP0, OP10, and OP20, which did not differ (p > 0.05). Six-week-old quail on OP10 treatment had the highest feed intake (84.35 g/bird) and the lowest feed intake was observed from birds on OP25 treatment (39.81 g/bird).
The regression results showed that overall BWG linearly decreased [y = 126 (± 2.58)–1.673 (± 1.909) x; R2 = 0.408, p = 0.036] as dietary OP levels increased. Likewise, dietary differences were observed in overall BWG, where quail reared on diets OP20 and OP25 recorded the lowest (p < 0.05) weight gain followed by those on diet OP15, and the highest weight gain was from those on diets OP0 and OP10.
Neither linear nor quadratic effects (p > 0.05) were recorded for overall G:F as dietary OP levels increased. Similarly, no significant differences were observed among the treatment groups on overall G:F.
Serum biochemistry
Regression results showed that there were no significant linear or quadratic effects (p > 0.05) for all serum biochemical parameters as dietary OP levels increased (Table 4). The inclusion of dietary OP also induced no significant dietary effects on all serum biochemical parameters of the Jumbo quail.
Carcass performance and internal organs
Table 5 shows that the inclusion of increasing dietary OP levels linearly reduced carcass yield [y = 65.57 (± 2.55)–0.15 (± 0.48) x; R2 = 0.167, p = 0.040], HCW [y = 78.99 (± 4.53)–0.55 (± 0.87) x; R2 = 0.395, p = 0.002] and CCW [y = 73.9 (± 4.10)–0.066 (± 0.779) x; R2 = 0.416, p = 0.0003]. Likewise, linear decreases were recorded for drumstick [y = 3.85 (± 0.23)–0.03 (± 0.04) x; R2 = 0.262, p = 0.007], thigh [y = 4.69 (± 0.37)–0.01 (± 0.07) x; R2 = 0.262, p = 0.008], wing [y = 4.84 (± 0.24)–0.04 (± 0.04) x; R2 = 0.386, p = 0.001], and breast weights [y = 11.60 (± 1.08)–0.03 (± 0.20) x; R2 = 0.165, p = 0.042]. However, neither linear nor quadratic (p > 0.05) effects were recorded for all the visceral organs as dietary OP levels were increased. The dietary treatments had significant effects on HCW, CCW, and wing weight only. Quail reared on diet OP25 had lighter (p < 0.05) HCW and wings than those on diet OP0, whose HCW and wings were statistically similar (p > 0.05) to those reared on diets OP10, OP15, and OP20. The control diet OP0 promoted the heaviest (p < 0.05) CCW (74.0 g) while the OP25 treatment resulted in the lightest CCW (47.3 g).
Meat quality
Table 6 shows that there were linear increases for 1-h breast meat yellowness (b*1) [y = 2.85 (± 0.55) + 0.014 (± 0.104) x; R2 = 0.270, p = 0.009] and 1-h hue angle [y = 0.39 (± 0.06) + 0.01 (± 0.01) x; R2 = 0.248, p = 0.010]. However, linear decreases were recorded for 24-h hue angle [y = 0.56 (± 0.08)–0.01 (± 0.01) x; R2 = 0.318, p = 0.006] and 24-h redness (a*24) [y = 6.81 (± 0.24)–0.11 (± 0.04) x; R2 = 0.256, p = 0.036] as dietary OP levels were increased. The dietary treatments had significant effects on a*1, b*1, chroma1, and hue angle24. Birds reared on diet OP25 had higher (p < 0.05) a*1 than those on diet OP10, but did not differ from those reared on OP0, OP15, and OP20. Quail reared on diet OP25 had higher b*1 than those on diets OP15 and OP20, whose b*1 were statistically similar (p > 0.05) to those reared on diets OP0 and OP10. Diet OP10 promoted lower chroma1 values than diets OP15, and OP20, which did not differ (p > 0.05). Quail reared on diet OP25 had higher meat hue Angle24 values than those reared on diet OP10, but statistically did not differ from those reared on OP0, OP15, and OP20 which did not vary.
Discussion
The adoption of agro-waste by-products as sources of nutrients and bioactive compounds is crucial in ensuring long-term intensive quail production while reducing environmental pollution24. Olive pomace is one such waste by-product that can be used to supply dietary energy and phytochemicals to the quail subsector Sayehban et al.13 and as such, provide an innovative and efficient strategy for recycling. To this end, no studies have evaluated the effect of OP inclusion in Jumbo quail diets, thus the current study investigated the optimum inclusion level of dietary OP in diets of Jumbo quail.
The results showed a significant diet × week interaction effect on feed intake, which demonstrates that the capacity of the birds to consume the dietary treatments varied with age. Including dietary OP up to 150 g/kg promoted similar feed consumption as the control diet, however, levels up to 250 g/kg linearly reduced feed consumption. This indicates that higher levels of dietary OP suppress feed utilization, which could be due to high fiber (24 g/kg DM) levels in OP. This finding agrees with the results of Saleh and Alzawqari25 who noted reduced feed intake when 200 g/kg olive cake meal was used to replace corn in broiler diets. Similarly, the diets containing OP at 200–250 g/kg promoted the lowest overall weight gain than those containing 0–100 g/kg OP, further confirming that the antinutritional effects of fiber in OP might have reduced nutrient absorption into body mass. The lack of adverse effects on feed intake and weight gain when OP was included up to 150 g/kg corroborates the findings of Sayehban et al.13, who reported that OP levels as high as 100 g/kg can be included in broiler diets without detrimental effects on performance. There were no dietary effects on overall G:F, which demonstrates that the inclusion of OP in the diets did not enhance the birds’ capacity to efficiently convert the feed into body mass. Numerically, birds in the control group had higher G:F values than those in the OP treatments, which confirms that the inclusion of OP in Jumbo quail diets compromised growth performance. Contrary to the results, Pappas et al.26 reported that the supplementation of a broiler finisher diet with OP at 80 g/kg resulted in poorer G:F than the control. The authors opined that the fiber content of OP was responsible for the poor G:F. The reported poor growth performance across research might be attributed to changes in nutrient composition, particularly the insoluble fiber and condensed tannin content of the olive by-products utilized.
Serum biochemical parameters provide an efficient evaluation of any pathophysiological aberrations in animals27. All serum biochemical indices showed no diet-induced variations as OP inclusion levels increased. These results disagree with previous studies that reported a decrease in serum cholesterol when an olive cake meal was used to replace maize at 100 g/kg in a broiler diet12,25. Since dietary OP contains antinutrients such as condensed tannins and insoluble fibers, it was hypothesized that dietary inclusion of OP would have a deleterious influence on serum biochemical parameters. Also, the lack of dietary effects on serum biochemical parameters measured could be because the diets were isonitrogenous and isocaloric. Albumin is a protein present in blood plasma, and low amounts indicate renal malfunction, liver damage, inflammation, or infections, whereas high levels suggest dehydration or severe diarrhea. Furthermore, serum total protein was similar which could be expected since the dietary treatments on analyses contained similar numerical crude protein values. Also, the liver enzyme (ALT) was unaltered, indicating that the birds did not suffer from diet-induced hepatotoxicity. Nonetheless, all the serum biochemical indices were within the normal ranges reported for a healthy quail28,29,30.
Variations in dietary fiber content have been linked to changes in the gastrointestinal morphology of poultry as an adaptive mechanism to utilize the high fiber levels31. Surprisingly, no dietary effects were recorded on all the examined visceral organs despite high levels of insoluble fiber in the OP-containing treatments. Similarly, Sayehban et al.13 observed no differences in the visceral organs of broilers supplemented with 100 g/kg olive pulp in their diets. It is probable that levels up to 250 g/kg of dietary OP were insufficient to induce physio-anatomical alterations in visceral organs. However, growth parameters were negatively impacted at 250 g/kg of dietary OP inclusion, indicating that there is a need to valorize OP with exogenous fibrolytic enzymes and/or polyethylene glycol to enable larger amounts of OP inclusion in Jumbo quail diets.
The viability and profitability of poultry production have been strongly linked to carcass yield enhancements. Understanding the elements that influence carcass characteristics (breasts, wings, thighs, and drumsticks) is thus critical for the success of any poultry business. Unfortunately, there is a limited understanding of such parameters in quail birds32, which could be because quail birds are mostly sold as live birds or whole carcasses. In this study, the inclusion of OP linearly decreased carcass yield, HCW, CCW, and weights of drumsticks, thigh, wing, and breast. This observation could be linked to the reduced feed intake observed among the OP-containing diets, which resulted in poor nutrient uptake for muscle development leading to poor weight gains. This indicates that the use of OP in quail diets could reduce the profitability and economic sustainability of quail enterprises. Furthermore, these results are inconsistent with the findings of Saleh and Alzawqari25 as well as Pappas et al.26, who recorded no significant differences on carcass characteristics of broilers when 80 and 100 g/kg of olive waste by-products were used to replace maize in diets.
Meat color, water holding capacity (WHC), cook losses, texture, and sensory properties all contribute to overall customer acceptance of meat and meat products33. Color differences from the expected norm on retail displays cause consumers to reject meat products. Color differences were found in all treatment groups, indicating that the inclusion of OP positively influenced the appearance of the meat. A linear increase was recorded for meat yellowness and hue angle measured 1 h post-mortem which could be due to the presence of carotene and other pigments in olive residues34 that are known to improve meat yellowness. Furthermore, meat redness and hue angle measured 24 h post-mortem linearly declined, which could be attributable to the exposure of the meat to oxygen upon storage that triggers oxidative metabolism, and as such, produces free radical by-products that ultimately cause myoglobin oxidation resulting in brown pigment metmyoglobin35. Meat pH is mostly associated with the biochemistry condition of the muscle at the point of slaughter, which causes rigor mortis to occur36. Chan et al.37 identified a link between meat pH and color, with lighter meat indicating a low pH value and dark meat indicating a high pH value. Nonetheless, the dietary treatments in this study had no influence on meat pH1 (5.84–6.04) and pH24 (6.06–6.26), which was within the normal meat pH (5.7–6.5) range reported by Mulaudzi et al.32. The ability of meat to retain water when subjected to external forces such as slicing, grinding, and compressing is referred to as WHC38. The amount of water lost or retained is mostly determined by the pH of the tissue; for instance, higher pH results in less water loss, whereas a lower pH value determines the amount of water retained by the meat39. When meat pH is low, protein denaturation occurs, which further lowers the meat's WHC40. The lack of dietary variations in meat pH may explain why the meat exhibited non-significant WHC values. Indeed, no variations in the cooking loss were identified, suggesting that OP supplementation did not impair the organoleptic (juiciness, tenderness, texture, smell, and color) and sensory aspects41,42 of quail meat.
Conclusion
Dietary olive pomace above 150 g/kg compromised growth and carcass performance, but not serum biochemical and meat quality parameters of the Jumbo quail. It is recommended that where higher dietary levels of the pomace are desired, fiber-degrading strategies can be employed prior to its inclusion in Jumbo quail diets.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Mulaudzi, A., Mnisi, C. M. & Mlambo, V. Enhancing the utility of dietary Moringa oleifera leaf meal for sustainable Jumbo quail (Coturnix sp.) production. Sustainability 14, 5067. https://doi.org/10.3390/su14095067 (2022).
Duman, A. K., Özgen, G. Ö. & Üçtuğ, F. G. Environmental life cycle assessment of olive pomace utilization in Turkey. Sustain. Prod. Consump. 22, 126–137. https://doi.org/10.1016/j.spc.2020.02.008 (2020).
Mahlake, S. K., Mnisi, C. M., Lebopa, C. & Kumanda, C. The effect of green tea (Camellia sinensis) leaf powder on growth performance, selected hematological indices, carcass characteristics and meat quality parameters of Jumbo quail. Sustainability 13, 7080. https://doi.org/10.3390/su13137080 (2021).
Wilson, W. C. et al. Integrating the soybean-maize-chicken value chains to attain nutritious diets in Tanzania. Food Secur. 13, 1595–1612. https://doi.org/10.1007/s12571-021-01213-4 (2021).
Adams, C. B. et al. Energy values of brewer’s grains and olive pomace waste for broiler chickens determined using the regression method. Agriculture 12, 444. https://doi.org/10.3390/agriculture12040444 (2022).
Katsinas, N., Enríquez-de-Salamanca, A., da Silva, A. B., Bronze, M. R. & Rodríguez-Rojo, S. Olive pomace phenolic compounds stability and safety evaluation: From raw material to future ophthalmic applications. Molecules 26, 6002. https://doi.org/10.3390/molecules26196002 (2021).
Khdair, A. & Abu-Rumman, G. Sustainable environmental management and valorization options for olive mill byproducts in the Middle East and North Africa (MENA) region. Processes 8, 671. https://doi.org/10.3390/pr8060671 (2020).
Gómez-Cruz, I., Cara, C., Romero, I., Castro, E. & Gullón, B. Valorisation of exhausted olive pomace by an eco-friendly solvent extraction process of natural antioxidants. Antioxidants 9, 1010. https://doi.org/10.3390/antiox9101010 (2020).
Ruschioni, S. et al. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio molitor L.) larvae. Foods 9, 317. https://doi.org/10.3390/foods9030317 (2020).
Papadomichelakis, G. et al. Effects of dietary dried olive pulp inclusion on growth performance and meat quality of broiler chickens. Livest. Sci. 221, 115–122. https://doi.org/10.1016/j.livsci.2019.01.023 (2019).
Sayehban, P. et al. Effects of different levels of two types of olive pulp with or without exogenous enzyme supplementation on broiler performance and economic parameters. Braz. J. Poultry Sci. 18, 489–500. https://doi.org/10.1590/1806-9061-2015-0060 (2016).
Sateri, S. et al. Effect of olive meal and supplemental enzymes on performance traits, blood biochemistry, humoral immunity response and caecal microbiota of broilers. S. Afr. J. Anim. Sci. 47, 804–812. https://doi.org/10.4314/sajas.v47i6.8 (2017).
Sayehban, P. et al. Olive pulp and exogenous enzymes feed supplementation effect on the carcass and offal in broilers: A preliminary study. Agriculture 10, 359. https://doi.org/10.3390/agriculture10080359 (2020).
Fadel, M. & El-Ghonemy, D. H. Biological fungal treatment of olive cake for better utilization in ruminants nutrition in Egypt. Int. J. Recycl. Org. Waste Agric. 4, 261–271. https://doi.org/10.1007/s40093-015-0105-3 (2015).
Fathy, S. A., Mahmoud, A. E., Rashad, M. M., Ezz, M. K. & Mohammed, A. T. Improving the nutritive value of olive pomace by solid state fermentation of Kluyveromyces marxianus with simultaneous production of gallic acid. Int. J. Recycl. Org. Waste Agric. 7, 135–141. https://doi.org/10.1007/s40093-018-0199-5 (2018).
NRC. Nutrient Requirements of Poultry (National Research Council, 2004).
AOAC. Official Methods of Analysis (Association of Official Analytical Chemists, 2005).
AgriLASA. Feed and Plant Analysis Methods (Agri Laboratory Association of Southern Africa, 1998).
Egbu, C. F., Motsei, L. E., Yusuf, A. O. & Mnisi, C. M. Evaluating the efficacy of Moringa oleifera seed extract on nutrient digestibility and physiological parameters of broiler chickens. Agriculture 12, 1102. https://doi.org/10.3390/agriculture12081102 (2022).
Egbu, C. F., Motsei, L. E., Yusuf, A. O. & Mnisi, C. M. Effect of Moringa oleifera seed extract administered through drinking water on physiological responses, carcass and meat quality traits, and bone parameters in broiler chickens. Appl. Sci. 12, 10330. https://doi.org/10.3390/app122010330 (2022).
Whiting, R. & Jenkins, R. Comparison of rabbit, beef, and chicken meats for functional properties and frankfurter processing. J. Food Sci. 46, 1693–1696. https://doi.org/10.1111/j.1365-2621.1981.tb04465.x (1981).
Honikel, K. O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49, 447–457. https://doi.org/10.1016/s0309-1740(98)00034-5 (1998).
Users guide (Statistical Analysis System Institute Inc, 2010).
Oyeagu, C. et al. The effect of feeding toasted Bambara nut (Vignasubterranea (L.) verdc) offal and supplementary enzyme on performance of broiler chicks. J. Trop. Agric 93, 271–283 (2016).
Saleh, A. & Alzawqari, M. Effects of replacing yellow corn with olive cake meal on growth performance, plasma lipid profile, and muscle fatty acid content in broilers. Animals 11, 2240. https://doi.org/10.3390/ani11082240 (2021).
Pappas, A. C. et al. Effects of olive pulp addition to broiler diets on performance, selected biochemical parameters and antioxidant enzymes. J. Hellenic Vet. Med. Soc. 70, 1687–1696. https://doi.org/10.12681/jhvms.21793 (2019).
Mokone, B., Motsei, L. E., Yusuf, A. O., Egbu, C. F. & Ajayi, T. O. Growth, physiological performance, and pork quality of weaner large white piglets to different inclusion levels of nano zinc oxide. Trop. Anim. Health Prod. 54, 1–8. https://doi.org/10.1007/s11250-021-03024-3 (2022).
Mehri, M., Sabaghi, V. & Bagherzadeh-Kasmani, F. Mentha piperita (peppermint) in growing Japanese quails’ diet: Serum biochemistry, meat quality, humoral immunity. Anim. Feed Sci. Technol. 206, 57–66. https://doi.org/10.1016/j.anifeedsci.2015.05.022 (2015).
Agina, O. A., Ezema, W. S. & Iwuoha, E. M. The haematology and serum biochemistry profile of adult Japanese quail (Coturnix coturnix japonica). Notulae Sci. Biol. 9, 67–72. https://doi.org/10.15835/nsb919928 (2017).
Moula, N., Sadoudi, A., Touazi, L., Leroy, P. & Geda, F. Effects of stinging nettle (Urtica dioica) powder on laying performance, egg quality, and serum biochemical parameters of Japanese quails. Anim. Nutr. 5, 410–415. https://doi.org/10.1016/j.aninu.2019.05.002 (2019).
Jha, R., Fouhse, J. M., Tiwari, U. P., Li, L. & Willing, B. P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 6, 48. https://doi.org/10.3389/fvets.2019.00048 (2019).
Mulaudzi, A., Mnisi, C. M. & Mlambo, V. Effect of pre-treating dietary Moringa oleifera leaf powder with fibrolytic enzymes on physiological and meat quality parameters in Jumbo quail. Poultry 1, 54–65. https://doi.org/10.3389/fanim.2022.960233 (2022).
Yu, Q. P. et al. Studies on meat color, myoglobin content, enzyme activities, and genes associated with oxidative potential of pigs slaughtered at different growth stages. Asian Australas. J. Anim. Sci. 30, 1739. https://doi.org/10.5713/ajas.17.0005 (2017).
Lazzerini, C. & Domenici, V. Pigments in extra-virgin olive oils produced in Tuscany (Italy) in different years. Foods 6, 25. https://doi.org/10.3390/foods6040025 (2017).
Corlett, M. T., Pethick, D. W., Kelman, K. R., Jacob, R. H. & Gardner, G. E. Consumer perceptions of meat redness were strongly influenced by storage and display times. Foods 10, 540. https://doi.org/10.3390/foods10030540 (2021).
Ding, Z., Wei, Q., Liu, C., Zhang, H. & Huang, F. The quality changes and proteomic analysis of cattle muscle postmortem during rigor mortis. Foods 11, 217. https://doi.org/10.3390/foods11020217 (2022).
Chan, J. T., Omana, D. A. & Betti, M. Effect of ultimate pH and freezing on the biochemical properties of proteins in turkey breast meat. Food Chem. 127, 109–117. https://doi.org/10.3382/ps.2010-01185 (2011).
Bowker, B. & Zhuang, H. Relationship between water-holding capacity and protein denaturation in broiler breast meat. Poult. Sci. 94, 1657–1664. https://doi.org/10.3382/ps/pev120 (2015).
Warner, R. D. Lawrie’s Meat Science 457–508 (Elsevier, 2022).
Singh, R. & Deshpande, D. Thermally induced changes in quality of chicken breast meat protein fractions. J. Nutr. Food Sci. 8, 1–5. https://doi.org/10.4172/2155-9600.1000709 (2018).
Mason, A. et al. Theoretical basis and application for measuring pork loin drip loss using microwave spectroscopy. Sensors 16, 182. https://doi.org/10.3390/s16020182 (2016).
Choi, Y. M., Garcia, L. G. & Lee, K. Correlations of sensory quality characteristics with intramuscular fat content and bundle characteristics in bovine longissimus thoracis muscle. Food Sci. Anim. Resourc. 39, 197. https://doi.org/10.5851/kosfa.2019.e15 (2019).
Acknowledgements
We are gratefully indebted to Natasha Snyman from Chemuniqué (Pty) Ltd (Lanseria, Gauteng) for her free-of-charge assistance with the feed formula used in this study.
Funding
Open access funding provided by North-West University.
Author information
Authors and Affiliations
Contributions
I.S.H.: Conceptualization, Investigation, Methodology. C.M.M.: Conceptualization, Investigation, Methodology, Data curation, Project administration, and Review. C.F.E.: Data curation, Writing, Review, and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hlatshwayo, I.S., Mnisi, C.M. & Egbu, C.F. Effect of dietary olive (Olea europea) pomace on productive performance, and physiological and meat quality parameters in Jumbo quail. Sci Rep 13, 6162 (2023). https://doi.org/10.1038/s41598-023-33495-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33495-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.