Abstract
Zeolite, as a natural mineral, could be a good additive for ducks, in line with pro-environmental trends. The study aimed to evaluate zeolite additives in feed for broiler ducks of both sexes on production results, meat quality, and the strength of the jejunum, tibia, and femur. The experiment used 200 Cherry Valley ducks, divided into a control group of males (CM) and females (CF) and an experimental group of males (ZM) and females (ZF). In the control groups, a commercial diet was used. In the experimental groups, 1% zeolite was added. The ZM group demonstrated higher body weight and weight gain than the CM group. Zeolite reduced the feed conversion ratio. A higher liver weight was found in the experimental group (ZM). Notably, zeolite influenced the weight of male pectoral muscles. Higher water loss in the pectoral muscles and higher protein content in the leg muscles were found in the same group. Females had a higher weight of neck and wings with skin. Female pectoral muscles had lower protein and water content. Zeolite in feed at a 1% level for broiler ducks could be recommended as a natural additive that positively affects the ducks' production results concerning good quality meat.
Similar content being viewed by others
Introduction
The production of broiler ducks in Poland in 2022 amounted to 67.8 thousand tons of carcass weight. It indicates a stable third position in the European Union for several years1. Duck meat is a product with high nutritional value, high content of fatty acids, and higher fat content than chicken meat (higher palatability). It is gaining popularity among consumers and may be competitive with meat from other poultry species2,3.
Feed additives have been used in poultry production for many years, which can result in better utilization of nutrients from feed, higher body weight gain, and lower feed consumption, as well as modulate the mechanisms of the immune system and optimize production efficiency. These include probiotics, prebiotics, synbiotics, eubiotics, phytobiotics, herbs, organic acids, essential oils, and minerals4,5,6,7. The use of substances with health-promoting properties is part of the European Green Deal strategy due to the potential reduction of antibiotics in animal production8.
Zeolites are a group of natural minerals belonging to aluminosilicates (analcime, chabazite, laumontite, and mordenite). These clay minerals with a porous structure have some properties that allow them to be used in many industrial sectors (agriculture, horticulture, household products)9. In animal production, aluminosilicates are used as feed additives as well as bedding additives10,11,12, primarily due to their high ability to absorb water and also harmful gases (especially ammonia) and mycotoxins, as well as reducing the population of harmful insects9,13,14.
Except for the positive impact of these substances on the environmental conditions in the livestock houses, the beneficial effect of aluminosilicates on selected production parameters of broiler chickens (higher body weight gain, lower mortality)15, and histomorphometry parameters of the jejunum, as the elongation of intestinal villi16 was notably found. In the study by Hcini et al.17, 1 and 2% zeolite were added to feed for turkeys. It was found that the experimental groups were characterized by higher growth efficiency and higher meat quality (including the content of polyunsaturated fatty acids). Feeding geese with the addition of zeolite, including nanostructured zeolite, may increase the body weight of birds and modify the content of selected chemical traits of meat18.
The beneficial effect of aluminosilicates on the body of birds may occur in the case of a protective effect on the liver and activation of metabolic processes or antitoxic effects19. Changes in the characteristics of blood serum, the weight of selected organs, and the chemical composition of muscles were also observed20. Many studies have found changes in selected carcass characteristics and physicochemical indicators of poultry meat11,21,22,23,24.
Research on the use of aluminosilicates in feeding broiler ducks is largely limited. In a pilot study, Biesek et al.25 showed that zeolite at a level supplementation of 4% in the feed influenced negative on body weight gain and water-holding capacity (7 weeks of rearing). It was also noticed that the yellowness was higher in leg muscles of ducks fed with zeolite. Simultaneously, it was concluded that too high an additive level in the feed may be a limiting factor. These results were inconclusive and required further experiments to verify the effect of zeolite thoroughly on the production results and meat quality of broiler ducks.
The study aimed to evaluate the use of zeolite addition at the level of 1% to the commercial diet of Cherry Valley broiler ducks and drakes on production results, carcass composition, meat quality, and strength of the jejunum, femur, and tibia.
Results
Growth performance
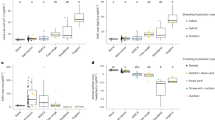
Significantly higher body weight of drakes was found in the zeolite group compared to the control group on days 28 (P = 0.048) and 42 (P = 0.041). Drakes fed with a diet supplemented with zeolite also achieved significantly higher (total) body weight gains (P = 0.040) and average daily body weight gains (P = 0.040). A significant effect of zeolite on the reduction of FCR was found when ducks were fed grower feed (P = 0.034) (Table 1).
Carcass features
Slaughter yield was similar in all groups and ranged from 65.04 to 67.27 g/100 g of pre-slaughter body weight. Significantly higher liver weight was found in ducks from the ZM group compared to ZF and CF (P < 0.001). Males had significantly higher liver’ weight compared to females (P < 0.001). Ducks from the CM group had the significantly highest weight of the gizzard (P = 0.001). Significantly lower weight of the neck was demonstrated in the ZM group compared to CM and ZF (P < 0.001). The weight of pectoral muscles was significantly the highest in group ZM (P = 0.045). Significantly lower skin with subcutaneous fat weight was demonstrated in the CM group compared to ZM (P = 0.039) (Table 2).
Meat quality traits of pectoral and leg muscles
The significantly highest pH was found in the meat of ducks from the CF group (P = 0.029, P = 0.014). This group also showed the lowest muscle lightness compared to CM and ZF (P = 0.003). The pectoral muscles from the ZM group were characterized by significantly higher WHC (P = 0.024; P = 0.004), protein content (P = 0.032; P = 0.014), and water content (P < 0.001). Significantly, the lowest salt content was found in the muscles of drakes of both groups compared to females (P < 0.001), as well as IMF compared to ZF (P = 0.002; P = 0.001). The leg muscles of ducks from CF had significantly the lowest WHC (P = 0.003; P = 0.011), protein content (P < 0.001), and water content (P < 0.001). The highest salt content was found in the muscles from the ZM group (P = 0.002), and the IMF content was found in the muscles of ducks from the ZF group (P < 0.001; P = 0.001) (Table 3).
Jejunum tensile strength and bones breaking strength
Significantly, the highest force needed for breaking the tibia was observed in the CM (292.30) and ZF (287.20) compared to the ZM (237.37) (P = 0.048). For other parameters, such as jejunum strength or femur strength, no statistically significant differences were found between the groups (P > 0.05) (Table 4).
Discussion
In our research, the BW and ADBWG of ducks in the ZM group on days 28 and 42 were significantly higher compared to the CM group. In the experimental group, ducks had lower FCR than the control group. The positive effect of natural minerals in poultry rearing has also been found in previously conducted studies. Banaszak et al.21 point out that broiler chickens' significantly highest BW and BWG were observed in the group fed with a mixture of halloysite and zeolite and added to the litter. According to Karamanlis et al.26, zeolite in feed (2%) and litter (2 kg/m2) also increased the BW of birds compared to the control group. Contrary to our research, zeolite at the level of 4% in the feed reduced BWG (by 0.34 kg) and increased FCR (by 0.50 kg/kg) in commercial ducks during 49 days of rearing25. An et al.27 have shown that feeding with 1% zeolite improved production results of Arbor Acres chickens' production results at raised temperatures. Zeolite may have protective properties against the harmful effects of heat stress in birds27.
Selected aluminosilicates may benefit the selected morphometric features of the jejunum. Halloysites in feed and litter increased the height and area of intestinal villi of broiler chickens16. Corresponding results were shown by supplementation with 3% zeolite in feed. Moreover, a higher thickness of the mucosa of the jejunum and ileum was noted28. The jejunum is the middle of the small intestine29, where digestion and intensive absorption of nutrients such as sugars, fats, amino acids, and minerals occur30. Zeolite can stimulate the secretion of digestive enzymes (amylase, lipase, and jejunal trypsin) and increase the digestibility of crude protein31. This ability may explain the improved BWG of broiler chickens. Similar conclusions were presented by Tang et al.32, in the study concerning the chickens feeding with zeolite containing zinc or Aigamo ducks fed with a mixture of zeolite, plant extract, and vermiculite33. The beneficial effect of zeolite on the digestion and absorption process has been demonstrated in other species of livestock, such as pigs34,35 and cows36. Due to the above properties, this could result in better production rates of ducks from the ZM group. In addition to the parameters determining the absorption of nutrients in the intestines, this mineral may be an immunomodulating factor of the immune system (expression of interleukin and interferon genes) and improve intestinal tightness37. It constitutes another health-promoting property, which could indirectly translate into better production results.
Zeolite significantly affected the weight of the liver and gizzard, unlike Pavlak et al.38, which did not indicate significantly different liver weights after using different doses of zeolite in broiler chickens' feed (0.25%, 0.5%, and 1%). Cited authors only indicated affection on the pancreas weight. Changes in the weight of the pancreas could result from the intensity of enzyme secretion. This relationship could indicate the highest liver weight in drakes fed with zeolite. Liver cells are the target site for neutralizing toxic substances absorbed in the gastrointestinal tract39. Zeolite is characterized by a porous structure that facilitates the absorption of toxic substances, e.g., mycotoxins40. A few studies have shown reduced liver weight by deoxynivalenol or zearalenone in chickens41,42. It constitutes a specific protective barrier for the liver and the entire body.
The significantly different weight of chicken gizzard depending on the method of zeolite application was confirmed by Basha et al.43. However, Banaszak et al.11 and Abdelrahman et al.24, did not noted any affect on the gizzard weight. Sand grains and natural minerals (grit) particles can affect digestion and gizzard weight44. In the presented research, the mineral was used in dusty form. Scientific research in this area is focused primarily on Gallinaceous poultry. Significantly, the neck's highest weight were found in the ZF group. Drakes from the ZM group were characterized by a significantly higher pectoral muscle weight than CM group (over 60.00 g).
The lowest weight of skin with subcutaneous fat occurred in the CM group. Contrary to our results, different doses of zeolite in the feed did not affect the weight of chicken pectoral muscles45. Similar results were shown by Saçakli et al.46 using only clinoptilolite or in combination with phytase. The pectoral muscle weight is a crucial element of the poultry carcass with high economic importance47. The increase in muscle weight depends on the rate of increase in the length and diameter of specific muscle fibers (the number of fibers is constant), which is determined, for example, by the diet48. The higher pectoral muscle weight could be influenced by better nutrient digestibility after using zeolite. Reports by other authors are inconsistent, as the protein content in the feed determines the highest performance of pectoral muscles, as opposed to digestibility49. Drakes were characterized by a higher weight of the liver neck but lower wing weight than females. Kaewtapee et al.50 found that Cherry Valley drakes had significantly higher weights of the gizzard and pectoral muscles. The influence of sex on carcass characteristics may be determined by differences in physiology (hormone secretion and metabolic processes).
The pH of the pectoral muscles of all groups was in the range of 5.83–5.95. It corresponds to the results of poultry meat pH from the other authors12,25. Pectoral muscles from the CM and ZF groups were characterized by significantly higher lightness. Lower WHC occurred in the pectoral muscles of the control group. The appropriate pH value of meat 24 h after slaughter indicates the course of the glycolysis process and the formation of H + ions during post-slaughter ripening51. It also affects the texture and color of the meat. Meat with a higher pH is characterized by a darker color52, which is confirmed by the results of meat from females from the control group (the highest pH value, the lowest L* value). Shabani et al.53 demonstrated the possibility of limiting lipid oxidation in the meat of slaughtered chickens through the action of aflatoxins after using zeolite (0.75 and 1%). This process affects changes in meat color—discoloration due to oxidation of heme dyes54, which could confirm a significantly higher L* value in the pectoral muscles of females of the experimental group compared to females of the control group. Similar to our research, Biesek et al.25 also found significantly higher water loss from ducks' pectoral and leg muscles at 6 weeks. WHC is an essential indicator of the technological suitability of meat for further processing and its sensory value55. It is related to the denaturation of muscle proteins during post-slaughter meat maturation (pH drop). Thus, more free water is created, increasing WHC56. The low pH of meat determines its lighter color, soft consistency and high water loss (low water absorption)—meat with a PSE defect (pH ≤ 5.8). Too high pH of the meat causes its darker color and slight drip loss (DFD meat; pH ≥ 6.3)57,58.
The chemical composition of the pectoral muscles indicates significant differences by sex (salt, IMF, and water content in the pectoral and leg muscles and protein content in the pectoral muscles). After zeolite application, drake pectoral muscles had the highest protein content, and female pectoral muscles had the highest salt and IMF content. A significantly higher protein content in leg muscles was found in the experimental groups. Safaei et al.59 revealed a reduction in the IMF content and no changes in the protein content in the meat of broiler chickens fed with aluminosilicates (zeolite, bentonite, kaolin). Other studies also indicated worse protein content in duck meat25. The chemical composition of meat largely depends on nutritional factors60 and affects its dietary value61 and sensory properties62.
The tibia of drakes fed with zeolite were characterized by lower fracture strength than the bones from the ZF and CM groups. Biesek et al.25 found no effect of zeolite addition on the strength parameters of tibia bones of ducks of various origins, or Eleroğlu et al.63 fed chickens with Ca–zeolite addition. According to Safaeikatouli et al.64, 3% bentonite or zeolite improved the properties of the chicken tibia (weight/length index, strength index), possibly due to higher calcium absorption.
To sum up, feeding broiler ducks with 1% zeolite addition positively affected selected production results, especially in the male group. In particular, lower FCR was found in the experimental group in both sexes. The critical aspect is in the features of pectoral muscles. Zeolite increased the WHC of pectoral muscles, and in leg muscles, the protein and IMF content improved, and the water content decreased. It is justified to recommend 1% zeolite in the feed for broiler ducks. It has been shown that the sex of ducks may differentiate the production results and carcass characteristics. When analyzing our results and considering the available literature, zeolite is crucial in the digestion of nutrients and the immunity of ducks; however, it should be additionally studied. Taking up the presented research issues allows for a new perception of natural additives such as zeolite in duck nutrition. Thus, its favorable properties can contribute to improving production efficiency indicators and obtaining good-quality meat.
Material and methods
The consent for research was obtained from the Institutional Animal Care and Use Committee of the Bydgoszcz University of Science and Technology (Local Ethical Committee, No. 2/2022). The ethics committee of Bydgoszcz University of Science and Technology approved the experimental protocols presented in this study. The National Ethical Committee for Animal Experiments recommendations and the ARRIVE guidelines were also considered. The experiment followed Directive 2010/63/EU of the European Parliament and the Council. All methods used in the experiment were applied in accordance with applicable guidelines and requirements.
Animals and diets
In the experiment, 100 females (ducks) and 100 males (drakes) of Pekin Cherry Valley SM3 Medium ducklings were used (Tulce, Greater Poland Voivodeship, Poland). Birds were divided into 4 groups of equal number, 5 repetitions (pens) each. There was a control group of males (CM), a control group of females (CF), and an experimental group of males (ZM) and females (ZF).
Rearing lasted 42 days. The environmental conditions in the broiler duck house were adapted to the recommendations for keeping broiler ducks. On the first day of rearing, the temperature was 26 °C, which was gradually lowered to 18 °C (after 4th week of life). An additional heat source with a temperature of 30 °C (and lowered within the time) was placed in each pen (for 4 weeks). The relative humidity was in the range of 60–70% and the air movement was 0.2–0.3 m/s. The concentration of harmful gases was monitored to ensure that it did not exceed 3000 ppm—CO2, 20 ppm—NH3 and 5 ppm—H2S, respectively. The pen area was 2 m2 and the stocking density did not exceed 17 kg/m2. Chopped wheat straw was used as bedding. Feed and water were provided ad libitum. Rearing was divided into two feeding periods. From day 1 to 28, the ducks were fed a commercial diet of the starter type. From day 29 to 42, a grower-type commercial diet was used. The commercial diet was isocaloric and isoprotein. The composition of commercial diets corresponded to the nutritional recommendations for broiler ducks65. The control groups were fed commercial diets without additives throughout the rearing period. In the experimental groups, birds were fed a commercial diet supplemented with 1% zeolite, previously thoroughly mixed mechanically66. Chemical composition of zeolite: SiO2 (silicon dioxide)—71.30%; Al2O3 (aluminum oxide)—13.10%; CaO (calcium oxide)—5.20%; K2O (potassium oxide)—3.40%; Fe2O3 (iron (III) oxide)—1.90%; MgO (magnesium oxide)—1.20%; Na2O (sodium oxide)—1.30%; TiO2 (titanium oxide)—0.30%; Si/Al (silicon / aluminum)—5.40%; Clinoptilolite—84.00%; Cristobalit—8.00%; Mica clay—4.00%; Plagioclases—3.50%; Rutile—0.20%. Specific surface area of 30–60 m2/g, a bulk density of 1.60–1.80 kg/m3 and weight of 2.20–2.44 kg/m3.
Diet composition
Starter feed contained: maize, wheat, soybean extraction meal, wheat bran, sunflower extraction meal, hulled sunflower seeds, barley, rapeseed extraction meal, wheat gluten feed, calcium carbonate, animal fat, monocalcium phosphate, vegetable oil and fat (raw sunflower), sodium chloride, and sodium sulfate, crude protein (19.5%), ether extract (3.9%), crude fiber (4.2%), lysine (0.93%), methionine (0.42%), threonine (0.72%), calcium (0.85%), phosphorus (0.69%), sodium (0.17%), vitamin A (10,000 IU), vitamin D3 (3000 IU), vitamin E (25 IU). Grower feed contained: maize, wheat, wheat bran, soybean extraction meal, sunflower extraction meal, from dehulled sunflower seeds, triticale, rapeseed extraction meal, animal fat, calcium carbonate, monocalcium phosphate, sodium chloride, calcium bicarbonate, crude protein (17.1%), ether extract (3.7%), crude fiber (4.5%), lysine (0.87%), methionine (0.37%), threonine (0.61%), calcium (0.81%), phosphorus (0.66%), sodium (0.16%), vitamin A (10,000 IU), vitamin D3 (3000 IU), vitamin E (25 IU) (De Heus, Łęczyca, Polska). Commercial diet from each feeding period and group was collected (approximately 500 g) into 5 string bags each. Laboratory analyses were conducted using methods specified by the Polish Committee for Standardization (www.pkn.pl). The content of dry matter (DM)67, crude ash (CA)68, and crude protein (CP)69 was assessed. Also, crude fiber (CF)70, crude fat (EE)71, starch72, NDF73, ADF, and ADL74 were analyzed. The samples were analyzed in 10 repetitions. The compliance of the content of nutrients declared by the manufacturer was verified, and minor deviations were considered acceptable (Table 5).
Growth performance
Ducks were weighed on days 1, 28, and 42 (Radwag, Radom, Poland). The body weight (BW) and feed intake (FI) were monitored. Based on these data, body weight gain (BWG), average daily body weight gain (ADBWG), and average daily feed intake (ADFI) were calculated. The feed conversion ratio (FCR) was calculated based on BW and FI. The parameters were determined for each feeding period and the entire rearing. Moreover, deaths were recorded during rearing (% viability), and production efficiency indicators were calculated, such as the European Production Efficiency Factor (EPEF) and European Broiler Index (EBI). For calculations, formulas were used:
-
\(BWG=final \, body \, weight \, \left(g\right)-initial \, body \, weight (g)\)
-
\(ADBWG=\frac{BWG\, in \, feeding \, period (g)}{number \, of \, days}\)
-
\(ADFI=\frac{total \, feed \, intake \, per \, one \, duck (g)}{42 \, days}\)
-
\(FCR=\frac{feed\, intake \, \left(g\right)}{body \, weight \, gain \, \left(g\right)}\)
-
\(EPEF=\frac{viability\, \left({\%}\right) \times BW \, (kg)}{\begin{array}{c}age\, \left(days\right) \times FCR \, \left(\frac{kg \, feed}{kg \, gain}\right)\end{array}}\times 100{\%}\),
-
\(EBI = \frac{{\begin{array}{*{20}c} {viability{\mkern 1mu} \left( \% \right) \times ADG\left( {\frac{{\frac{g}{{chick}}}}{{day}}} \right)} \\ \end{array} }}{{FCR\left( {\frac{{kg{\mkern 1mu} feed}}{{kg{\mkern 1mu} gain}}} \right) \times 10}} \times 100\%\),
Samples collection
On day 42, 10 birds per group (2 ducks or drakes per pen) with a body weight similar to the average body weight of birds in each pen were selected for slaughter. After previously stunning the birds (with an electric current), they were slaughtered by cutting the spinal cord between the first cervical vertebra and the occipital condyle. After bleeding, the carcasses were dipped in water at 65 °C for 10 s and plucked with a mechanical plucker. Feather leftovers were removed using food-grade wax (Polwax, Jasło, Poland), and the carcasses were eviscerated. The feet were cut off at the ankle joint. Heart, liver, and gizzard were also collected. The prepared carcasses with offal were chilled in a refrigerator (Hendi, Poznań, Poland) at 4 °C for 24 h for further meat quality analyses.
From the digestive tract, approximately a 10 cm long section of the jejunum (from Meckel's diverticulum). The jejunum samples were frozen (Gorenje, Velenje, Slovenia) at − 18 °C.
Carcass features
Carcasses and offal were weighed. The carcasses were dissected75 and weighed. Carcass elements included the neck, pectoral muscles (m. pectoralis major and minor), leg muscles (deboned), skin with subcutaneous fat, wings with skin, abdominal fat, and carcass remains (trunk, tibia, and femur). The femur and tibia bones from the right leg were collected and frozen (Gorenje, Velenje, Slovenia) at − 18 °C for further analysis. Based on the results obtained, slaughter yield with or without offal and the percentage of individual carcass elements were calculated.
Meat quality
During the weighing of the post-chilled carcasses, the pH of the pectoral muscle was measured using a pH meter (Elmetron, Zabrze, Poland) with a dagger electrode. The device was calibrated in standard pH 4.00, 7.00, and 9.00 buffers.
The color of the pectoral and leg muscles was measured with a CR-400 colorimeter (Konica Minolta, Tokyo, Japan) from the inner side of the muscle. The following parameters were determined on the CIE Lab scale: lightness (L*), redness (a*), and yellowness (b*).
The pectoral muscle (previously weighed) was placed in a string bag (notched at the bottom) and then placed in a larger bag to allow water to leak out. It was transferred to a cold store (Hendi, Poznań, Poland) to a temperature of 4 °C for 24 h. After this time, it was re-weighed, and the drip loss was calculated76.
Each group's pectoral and leg muscles were ground in a meat grinder (Hendi, Poznań, Poland). Meat samples weighing 0.300 g (± 0.005 g) were weighed. The samples were placed between two pieces of Whatman 1 paper and kept under a load of 2 kg for 5 min. The meat samples were re-weighed, and the water holding capacity (WHC) was calculated77.
The chemical composition of pectoral and leg muscles (protein, collagen, salt, intramuscular fat (IMF), and water content) was analyzed, and 90 g of ground meat from each group was weighed. The analyses were performed using the FoodScan apparatus (FOSS, Hillerød, Denmark) using spectrophotometry using near-infrared transmission (NIT)78.
Each qualitative analysis of physiochemical features was performed in 10 replicates. For calculations, formulas were used:
-
Dressing percentage \(=\frac{carcass\, weight (g)}{live\, body\, weight \left(g\right) }\times 100\)
-
Drip loss \(=100-\left(\frac{M2}{M1}\right)\times 100\%\) where M2—breast muscle weight after 24 h, M1—initial breast muscle weight.
-
\(WHC=100-\left(\frac{M2}{M1}\right)\times 100\%\) where, M2—ample weight after 5 min, M1—initial sample weight.
Jejunum tensile strength and bones’ breaking strength
The jejunum samples were thawed at 4 °C for 24 h. Tensile strength was analyzed using an Instron 3345 device (Instron, Buckinghamshire, England) with Bluehill 3 software. It was defined as the highest force necessary to rupture a sample. Each end of the intestinal sample was placed in pneumatic holders and stretched at a speed of 500 mm/min (Meckel's diverticulum was taken as the standardization point).
The bones were thawed in the same conditions as in the case of the jejunum. Then, they were thoroughly cleaned of meat residues and weighed. Femoral and tibial strength measurements were performed by the Instron Bend Fixture 10 mm Anvil adapter. Each bone was placed on an attachment, and measurements were taken at 250 mm/min speed. The maximum force to fracture a bone and the force per 1 g of bone (N/g) were investigated25.
Statistical analyzes
The numerical data were calculated using a statistical program (Statistica, ver. 13.3.0., TIBCO, Software, Kraków, Poland, 2017). Mean values and SEM were calculated. A one-way analysis of variance for zeolite and sex and a two-way analysis of variance were used, considering the interaction of zeolite and sex. Statistical models were used: a one-way analysis of variance (Yz/s = µ + Cz/Ds + ez/s, where Yz/s—the dependent variable; µ—the overall mean, Cz—zeolite in feed, Ds—sex, e—residual error) for each experimental factor (zeolite addition or sex) and a two-way analysis of variance (Yz/s = µ + Cz + Ds + CDzs + ez/s, where Yz/s—the dependent variable; µ—the overall mean, Cz—zeolite in feed, Ds—sex, CDzs—interaction between sex and zeolite in feed, e—residual error) for interaction between zeolite addition and sex of birds. Statistically significant differences were verified with the Tukey test with a probability of P < 0.05.
Ethical approval
The consent for research was obtained from the Institutional Animal Care and Use Committee of the Bydgoszcz University of Science and Technology (Local Ethical Committee, No. 2/2022). The ethics committee of Bydgoszcz University of Science and Technology approved the experimental protocols presented in this study. The National Ethical Committee for Animal Experiments recommendations and the ARRIVE guidelines were also considered. The experiment followed Directive 2010/63/EU of the European Parliament and the Council. All methods used in the experiment were applied in accordance with applicable guidelines and requirements.
Data availability
None of the data was deposited in an official repository. All of the data obtained in the research are presented in this paper. The data that support the study findings are available from the corresponding author upon reasonable request.
References
AVEC Annual Report. Retrieved on 10 November 2023 from https://avec-poultry.eu/resources/annual-reports/ (2023).
Biswas, S. et al. Technological investigation into duck meat and its products—a potential alternative to chicken. World’s Poult. Sci. J. 75, 609–620. https://doi.org/10.1017/S004393391900062X (2019).
Arshad, M. S. et al. Influence of E-beam irradiation on microbiological and physicochemical properties and fatty acid profile of frozen duck meat. Food Sci. Nutr. 8, 1020–1029. https://doi.org/10.1002/fsn3.1386 (2020).
Dankowiakowska, A., Kozłowska, I. & Bednarczyk, M. Probiotics, prebiotics and snybiotics in poultry—mode of action, limitation, and achievements. J. Cent. Eur. Agric. 14, 467–478. https://doi.org/10.5513/JCEA01/14.1.1222 (2013).
Al-Khalaifah, H. S. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 97, 3807–3815. https://doi.org/10.3382/ps/pey160 (2018).
El-Deek, A. A. et al. Alternative feed ingredients in the finisher diets for sustainable broiler production. Sci. Rep. 10, 17743. https://doi.org/10.1038/s41598-020-74950-9 (2022).
Wlaźlak, S., Pietrzak, E., Biesek, J. & Dunisławska, A. Modulation of the immune system of chickens a key factor in maintaining poultry production—a review. Poult. Sci. 102, 102785. https://doi.org/10.1016/j.psj.2023.102785 (2023).
Prandecki, K., Wrzaszcz, W. & Zieliński, M. Environmental and climate challenges to agriculture in Poland in the context of objectives adopted in the European Green Deal Strategy. Sustainability 13, 10318. https://doi.org/10.3390/su131810318 (2021).
Eroglu, N., Emekci, M. & Athanassiou, C. G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 97, 3487–3499. https://doi.org/10.1002/jsfa.8312 (2017).
Gilani, A., Kermanshahi, H., Golian, A. & Seifi, S. Appraisal of the impact of aluminosilicate use on the health and performance of poultry. Turk. J. Vet. Anim. Sci. 40, 255–262. https://doi.org/10.3906/vet-1501-103 (2016).
Banaszak, M., Biesek, J. & Adamski, M. Wheat litter and feed with aluminosilicates for improved growth and meat quality in broiler chickens. PeerJ 9, e11918. https://doi.org/10.7717/peerj.11918 (2021).
Banaszak, M., Biesek, J. & Adamski, M. Aluminosilicates at different levels in rye litter and feed affect the growth and meat quality of broiler chickens. Vet. Res. Commun. 46, 37–47. https://doi.org/10.1007/s11259-021-09827-x (2022).
Adamović, M., Stojanović, M., Grubišić, M., Ileš, D. & Milojković, J. Importance of aluminosilicate minerals in safe food production. Maced. J. Anim. Sci. 1, 175–180 (2011).
Cook, D. F. et al. Amending poultry broiler litter to prevent the development of stable fly, Stomoxys calcitrans (Diptera: Muscidae) and other nuisance flies. J. Econ. Entomol. 111, 2966–2973. https://doi.org/10.1093/jee/toy277 (2018).
Burmańczuk, A., Roliński, Z., Kowalski, C., Burmańczuk, N. & Markiewicz, W. Possible use of natural zeolites in animal production and environment protection. J. Elem. 20, 803–811 (2015).
Banaszak, M. et al. Impact of aluminosilicates on productivity, carcass traits, meat quality, and jejunum morphology of broiler chickens. Poult. Sci. 99, 7169–7177. https://doi.org/10.1016/j.psj.2020.08.073 (2020).
Hcini, E. et al. Does supplemental zeolite (clinoptilolite) affect growth performance, meat texture, oxidative stress and production of polyunsaturated fatty acid of Turkey poults?. Lipids Health Dis. 17, 1–9. https://doi.org/10.1186/s12944-018-0820-7 (2018).
Larina, Y., Ezhkov, V., Fayzrakhmanov, R., & Ezhkova, A. Meat productivity and quality of goose meat when using nanostructural zeolite in feeding. In BIO Web of Conferences 27, 00028; EDP Sciences. https://doi.org/10.1051/bioconf/20202700028 (2020).
Semenenko, M., Kuzminova, E., Grin, V., Rogaleva, E., & Semenenko, K. Possibilities of using natural aluminosilicates in the development of medicines at hepatosis in poultry. In E3S Web of Conferences 175, 04002 EDP Sciences. https://doi.org/10.1051/e3sconf/202017504002 (2020).
Prvulovic, D., Kojic, D., Grubor-Lajsic, G. & Kosarcic, S. The effects of dietary inclusion of hydrated aluminosilicate on performance and biochemical parameters of broiler chickens. Turk. J. Vet. Anim. Sci. 32, 183–189 (2008).
Banaszak, M., Biesek, J. & Adamski, M. Growth performance and meat quality from broiler chickens reared with zeolite and halloysite in feed and straw pellet. Anim. Sci. J. 92, 13649. https://doi.org/10.1111/asj.13649 (2021).
Biesek, J., Dunisławska, A., Banaszak, M., Siwek, M. & Adamski, M. The impact of hydrated aluminosilicates supplemented in litter and feed on chicken growth, muscle traits and gene expression in the intestinal mucosa. Animals 1, 2224. https://doi.org/10.3390/ani11082224 (2021).
Nadziakiewicz, M., Micek, P. & Wojtysiak, D. Effects of dietary halloysite supplementation on broiler chicken’s blood parameters, carcass and meat quality, and bone characteristics: a preliminary study. Ann. Anim. Sci. 23, 129–139. https://doi.org/10.2478/aoas-2022-0037 (2023).
Abdelrahman, M. M. et al. Using natural zeolite as a feed additive in Broilers’ diets for enhancing growth performance, carcass characteristics, and meat quality traits. Life 13, 1548. https://doi.org/10.3390/life13071548 (2023).
Biesek, J., Banaszak, M. & Adamski, M. Ducks’ growth, meat quality, bone strength, and jejunum strength depend on zeolite in feed and long-term factors. Animals 11, 1015. https://doi.org/10.3390/ani11041015 (2021).
Karamanlis, X. et al. The effect of a natural zeolite (clinoptilolite) on the performance of broiler chickens and the quality of their litter. Asian-Austral. J. Anim. Sci. 21, 1642–1650. https://doi.org/10.5713/ajas.2008.70652 (2008).
An, J. et al. Effects of supplemental different clay minerals in broiler chickens under cyclic heat stress. J. Anim. Sci. Technol. 65, 113. https://doi.org/10.5187/jast.2022.e94 (2023).
Wawrzyniak, A. et al. The effect of dietary supplementation of transcarpathian zeolite on intestinal morphology in female broiler chickens. J. Appl. Poult. Res. 26, 421–430. https://doi.org/10.3382/japr/pfx011 (2017).
Jamroz, D., Wertelecki, T., Houszka, M. & Kamel, C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Anim. Physiol. Anim. Nutr. 90, 255–268. https://doi.org/10.1111/j.1439-0396.2005.00603.x (2006).
Fetterer, R. H., Miska, K. B., Jenkins, M. C. & Wong, E. A. Expression of nutrient transporters in duodenum, jejunum, and ileum of Eimeria maxima-infected broiler chickens. Parasitol. Res. 113, 3891–3894 (2014).
Zhou, P. et al. Effects of dietary supplementation with the combination of zeolite and attapulgite on growth performance, nutrient digestibility, secretion of digestive enzymes and intestinal health in broiler chickens. Asian-Austral. J. Anim. Sci. 27, 1311. https://doi.org/10.5713/ajas.2014.14241 (2014).
Tang, Z., Wen, C., Li, P., Wang, T. & Zhou, Y. Effect of zinc-bearing zeolite clinoptilolite on growth performance, nutrient retention, digestive enzyme activities, and intestinal function of broiler chickens. Biol. Trace Elem. Res. 158, 51–57 (2014).
Khambualai, O. et al. Effects of dietary natural zeolite including plant extract on growth performance and intestinal histology in Aigamo ducks. Br. Poult. Sci. 50, 123–130. https://doi.org/10.1080/00071660802662788 (2009).
Ly, J., Grageola, F., Lemus, C. & Castro, M. Ileal and rectal digestibility of nutrients in diets based on leucaena (Leucaena leucocephala (Lam.) de Wit) for pigs Influence of the inclusion of zeolite. J. Anim. Vet. Adv. 6, 371–376 (2007).
Wang, H., Yin, J. & Kim, I. H. Experimental study on the effect of zeolite (clinoptilolite) on the growth performance, nutrient digestibility, and faecal microbiota of finishing pigs. J. Appl. Anim. Res. 49, 154–157. https://doi.org/10.1080/09712119.2021.1914063 (2021).
Osman, A. A., Soliman, S. A. & Doaa, E. S. Effects of dietary zeolite supplementation on milk yield, milk composition, digestion coefficients and nutritive values in Holsten cows. J. Anim. Poult. Fish Prod. 10, 17–20 (2021).
Dunislawska, A., Biesek, J., Banaszak, M., Siwek, M. & Adamski, M. Effect of zeolite supplementation on gene expression in the intestinal mucosa in the context of immunosafety support in poultry. Genes 13, 732. https://doi.org/10.3390/genes13050732 (2022).
Pavlak, M. S. D. et al. Impact of various dietary levels of zeolite on broiler performance, digestibility, and carcass traits. S. Afr. J. Anim. Sci. 52, 400–408. https://doi.org/10.4314/sajas.v52i3.15 (2022).
Ditta, Y. A., Mahad, S. & Bacha, U. Aflatoxins: their toxic effect on poultry and recent advances in their treatment. Mycotoxins Impact Manage. Strat. 20, 1–153 (2018).
Shariatmadari, F. The application of zeolite in poultry production. World’s Poult. Sci. J. 64, 76–84 (2008).
Lucke, A., Doupovec, B., Paulsen, P., Zebeli, Q. & Böhm, J. Effects of low to moderate levels of deoxynivalenol on feed and water intake, weight gain, and slaughtering traits of broiler chickens. Mycotoxin Res. 33, 261–271 (2017).
Chen, Y. et al. The protective effects of modified palygorskite on the broilers fed a purified zearalenone-contaminated diet. Poult. Sci. 98, 3802–3810. https://doi.org/10.3382/ps/pez085 (2019).
Basha, H. A., Goma, A. A., Taha, A. E. & AbouElkhair, R. Effect of different forms of natural zeolite (clinoptilolite) on productive performance and behavioral patterns of broiler chickens. Int. J. Agric. Sci. Vet. Med. 4, 1–11 (2016).
Svihus, B. et al. Performance and digestive function of broiler chickens given grit in the diet. Brit. Poult. Sci. 58, 530–535. https://doi.org/10.1080/00071668.2017.1332404 (2017).
Pavlak, M. S. et al. Zeolite and corn with different compositions in broiler chickens feeding. Poult. Sci. 102, 10249. https://doi.org/10.1016/j.psj.2023.102494 (2023).
Saçakli, P., Calik, A., Bayraktaroğlu, A. G., Ergün, A. & Şahan, Ö. Effect of clinoptilolite and/or phytase on broiler growth performance, carcass characteristics, intestinal histomorphology and tibia calcium and phosphorus levels. Kafkas Üniv. Vet. Fak. Derg. 21, 729–737 (2015).
Lorentz, L. et al. Production and body composition traits of broilers in relation to breast weight evaluated by path analysis. Sci. Agric. 68, 320–325. https://doi.org/10.1590/S0103-90162011000300008 (2011).
Chen, X. D., Ma, Q. G., Tang, M. Y. & Ji, C. Development of breast muscle and meat quality in Arbor Acres broilers, Jingxing 100 crossbred chickens and Beijing fatty chickens. Meat Sci. 77, 220–227. https://doi.org/10.1016/j.meatsci.2007.03.008 (2007).
Widyaratne, G. P. & Drew, M. D. Effects of protein level and digestibility on the growth and carcass characteristics of broiler chickens. Poult. Sci. 90, 595–603. https://doi.org/10.3382/ps.2010-01098 (2011).
Kaewtapee, C., Prahkarnkaeo, K. & Bunchasak, C. Effect of sex on growth curve, production performance and carcass quality of Cherry Valley ducks. J. Appl. Anim. Sci. 11, 9–18 (2018).
Chauhan, S. S. et al. Glycolysis and pH decline terminate prematurely in oxidative muscles despite the presence of excess glycogen. Meat Muscle Biol. 3, 254–264 (2019).
Wideman, N., O’bryan, C. A. & Crandall, P. G. Factors affecting poultry meat colour and consumer preferences—a review. World’s Poult. Sci. J. 72, 353–366 (2016).
Shabani, A., Dastar, B., Hassani, S., Khomeiri, M. & Shabanpour, B. Decreasing the effects of aflatoxins on color and oxidative stability of broiler meats using nanozeolite. J. Agric. Sci. Technol. 18, 109–121 (2016).
Huang, X. & Ahn, D. U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 28, 1275–1285. https://doi.org/10.1007/s10068-019-00631-7 (2019).
Szmańko, T., Lesiów, T. & Górecka, J. The water-holding capacity of meat: A reference analytical method. Food Chem. 357, 129727. https://doi.org/10.1016/j.foodchem.2021.129727 (2021).
Bowker, B. & Zhuang, H. Relationship between water-holding capacity and protein denaturation in broiler breast meat. Poult. Sci. 94, 1657–1664. https://doi.org/10.3382/ps/pev120 (2015).
Jung, S. et al. Comparison of pH, water holding capacity and color among meats from Korean native chickens. Korean J. Poult. Sci. 42, 101–108. https://doi.org/10.5536/KJPS.2015.42.2.101 (2015).
Lesiów, T. & Kijowski, T. Impact of PSE and DFD meat on poultry processing-a review. Pol. J. Food Nutr. Sci. 12, 3–8 (2003).
Safaei, M. Effects of inclusion kaolin, bentonite and zeolite in dietary on chemical composition of broiler chickens meat. Asian J. Anim. Vet. Adv. 9, 56–63 (2014).
Barteczko, J. & Lasek, O. Effect of varied protein and energy contents in mixture on meat quality of broiler chicken. Slovak J. Anim. Sci. 41, 173–178 (2008).
Bordoni, A. & Danesi, F. Poultry Meat Nutritive Value and Human Health (Woodhead Publishing, 2017).
Chartrin, P. et al. Effects of intramuscular fat levels on sensory characteristics of duck breast meat. Poult. Sci. 85, 914–922. https://doi.org/10.1093/ps/85.5.914 (2006).
Eleroğlu, H., Yalçın, H. & Yıldırım, A. Dietary effects of Ca-zeolite supplementation on some blood and tibial bone characteristics of broilers. S. Afr. J. Anim. Sci. 41, 319–330. https://doi.org/10.4314/sajas.v41i4.1 (2011).
Safaeikatouli, M., Boldaji, F., Dastar, B. & Hassani, S. Growth response and tibia bone characteristics in broilers fed diets containing kaolin, bentonite and zeolite. J. Anim. Feed Sci. 21, 334–344 (2012).
Smulikowska, S., & Rutkowski, A. Nutritional recommendations and nutritional value of poultry feeds. In Cooperative Work. Fifth Edition—Changed and Supplemented. Polish Academy of Science, Institute of Physiology and Animal Nutrition, Jabłonna, Poland, 2019, 58–65 ISBN 978-83-951612-1-6 (in Polish) (2018).
Biesek, J., Banaszak, M., Kądziołka, K., Wlaźlak, S. & Adamski, M. Growth of broiler chickens, and physical features of the digestive system, and leg bones after aluminosilicates used. Sci. Rep. 12, 20425. https://doi.org/10.1038/s41598-022-25003-w (2022).
PN-ISO 6496:2002 (2002) Pasze—oznaczanie wilgotności i zawartości innych substancji lotnych (in Polish) (2002).
Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed (Text with EEA relevance).
PN-EN ISO 5983-1:2006 Pasze—oznaczanie zawartości azotu i obliczanie zawartości białka surowego—Część 1: Metoda Kjeldahla (in Polish) (2006).
PN-ISO 6865:2002 Pasze—oznaczanie zawartości włókna surowego—metoda z pośrednią fltracją (in Polish) (2002).
PN-EN ISO 6492:2005 Pasze—oznaczanie zawartości tłuszczu (in Polish) (2005).
PN-R-64785: 1994—Pasze. Oznaczanie zawartości skrobi metodą polarymetryczną (1994).
PN-EN ISO 16472:2007 Pasze—oznaczanie zawartości włókna obojętnodetergentowego po traktowaniu amylazą (aNDF) (in Polish) (2007).
PN-EN ISO 13906:2009 Pasze—oznaczanie zawartości włókna kwaśnodetergentowego (ADF) i ligniny kwaśnodetergentowej (ADL) (in Polish) (2009).
Ziołecki, J. & Doruchowski, W. Methods for Assessing Slaughter Value COBRD Publisher (Poznań, 1989).
Honikel, K. O. The water binding of meat. Fleischwirtschaft 67, 1098–1102 (1987).
Grau, R. & Hamm, R. Eine einfache Methode zur Bestimmung der Wasserbindung in Fleisch. Fleischwirtschaft 4, 295–297 (1952).
PN-A-82109:2010 Meat and meat preparations—determination of fat, protein, and water content. Near Infrared Transmission Spectrometry (NIT) using Artifcial Neural Network (ANN) calibration, Lublin, Poland (in Polish) (2010).
Acknowledgements
The authors thank the Laboratory of Chemical Research and Instrumental Analyzes and the Department of Animal Breeding and Nutrition team for technical assistance in the chemical analyses. The research was carried out as part of project No. UMO-2021/43/D/NZ9/01756, financed by the National Science Center (Poland). Financially supported by the Minister of Science under the program “Regional Initiative of Excellence” (RID/SP/0017/2024/01).
Funding
The research was carried out as part of project No. UMO-2021/43/D/NZ9/01756, financed by the National Science Center (Poland). The project No. UMO-2021/43/D/NZ9/01756 also financed the APC. Financially supported by the Minister of Science under the program “Regional Initiative of Excellence” (RID/SP/0017/2024/01).
Author information
Authors and Affiliations
Contributions
All authors participated in the described experiment. S.W., J.B., M.B.—conceptualization; S.W., J.B.—data curation; S.W. J.B.—formal analysis; M.B.—funding acquisition; S.W., J.B., M.B.—investigation; J.B., M.B.—methodology; M.B.—project administration; S.W.—resources; S.W., J.B.—software; J.B., M.B.—supervision; S.W.—validation; S.W., J.B.—visualization; S.W.—writing—original draft; J.B., M.B.—writing—review and editing. All authors participated in and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wlaźlak, S., Biesek, J. & Banaszak, M. Growth performance, meat quality, strength of jejunum and leg bones of both sexes Cherry Valley ducks fed with zeolite. Sci Rep 14, 3938 (2024). https://doi.org/10.1038/s41598-024-54393-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54393-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.