Abstract
Coral cover has declined worldwide due to anthropogenic stressors that manifest on both global and local scales. Coral communities that exist in extreme conditions can provide information on how these stressors influence ecosystem structure, with implications for their persistence under future conditions. The Port of Miami is located within an urbanized environment, with active coastal development, as well as commercial shipping and recreational boating activity. Monitoring of sites throughout the Port since 2018 has revealed periodic extremes in temperature, seawater pH, and salinity, far in excess of what have been measured in most coral reef environments. Despite conditions that would kill many reef species, we have documented diverse coral communities growing on artificial substrates at these sites—reflecting remarkable tolerance to environmental stressors. Furthermore, many of the more prevalent species within these communities are now conspicuously absent or in low abundance on nearby reefs, owing to their susceptibility and exposure to stony coral tissue loss disease. Natural reef frameworks, however, are largely absent at the urban sites and while diverse fish communities are documented, it is unlikely that these communities provide the same goods and services as natural reef habitats. Regardless, the existence of these communities indicates unlikely persistence and highlights the potential for coexistence of threatened species in anthropogenic environments, provided that suitable stewardship strategies are in place.
Similar content being viewed by others
Introduction
Populations of reef-building corals have been declining world-wide due to a combination of global and local stressors1. This macabre phenomenon is especially apparent in South Florida and the Florida Keys, where urban populations and active coastal development are in close proximity to coral reefs, influencing the goods and services they provide. Warming, acidification, poor water quality, disease, and unsustainable exploitation have contributed to a precipitous > 80% drop in coral cover throughout the Caribbean since the 1970s2. Currently in Southeast Florida, coral cover is less than 1%, while reefs in the Keys currently exhibit roughly 6%3.
The influences of the numerous stressors within the region are both temporally and spatially complex, dependent on benthic composition, hydrography, and proximity to urban development and runoff. This can lead to local refugia where, for example, seagrass photosynthesis ameliorates acidification4. The antithesis, however, is also true and hotspots exist whereby coral communities are exposed to extreme levels of stressors5. The latter situation often leads to ecosystem degradation but, in unique circumstances, extreme environments can prove valuable for science and management.
Characterization of areas with acute acidification, high temperatures, and elevated nutrients have led to insights on how these stressors may impact reefs on scales ranging from the organismal6 to the ecosystem7. In these areas, unique combinations of environmental factors can lead to outcomes that are difficult to predict using single-taxon, single-stressor studies. Particularly persistent coral species or genotypes may reveal mechanisms of resilience in the genetics of the coral host and/or the associated algal symbionts of the family Symbiodiniaceae8, as well as the wider microbiome9. These natural associations and molecular mechanisms may ultimately prove useful for increasing the efficacy and efficiency of restoration efforts and are likely critical for maintaining ecosystem function in a rapidly changing world.
While naturally-extreme coral environments have been identified worldwide in areas ranging from volcanic CO2 seeps10 and upwelling regions11, to mangrove channels12 and tide pools13, recent attention has focused on corals living in close proximity to metropolitan areas14,15,16,17. These so-called urban coral communities, which can develop on anthropogenic substrates and structures, are by their very nature relevant to reef coexistence in the Anthropocene. They are well-documented to have poor water quality, including higher turbidity, sedimentation and nutrients compared with less-impacted offshore reef systems18,19,20. It is also likely that numerous, less-studied stressors such as light and noise pollution, abrasion from litter and debris, as well as increased resource use and fishing also impact organisms in these environments14.
The Port of Miami is an urbanized waterway located in Southeast Florida, connecting Biscayne Bay to the western Atlantic. It is adjacent to downtown Miami and Miami Beach, and its waterways are highly engineered, incorporating seawalls, riprap, dredged canals, bridges, pilings, and piers. In addition to recreational boat traffic, it supports an active cruise industry with a total of 1220 cruise ships docked in 2019, accounting for 5,592,000 passengers21. In the same year, 1000 cargo ships docked in the port, moving more than a million containers and 9.6 million tons of goods valued at 27 billion USD21.
Expansion of the port to accommodate larger Neopanamax ships was conducted from 2012 to 2015, and included large-scale dredging extending out through the offshore reef tract that contributed to turbidity plumes reaching an extent of 228 km222. Sedimentation associated with these efforts led to the burial and mortality of nearby corals, likely resulting in the loss of over 500,000 individuals23. The now Caribbean-wide stony coral tissue loss disease (SCTLD) was observed to originate in the area in 2014, causing rapid mortality of colonies24. Coral bleaching, owing to region-wide heat stress, also occurred in 2014 and 20158,25. These more-acute disturbances have occurred on top of chronic water quality issues including eutrophication, high phytoplankton biomass, low dissolved oxygen, and acidification5,26.
Despite all these chronicled and presumed deleterious factors, corals persist. Here we document diverse coral communities living on artificial structures in the Port of Miami (Fig. 1). We describe the temporal dynamics and control of important environmental and water-quality parameters, relative to a more-natural offshore reef. These communities demonstrate remarkable resilience and represent an important source of information for understanding, predicting, and managing coral communities in a world increasingly affected by human influence.
Map showing South Florida and the Port of Miami study region including the location of three inshore, urban monitoring sites (Star Island, MacArthur North, MacArthur South), the Coral City Camera site used for fish monitoring, and the more-natural Emerald Reef site. Figure produced using Illustrator (v24.3, Adobe).
Results
Environmental conditions
While mean temperatures were comparable across sites (Table 1), significant differences were detected between sites and seasons (Table S1). Higher variability was observed at urban sites (Fig. 2), with more extreme low temperatures observed in the dry season and more extreme high temperatures in the wet season. The lowest (18.54 °C) and highest (32.77 °C) temperature were recorded at MacArthur North, more than two degrees colder and one degree warmer than the extremes recorded at Emerald Reef. Significant differences in salinity, photosynthetically active radiation (PAR), and flow were also detected by site and season (Table S1). In general, inshore urban sites had lower salinity than offshore, and lower values were observed in the wet season, loosely corresponding to precipitation and river flow (Table 1, Fig. 2). Light and flow were higher in the shallower, tidally-flushed urban sites than at Emerald (Table 1, Fig. 2). Currents in these sites were largely bi-directional, with MacArthur North experiencing the most uniform and MacArthur South experiencing the highest velocities (Fig. 3). Temperature and salinity fluctuations were observed to correspond to tide rather than time at inshore sites (Figs. S1–S3). While oxygen concentrations were not measured at Emerald, mean percent saturation was in the mid-90’s at the urban sites (Table 1), with high variability observed in the summer of 2020 (Fig. 2).
Environmental conditions at a natural coral reef (pink) and three inshore urban sites (blue) over the study’s duration. Continuous data are shown as lines, while discrete samples are shown as points (see salinity and pH data). Precipitation (cm), river flow (1000 L s−1) and tidal height (m) are not site-specific and are shown in black. Zeros in river flow may reflect an absence of data, rather than no flow. PAR is photosynthetically active radiation expressed as a daily dose (mol d−1). Wet seasons are overlaid on precipitation data in gray.
There were significant differences in pH between sites and seasons (Fig. 2, Table 1, Table S2). Urban coral sites were generally more acidic than the natural offshore site, especially during the wet season when the pH (spectrophotometric) averaged 7.886 ± 0.087 (mean ± stdev.) at MacArthur North (Fig. 2, Table 1). Urban sites also had higher pH variability, which appeared to be influenced by both light and tidal state (Table 1, Fig. 2, Fig. S1). Total alkalinity (TA) and dissolved inorganic carbon (DIC) were correlated with salinity across all sites and seasons (Fig. S2) and no diel trends were observed (Fig. S3). A property-property plot of salinity-normalized nTA and nDIC revealed a significant linear relationship with a slope of 0.76 (Fig. S4). Extremes in these parameters were observed primarily at the inshore urban sites, with lower values in the wet season and higher in the dry (Fig. S4). While TA and nTA were significantly different between seasons (higher in dry), no difference was detected between sites (Table 1, Table S2). DIC, however, was significantly different across sites and seasons (higher in dry), while nDIC was only significantly different across seasons (Table 1, Table S2). These parameters contributed to significant differences in the calculated partial pressure of CO2 (pCO2) across sites and seasons, with higher values observed at inshore urban sites during the wet season, and the most extreme of which was MacArthur North (dry 536 ± 85, wet 672 ± 194 µatm; Table 1, Table S2). The saturation state of aragonite (Ωarag) was significantly different across sites, but not seasons, with the highest values at Emerald Reef (dry 3.56 ± 018, wet 3.54 ± 0.36) and lowest values at MacArthur North (dry 3.09 ± 0.33, wet 3.09 ± 0.55; Table 1, Table S2).
With respect to nutrients, Si and NO2 were significantly different across sites and seasons, with higher values typically measured at inshore urban sites (Table 2, Table S3). At these sites, however, SI and NO2 generally had mirrored behavior, with the wet season characterized by higher SI and lower NO2 (Table 2, Table S3). The concentration of NO3 was significantly different across sites, but not seasons, with higher values again measured at inshore urban sites (Table 2, Table S3). As with the seasonal trends of the other measured nutrients, NH4 was higher during the wet season; however, there was no difference among sites in NH4 (Table 2, Table S3). PO4 was similar across all sites and seasons. N:P ratios ranged from 4.8 to 139.0 and were generally lower in the dry season (Table 2). Wet season values all suggested P limitation and means were greater than 120, with the exception of MacArthur North, which was 36.4.
Benthic community
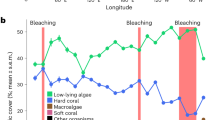
Urban sites hosted relatively diverse coral communities with significantly higher scleractinian coral cover than Emerald Reef. Urban coral communities were largely restricted to artificial hard substrates, which contributed to their linear nature (Fig. 4). Benthic communities were distinct at each of the four sites surveyed (PERMANOVA: F3,391 = 28.9, R2 = 0.183, p < 0.001), with all pairwise site comparisons significant (Fig. 5, Table S4). The relative cover of sediments was the most influential benthic class between all pairwise comparisons of site (percent variation ranging from 18.2 to 23.3%), with turf algae the next most influential class. When analyzing scleractinian taxa only, there was once again a significant difference in community composition among sites (F3,130 = 18.5, R2 = 0.304, p < 0.001), with all pairwise site comparisons significant except for Emerald Reef versus MacArthur North (Fig. 5, Table S4). SIMPER analysis indicated that few scleractinian taxa contributed to variation between sites, with Siderastrea siderea, Porites astreoides, Colpophyllia natans, and Pseudodiploria strigosa being the most differentially abundant taxa.
Benthic community composition and Shannon diversity metrics across offshore and urban sites (left column), with subset datasets for scleractinian taxa only (right column). Percent cover statistics represent results of PERMANOVAs, and diversity statistics represent ANOVA results. Groups which share a letter are not significantly different.
Overall benthic diversity was significantly higher at the natural reef site Emerald Reef compared to the three inshore urban sites (ANOVA: F3,391 = 17.8, p < 0.001), and analysis of scleractinian diversity only demonstrated significant variation across sites as well (F3,391 = 5.6, p < 0.001; Fig. 5, Table S4). Significant pairwise differences were driven by differences between MacArthur South and Emerald Reef as well as between MacArthur South and Star Island, and were likely the result of sporadic coral abundance among photos within sites. Urban coral assemblages were dominated by relatively stress-tolerant species such as S. siderea, S. radians, and P. astreoides; additionally, SCTLD-susceptible species, including C. natans, Pseudodiploria strigosa, and P. clivosa, were mainly found at the urban sites (Fig. 5). Thirteen scleractinian were observed across all urban sites, compared to eight species at Emerald Reef; additionally, coral colonies were in higher abundance at the urban sites except for Agaricia agaricites, which was only observed at Emerald Reef. Colony size-frequency distributions were skewed towards smaller size-classes, especially at urban sites, with the exception of C. natans which was sparsely distributed but reached sizes in excess of 2000 cm2 (Fig. 6). Massive and encrusting morphologies were dominant at the inshore sites, though abundant Porites porites (branching) was recorded at MacArthur South. Unlike scleractinians, gorgonians were observed to be more abundant at Emerald Reef versus inshore sites.
Fish composition
A total of 139 species of fish were identified, belonging to 48 families (Table S5). Many of these species are known associates of coral reefs in the area. While 28 of these species were observed on a near-daily basis, 31 were observed five or fewer times including the critically-endangered smalltooth sawfish (Pristis pectinata).
Discussion
The coral cover at the urban sites (2.50–5.90%) was higher than at the natural coral reef control site (0.04% ± 0.30) and higher than the mean cover of Southeast Florida coral reefs in 2020 (0.8% ± 0.21)3 regardless of the multitude anthropogenic stressors. Reefs in the region stopped accreting between 3700 and 8000 years ago27 and are, therefore, more accurately characterized as coral communities today. While Montastraea carvernosa were dominant in the region in the early 2000’s28, today S. siderea and S. radians, as well as P. astreoides and P. porites, are among the most common species, similar to the urban sites recorded herein3. These species, with the exception of S. siderea, have been previously documented within Biscayne Bay29. Despite significantly higher coral cover and species diversity at the urban sites relative to the natural reef site, coral diversity was still near-zero for all sites examined. This highlights the sporadic nature of coral benthic cover on the artificial substrates in the Port of Miami, and conversely emphasizes the relative low abundance of reef-building species at the reef site.
Notably absent or rare at our reef control site and at many offshore reefs in the region3 are massive species that are especially susceptible to SCTLD (e.g. C. natans, P. strigosa, P. clivosa)30, which is believed to have originated in the region during the time of Port of Miami dredging in 201424,31. By contrast, we recorded numerous colonies of C. natans and Pseudodiploria spp. living on the artificial substrates of the port, including the largest individuals observed in this study. While their persistence relative to offshore populations is notable, massive and encrusting morphologies are known to dominate in turbid, human-impacted reefs elsewhere14,32,33.
Coral cover at urban sites was comparable to that of the Florida Keys (6.1% ± 0.87, 2018)3 and is likely too low to lead to the development of frameworks through active reef accretion34. While species may continue to thrive in these habitats, it is therefore likely that they will not provide the same level of anthropocentric ecosystem goods and services as natural reef systems14,35. We observed, however, numerous species of fishes, including many known to be reef-associates, within the port, suggesting that these urban coral communities may be providing some of the same ecological services as natural systems.
Unfavorable environmental conditions
The occurrence and persistence of coral communities in these urban environments is especially notable given the marginal environmental conditions. While temperatures between the sites were significantly different (Table S1), means were similar across sites within seasons (~ 25 °C, Dry; ~ 29 °C, Wet). MacArthur North and Star Island, however, experienced particularly extreme cold temperatures (~ 18 °C) in the dry season, and all maximum temperatures experienced during the wet season at all urban sites exceeded those at Emerald by up to 1 °C. Extreme temperatures at these sites were correlated with low tide (Fig. S1), when shallow depths and reduced water movement would have resulted in a greater influence from local weather conditions. Higher seasonal temperature variability has been documented in nearby Biscayne Bay and is also likely influenced by reduced water exchange29. A similar pattern of temperature extremes in navigational inlets versus offshore reef sites has been documented in South Florida, though the trend was not significant, likely due to low sample sizes5. Interestingly, MacArthur South, which is located within the Port of Miami navigational inlet, experienced thermal conditions more similar to offshore sites, likely due to greater water exchange versus MacArthur North and Star Island, which are separated from the open ocean via the MacArthur Causeway.
Both warm36 and cold-water37 events are well-known to cause bleaching and coral mortality. Global warming has resulted in a significant increase in extreme warm events since the 1970’s when in situ records were initiated on coral reefs in the Florida Keys, and if trends persist, annual bleaching may occur in the region before 203425. If biological and environmental resilience mechanisms are not sufficient to keep pace with this level of warming, these coral communities may succumb to warming, bleach, and eventually die.
Urban coral communities were exposed to more acidic conditions than natural reef environments in the region, with mean conditions that are not expected to occur globally until decades in the future. These data are supported by Enochs et al.5, who documented acidification in water emanating from navigational inlets in South Florida, including the Port of Miami. Our data also indicate a negative correlation of TA and DIC with salinity, and a nearly identical TA/DIC slope. This study extends further inshore and, unlike the prior study, used high-frequency instrumentation to document that this acidification is highly variable in nature, and in addition to diel fluctuation, is influenced by tidal state (Fig. S1). These data, therefore, support the hypothesis that the acidification may be driven by organic matter enrichment and nutrients from interconnected freshwater canals and rivers5.
Regardless of the particular driver(s) of this coastal acidification, low-pH conditions are known to impede calcification and accelerate bioerosion while encouraging macroalgae proliferation, thereby limiting framework development at urban sites7,38. The high variability measured at these sites, however, may provide a respite from acidification stress and result in physiological responses different than under sustained low pH39,40.
In addition to coastal acidification, the urban coral environments had higher concentrations of Si, NO2, and NO3. Poor water quality may also affect community composition through elevated nutrients, organic matter, pathogens, contaminants, turbidity, and/or sedimentation41. The particular agent(s) involved may be difficult or impossible to separate and identify41. High N:P ratios across all sites, particularly in the wet season, may indicate phosphate limitation, which has been linked to reduced photosynthetic efficiency, paling, low symbiont density, as well as reduced growth and greater density skeletons in corals42.
Previous work in the region has documented that five canals, Munisport Landfill, and the surrounding urban environment contribute to high turbidity, phytoplankton, and nutrients26,43. These waters transit through the Port of Miami, where they contribute to poorer water quality outside of the navigational inlet5. Sedimentation and subsequent burial of corals, along with salinity fluctuations, have been shown to limit coral community development in Biscayne Bay, and it is likely that similar processes influence the urban coral environments described herein29.
Similarly poor water quality conditions appear to be a common characteristic of urban coral environments, though their impact can vary14. Seminal studies in Hawaii have shown how improperly treated sewage effluent can lead to the degradation of nearby coral reefs44. Water quality parameters influenced by nearby human activity were found to explain variation in coral condition in Singapore45 and benthic composition in Borneo33. In Colombia, coral reef communities have been discovered at the mouth of Cartagena Bay, with high industrial and sewage pollution, as well as sedimentation46, though poor water quality has contributed to mortality and physiological impairment47.
Environmental mechanisms of persistence
The proliferation of anthropogenic coastlines, termed ocean sprawl, has transformed the ecology of coastal marine ecosystems. These artificial structures are fundamentally different in both their structure and orientation, often manifesting as homogeneous vertical hardscapes versus gradually sloping shorelines with heterogeneous microhabitats and soft sediments48. This is especially true in South Florida where natural rocky intertidal areas are relatively uncommon. While the vertical nature can reduce the intertidal zone, it may confer benefits to sessile subtidal invertebrates such as corals. For example, benthic stability is critical for slow-growing, long-lived organisms. Disturbance of unconsolidated substrates from wave action and storms can lead to burial, mortality, and the availability of uncolonized substrate, promoting faster growing non-calcareous species. Vertical surfaces are less likely to accumulate sediments, and organisms growing on their surfaces may in turn be conferred the same benefit, resulting in less burial and a reduced metabolic cost of clearing surfaces. Finally, vertical and overlying structures may help to shade corals, reducing the potential for bleaching under elevated temperature49.
Increased turbidity, driving reduced water clarity, may serve the same shading function and provide indirect refugia from bleaching or reduce algae proliferation32. Areas such as northwest Borneo that have high turbidity due to land-based sources of pollution and development, have had lower bleaching and high recovery following thermal stress33. It is, however, unlikely that this is the principal driver of community persistence in the Port of Miami considering that urban habitats are shallower and have higher light (Table 1), coupled with greater temperature extremes.
Biological mechanisms of persistence
Biological mechanisms may be contributing to coral persistence in the Port of Miami, despite temperature extremes, acidification, salinity fluctuations, turbidity and sedimentation, as well as eutrophication, and the potential for pathogens and contaminants.
Supplemental nutrition through heterotrophy contributes to colony health50 and has been identified as a means of ameliorating acidification51 and thermal stress52. High plankton biomass in urban environments may, therefore, contribute to the growth and persistence of corals in these settings, as observed through isotopic source tracking of autotrophic vs. heterotrophic energy sources53. Indeed, a prior study on the gene expression of P. strigosa in the Port of Miami found upregulation of genes that allow them to detect, recognize, and digest food54, and continued examination of trophic strategies in Miami’s urban coral populations is warranted. In addition to corals, however, high food availability may stimulate populations of suspension-feeding organisms that are less-desirable for reef development, such as the bioeroding clionaid sponges that were found to be present at urban sites herein (Fig. 5, Table 3)11.
Urban coral environments are characterized by especially high environmental variability, which may be instrumental in the resilience of reef organisms under climate change55. Temperature fluctuations were observed to be correlated with the tidal cycle (Fig. S1) and similarly influenced back reef environments in American Samoa have been shown to be home to more thermally-tolerant corals13. The benefit of this temperature variability is likely due, in part, to mechanisms of acclimatization that are not directly related to symbiont shuffling, as has since been demonstrated experimentally both in the field56 and laboratory57. For example, transcriptomic examination of Port of Miami corals found higher expression of immune-related pathways relative to natural reef corals, suggestive of immune/stress front-loading or priming54. Seawater carbonate chemistry in the Port of Miami was also observed to be highly variable across seasonal and diel cycles, with periodic exposure to pH conditions more extreme than those projected to occur in open ocean waters at the end of the century due to global OA (Table 1)58. Under contemporary conditions, these fluctuations may lead to enhanced calcification and coral growth, contributing to their persistence39, as has been postulated at other acidified reefs with high variability59.
The resilience of urban coral communities may be due in part to the composition and physiology of their symbiotic associates. For instance, Rubin et al.54 found that P. strigosa in the Port of Miami primarily hosted more heat-tolerant zooxanthellae in the genus Durusdinium vs. those at our natural control site, Emerald Reef, which primarily hosted Breviolum. Similar associations with stress-tolerant symbionts have been observed in urbanized habitats in Singapore60,61, as well as in environmentally-variable lagoon environments62. Prokaryotic communities also tend to reflect the environment in which the coral resides, where corals in marginal habitats can host divergent mucus-associated bacteria63. While some host-symbiont associations in urbanized environments may ultimately be beneficial to the coral host, shifts in prokaryotic communities due to urbanization (i.e. loss of microbial diversity compared to natural reef corals) may instead be detrimental64. Likewise, the previous study also observed disruptions to seasonal and gametogenic cycles in the coral host, which may have implications for the ability of urban corals to contribute larvae to persistence of local populations.
Beyond specialized host/symbiont interactions in urbanized environments, it is generally less well known how host genotype identity may contribute to coral resilience due to difficulties in identifying molecular bioindicators of resilience65. Further, genotype x environment effects are often confounded in wild populations without the use of manipulative experiments66. In at least one case reported in the Red Sea, however, consistent exposure to temperature extremes was hypothesized to have resulted in the selection of heat-resilient coral genotypes through time67. In the Port of Miami, where SCTLD was observed to decimate nearby offshore populations starting in 2014 to the present24,68, urban coral populations have been largely spared from disease-related mortality. Whether this observed phenomenon is due to proximity within the Port, disease-resistant urban genotypes, or algal symbiont/microbial communities remains to be tested. It is likely that there exists a hierarchy of resilience vs. susceptibility to stress within and among coral species16, in part due to host genotype, algal symbiont and microbial associations, and physiological and molecular mechanisms described above. This, in turn, requires a holistic understanding of coral resilience and accurate methods to evaluate genotype performance through the use of high-resolution genotyping and symbiont typing approaches, examination of physiological tolerance and disease resistance, and evaluation of phenotypic plasticity vs. adaptation through manipulative experimentation.
Broader context and future research needs
Coral mortality and declines in reef ecosystem health have become commonplace the world over largely due to anthropogenic climate change and unsustainable development. The existence of urban coral communities in the Port of Miami, an apparently adverse environment engineered for industry, is therefore remarkable. These corals may be uniquely suited to exist in these conditions due to their genetics, or they may be capitalizing on more ubiquitous mechanisms of resilience. They may, therefore, be at the brink of peril on the edge of their environmental tolerance or provide a glimpse into persistence and coexistence. Regardless, they provide insights that could be applicable to ever-increasingly urbanized marine environments, the so-called ocean sprawl48.
While the unique ecology and behavior of animals living in terrestrial urban environments has been well studied, along with their management and conservation69, these topics are poorly understood in the marine realm. Further, city planning and urban development are often conducted with principles of conservation that provide services to wildlife and human populations alike70. Urban marine environments can likely be engineered with an ecological perspective that leverages coexistence towards structural persistence, as well as ecosystem services71. For example, where urban habitats may host sexually mature coral populations, they may constitute refugia through self-seeding and potentially the export of larvae to nearby reef environments.
In order for urban areas in the Port of Miami to serve as refugia, hydrodynamic and genetic connectivity must be established with more natural reef environments72. While offshore reefs have the capacity to seed urban substrates, as evidenced by coral recruitment on artificial substrates, the ability for these populations to serve as a source has yet to be established. Complex hydrodynamic patterns, such as those driven by tidal cycles within inshore and port environments, may result in low gene flow and the development of cryptic urban lineages. In Singapore, for example, urban populations were found to be genetically distinct from natural reef populations, with a majority of individuals representing nonmigrants73. Urban populations may conversely be largely self-seeding with little gene flow to offshore reefs, but this hypothesis requires examination with population genetics approaches. Further, it is not yet known whether coral genotypes currently present within the Port are more resilient to future environmental conditions than their offshore counterparts, and if they are, whether it is persistent due to inherent genetic advantages or alternatively fleeting due to unique environmental circumstances. Increasing connectivity could help to confer advantageous traits to populations in natural reef environments, though potentially deleterious factors such as the spread of disease should be considered.
Finally, while the resilience and prevalence of coral communities in the Port of Miami are remarkable, their long-term persistence, and therefore capacity to serve as a future source of genetic material is, at this time, questionable. By their very nature and proximity, they are more subject to urban development. Maintenance or enhancement of the same infrastructure that originally provided habitat has the potential to destroy it. This was particularly evident in June 2022 when the seawall at the Star Island site was observed to have toppled, resulting in complete mortality of the associated communities including the ESA threatened species Orbicella faveolata and disease-susceptible brain coral species, and partial mortality of colonies in adjacent communities due to siltation and burial. Regardless, these coral communities serve as a reminder of the potential for coexistence on an otherwise depressing tableau of global change, widespread reef degradation, and mass extinction. Going forward, the close collaboration of permitting and governing agencies, environmental stewards, and developers is critical for ensuring that the current and future environmental value of these ecosystems is responsibly balanced with the economic value of the infrastructure on which they have developed.
Methods
Three study sites were located along engineered seawalls and riprap (~ 0.5–2 m depth) within the highly urbanized Port of Miami (MacArthur South, MacArthur North, Star Island, Fig. 1). A fourth site, indicative of a more-natural coral reef, was located roughly 12 km away at Emerald Reef (~ 7 m depth, Fig. 1). Monitoring was conducted from December 2018 to July 2020 in order to characterize the unique environmental conditions and biological communities present at each site. Observations spanned both wet and dry seasons, occurring approximately late May through early October and mid-October through mid-May, respectively, though not all datasets were continuous throughout the duration due to various interruptions.
Environmental conditions
Temperature was measured at each site every 5 min using high-accuracy thermistors (SBE56, Seabird Scientific). Salinity was inferred from conductivity and temperature loggers (SBE 16plus V2 SeaCAT, Seabird Scientific), as well as from bottle samples analyzed with a densitometer (DMA 5000 M, Anton Paar). PAR was measured every 30 min at each site using ECO-PAR™ loggers (Seabird Scientific), which includes a sensor-cleaning mechanism to reduce measurement drift associated with fouling during long-term deployments. Instantaneous PAR readings, which are time-dependent, were summed between the hours of 10 am and 3 pm, to calculate a daily dose. Dissolved oxygen was measured during select deployments of the SBE 16Plus meters, using attached dissolved oxygen optodes (SBE 63, Seabird Scientific). Current speed and direction were recorded every 15 min using tilt current meters (Lowell Instruments).
Carbonate chemistry was characterized at each site using discrete water samples and SeaFET™ pH loggers (Seabird Scientific). Discrete water samples were collected at depth by divers directly into borosilicate glass bottles which were immediately fixed at the surface using 200 µL of HgCl2 and sealed using Apiezon grease and ground glass stoppers. Discrete seawater samples were also collected in between field excursions using Subsurface Automated Samplers74 programmed to collect water 2 h after the first high tide of the day. Samples were immediately fixed using 200 µL of HgCl2 and kept in CO2-impermeable sampling bags until retrieval (< 1 mo), when they were transferred to, and sealed in borosilicate glass bottles as above. Total-scale pH was measured spectrophotometrically. TA was measured using automated Gran titration (AS-ALK2, Apollo SciTech) and DIC was determined by stripping and measuring the CO2 gas in the water sample (AS-C3, Apollo SciTech). TA and DIC values were measured twice and corrected using certified reference materials following Dickson et al.75. ΩArag, pCO2, and pH (when not measured directly) were calculated using Seacarb76 with the constants recommended in Dickson et al.75. TA and DIC were regressed against salinity and then salinity-standardized using a non-zero endmember following Friis et al.77.
SeaFET sensor performance was evaluated and data were only used during periods of continuous normal operation. Sensor readings were corrected using offsets calculated from the mean of discrete water samples collected at each site during the same deployment (n = 2–9). Offset calculations included water samples that were not used for the comparison of carbonate chemistry between sites.
Samples for nutrient analysis were collected by hand at the same time as discrete carbonate chemistry samples. Samples were first filtered using a nylon syringe filter (0.45 µm) and 50 mL was transferred to each of two seawater-rinsed 60 mL plastic vials. Both samples were kept on ice in the field. The vial reserved for analysis of ammonium was fixed with chloroform (~ 0.2 mL) and was transferred to a refrigerator within hours of collection. The vial reserved for analysis of other nutrients was frozen within hours of collection.
Dissolved nutrients were measured using a five-channel automated continuous flow analytical system with segmented flow and colorimetric detection (SEAL Analytical). Analysis was carried out for nitrate (NO3−), nitrite (NO2−), phosphate (PO4−3), orthosilicic acid (H4SiO4), and total ammonia (NH3 and NH4+). Nitrite was determined by diazotizing the sample with sulfanilamide and coupling with N-1 naphthyl ethylenediamine dihydrochloride to form an azo dye. Samples for nitrate analysis were passed through a cadmium column, which reduced nitrate to nitrite, and the resulting nitrite concentration (i.e. nitrate + nitrite, N + N) was then determined as described above. Nitrate concentrations were determined from the difference between N + N and nitrite78. Phosphate was determined by reacting the sample with molybdic acid to form phosphomolybdic acid. This complex was subsequently reduced with hydrazine, and the absorbance of the resulting phosphomolybdous acid was measured79. Silicic acid was analyzed by adding an acidic solution of ammonium molybdate to seawater to produce silicomolybdic acid80. Oxalic acid was added to inhibit a secondary reaction with phosphate and ascorbic acid was added to form silicomolybdous acid, which was measured colorimetrically. Ammonia in solution reacts with alkaline phenol and NaDTT at 37 °C to form indophenol blue in the presence of sodium nitroferricyanide as a catalyst81. The absorbance of indophenol blue at 660 nm was measured to determine the concentration of ammonia. N:P ratios were calculated as a single value averaged per site and season, owing to the different number of samples analyzed for ammonia.
Daily precipitation and river flow were obtained from the South Florida Water Management District’s DBHYDRO database and data from Station S-26, located on the Miami Canal (25.807628N, 80.260841W). Tidal height was obtained from the tidal station on Virginia Key (#8723214, NOAA/NOS/CO-OPS).
Benthic community
Photomosaics were created at each site to capture and describe the benthic composition. Images were collected by divers swimming in a back and forth “lawnmower pattern” using two cameras (Hero 6 Black, GoPro) affixed to an aluminum bar (~ 60 cm apart) that were programmed to collect images every second. Field collection was conducted at MacArthur North in November 2018, Emerald Reef and MacArthur South in July 2019, and at Star Island in November 2019. Images were assembled into mosaics using Photoscan v1.3.4 (Agisoft). To determine the composition of benthic communities and substrate cover at each site, 98 0.25 m2 images were randomly sampled from each mosaic using ArcMap (VXX, ESRI). Fifty random points were overlaid on each image and the underlying taxon was manually identified using CoralNet82. To determine the species richness and size frequency of hard corals at each site, all mosaics were first cropped to a consistent 60 m2. Scleractinian corals were identified to species and their planar surface area was measured using spatial analysis tools in ArcMap.
Fish composition
The community composition of fishes present in the Port of Miami was determined using photograph and video clips obtained from a live underwater camera (Octopus, View into the Blue) located off the Pilot House within the port (Fig. 1), and served at coralcitycamera.com. Fish monitoring was conducted from April 2020 to January 2021. These data are not directly tied to the benthic and environmental monitoring sites and simply describe the diverse communities of fishes present with the port. Fish abundances were categorized as abundant, seen on an hourly basis; common, seen on a daily basis; uncommon, seen on a weekly basis; rare, seen monthly or less; or seasonally common, observed as common on a seasonal basis.
Data analysis
All data manipulation, calculations, and statistics were performed in R Studio83. Environmental and water quality data were analyzed using linear models with site and season as factors. Data were log transformed prior to analysis, with the exception of pH data which are already on a log scale. Time series of environmental data were produced using the ggplot2 package84, and the frequencies of current vectors was plotted using the openair package85. Multivariate differences in benthic community composition across all benthic classes and with a filtered dataset of just scleractinian taxa were tested across sites using PERMANOVAs on square-root-transformed data in the vegan package86. SIMPERs were conducted in the same package to determine the benthic classes/taxa driving significant differences between sites, with a 70% cutoff threshold. Shannon diversity was calculated for datasets of all biota and for scleractinians only using relative abundance of benthic taxa (excluding abiotic classes), and compared among sites using one-way ANOVAs. Finally, size-frequency data were examined for the six most abundant scleractinian species across sites.
Data availability
Data produced as part of this study are publicly available at the National Centers for Environmental Information (NCEI, Accession 0276830) at https://www.ncei.noaa.gov/access/metadata/landing-page/bin/iso?id=gov.noaa.nodc:0276830.
References
Hoegh-Guldberg, O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. https://doi.org/10.1126/science.1152509 (2007).
Gardner, T. A., Cöté, I. M., Gill, J. A., Grant, A. & Watkinson, A. R. Long-term region-wide declines in Caribbean corals. Science 301, 958–960. https://doi.org/10.1126/science.1086050 (2003).
Grove, L. J. W. et al. National Coral Reef Monitoring Program, Biological monitoring summary—Florida: 2020–2021. NOAA Tech Memo 44, 47 (2022).
Manzello, D. P., Enochs, I. C., Melo, N., Gledhill, D. K. & Johns, E. M. Ocean acidification refugia of the Florida Reef Tract. PLoS ONE 7, 1–10. https://doi.org/10.1371/journal.pone.0041715.g001 (2012).
Enochs, I. C., Manzello, D. P., Jones, P. R., Stamates, S. J. & Carsey, T. P. Seasonal carbonate chemistry dynamics on Southeast Florida coral reefs: Localized acidification hotspots from navigational inlets. Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00160 (2019).
Prada, F. et al. Coral micro- and macro-morphological skeletal properties in response to life-long acclimatization at CO2 vents in Papua New Guinea. Sci. Rep. 11, 19927. https://doi.org/10.1038/s41598-021-98976-9 (2021).
Enochs, I. et al. Shift from coral to macroalgae dominance on a volcanically acidified reef. Nat. Clim. Chang. 5, 1083–1089. https://doi.org/10.1038/nclimate2758 (2015).
Manzello, D. P. et al. Role of host genetics and heat-tolerant algal symbionts in sustaining populations of the endangered coral Orbicella faveolata in the Florida Keys with ocean warming. Glob. Chang. Biol. 25, 1016–1031. https://doi.org/10.1111/gcb.14545 (2019).
Rosales, S. M., Sinigalliano, C., Gidley, M., Jones, P. R. & Gramer, L. J. Oceanographic habitat and the coral microbiomes of urban-impacted reefs. PeerJ 7, e7552. https://doi.org/10.7717/peerj.7552 (2019).
Fabricius, K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 1, 165–169. https://doi.org/10.1038/nclimate1122 (2011).
Enochs, I. C. et al. Upwelling and the persistence of coral-reef frameworks in the eastern tropical Pacific. Ecol. Mon. 91, e01482. https://doi.org/10.1002/ecm.1482 (2021).
Camp, E. F. et al. Mangrove lagoons of the Great Barrier Reef support coral populations persisting under extreme environmental conditions. Mar. Ecol. Prog. Ser. 625, 1–14. https://doi.org/10.3354/meps13073 (2019).
Oliver, T. A. & Palumbi, S. R. Do fluctuating temperature environments elevate coral thermal tolerance?. Coral Reefs 30, 429–440. https://doi.org/10.1007/s00338-011-0721-y (2011).
Heery, E. C. et al. Urban coral reefs: Degradation and resilience of hard coral assemblages in coastal cities of East and Southeast Asia. Mar. Pollut. Bull. 135, 654–681. https://doi.org/10.1016/j.marpolbul.2018.07.041 (2018).
Martínez-Castillo, V., Rodríguez-Troncoso, A. P., Santiago-Valentín, J. D. & Cupul-Magaña, A. L. The influence of urban pressures on coral physiology on marginal coral reefs of the Mexican Pacific. Coral Reefs 39, 625–637. https://doi.org/10.1007/s00338-020-01957-z (2020).
Ng, C. S. L. et al. Responses of urban reef corals during the 2016 mass bleaching event. Mar. Pollut. Bull. 154, 111111. https://doi.org/10.1016/j.marpolbul.2020.111111 (2020).
Zhao, Y. et al. Urban coral communities and water quality parameters along the coasts of Guangdong Province, China. Mar. Pollut. Bull. 180, 113821 (2022).
Lirman, D. & Fong, P. Is proximity to land-based sources of coral stressors an appropriate measure of risk to coral reefs? An example from the Florida Reef Tract. Mar. Pollut. Bull. 54, 779–791. https://doi.org/10.1016/j.marpolbul.2006.12.014 (2007).
Lapointe, B. E., Herren, L. W. & Bedford, B. J. Effects of hurricanes, land use, and water management on nutrient and microbial pollution: St. Lucie Estuary, Southeast Florida. J. Coast. Res. 285, 1345–1361. https://doi.org/10.2112/jcoastres-d-12-00070.1 (2012).
Gregg, K. L. Literature review and synthesis of land-based sources of pollution affecting essential fish habitats in southeast Florida. NOAA Fisheries Southeast Regional Office, West Palm Beach FL, 1–55 (2013).
POM. Port Guide 2018–2019. (2019).
Barnes, B. B., Hu, C., Kovach, C. & Silverstein, R. N. Sediment plumes induced by the Port of Miami dredging: Analysis and interpretation using Landsat and MODIS data. Rem. Sens. Environ. 170, 328–339. https://doi.org/10.1016/j.rse.2015.09.023 (2015).
Cunning, R., Silverstein, R. N., Barnes, B. B. & Baker, A. C. Extensive coral mortality and critical habitat loss following dredging and their association with remotely-sensed sediment plumes. Mar. Pollut. Bull. 145, 185–199. https://doi.org/10.1016/j.marpolbul.2019.05.027 (2019).
Gintert, B. E. et al. Regional coral disease outbreak overwhelms impacts from a local dredge project. Environ. Monit. Assess 191, 630. https://doi.org/10.1007/s10661-019-7767-7 (2019).
Manzello, D. P. Rapid recent warming of coral reefs in the Florida Keys. Sci. Rep. 5, 16762. https://doi.org/10.1038/srep16762 (2015).
Caccia, V. G. & Boyer, J. N. Spatial patterning of water quality in Biscayne Bay, Florida as a function of land use and water management. Mar. Pollut. Bull. 50, 1416–1429. https://doi.org/10.1016/j.marpolbul.2005.08.002 (2005).
Banks, K. W., Riegl, B. M., Shinn, E. A., Piller, W. E. & Dodge, R. E. Geomorphology of the Southeast Florida continental reef tract (Miami-Dade, Broward, and Palm Beach Counties, USA). Coral Reefs 26, 617–633. https://doi.org/10.1007/s00338-007-0231-0 (2007).
Moyer, R. P., Riegl, B., Banks, K. & Dodge, R. E. Spatial patterns and ecology of benthic communities on a high-latitude South Florida (Broward County, USA) reef system. Coral Reefs 22, 447–464. https://doi.org/10.1007/s00338-003-0334-1 (2003).
Lirman, D. et al. Coral communities of Biscayne Bay, Florida and adjacent offshore areas: Diversity, abundance, distribution, and environmental correlates. Aquat. Cons. Mar. Fresh Ecosys. 13, 121–135. https://doi.org/10.1002/aqc.552 (2003).
Aeby, G. S. et al. Pathogenesis of a tissue loss disease affecting multiple species of corals along the Florida Reef Tract. Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00678 (2019).
Precht, W. F., Gintert, B. E., Robbart, M. L., Fura, R. & van Woesik, R. Unprecedented disease-related coral mortality in Southeastern Florida. Sci. Rep. 6, 31374. https://doi.org/10.1038/srep31374 (2016).
Guest, J. R. et al. 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Sci. Rep. 6, 36260. https://doi.org/10.1038/srep36260 (2016).
Browne, N., Braoun, C., McIlwain, J., Nagarajan, R. & Zinke, J. Borneo coral reefs subject to high sediment loads show evidence of resilience to various environmental stressors. PeerJ 7, e7382. https://doi.org/10.7717/peerj.7382 (2019).
Perry, C. T. et al. Caribbean-wide decline in carbonate production threatens coral reef growth. Nat. Commun. 4, 1402. https://doi.org/10.1038/ncomms2409 (2013).
Moberg, F. & Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 29, 215–233 (1999).
Baker, A. C., Glynn, P. W. & Riegl, B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast Shelf Sci. 80, 435–471. https://doi.org/10.1016/j.ecss.2008.09.003 (2008).
Lirman, D. et al. Severe 2010 cold-water event caused unprecedented mortality to corals of the Florida reef tract and reversed previous survivorship patterns. PLoS ONE 6, e23047. https://doi.org/10.1371/journal.pone.0023047 (2011).
Enochs, I. C. et al. Enhanced macroboring and depressed calcification drive net dissolution at high-CO2 coral reefs. Proc. R. Soc. B 283, 20161742. https://doi.org/10.1098/rspb.2016.1742 (2016).
Enochs, I. C. et al. The influence of diel carbonate chemistry fluctuations on the calcification rate of Acropora cervicornis under present day and future acidification conditions. J. Exp. Mar. Biol. Ecol. 506, 135–143. https://doi.org/10.1016/j.jembe.2018.06.007 (2018).
Morris, J. et al. The influences of diurnal variability and ocean acidification on the bioerosion rates of two reef-dwelling Caribbean sponges. Glob. Chang. Biol. 28, 7126–7138. https://doi.org/10.1111/gcb.16442 (2022).
Fabricius, K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 50, 125–146. https://doi.org/10.1016/j.marpolbul.2004.11.028 (2005).
Buckingham, M. C. et al. Impact of nitrogen (N) and phosphorus (P) enrichment and skewed N: P stoichiometry on the skeletal formation and microstructure of symbiotic reef corals. Coral Reefs 41, 1147–1159. https://doi.org/10.1007/s00338-022-02223-0 (2022).
Caccia, V. G. & Boyer, J. N. A nutrient loading budget for Biscayne Bay, Florida. Mar. Pollut. Bull. 54, 994–1008. https://doi.org/10.1016/j.marpolbul.2007.02.009 (2007).
Grigg, R. W. Coral reefs in an urban embayment in Hawaii: A complex case history caused by natural and anthropogenic stress. Coral Reefs 14, 253–266 (1995).
Browne, N. K., Tay, J. K., Low, J., Larson, O. & Todd, P. A. Fluctuations in coral health of four common inshore reef corals in response to seasonal and anthropogenic changes in water quality. Mar. Environ. Res. 105, 39–52. https://doi.org/10.1016/j.marenvres.2015.02.002 (2015).
Pizarro, V. et al. Unraveling the structure and composition of Varadero Reef, an improbable and imperiled coral reef in the Colombian Caribbean. PeerJ 5, e4119. https://doi.org/10.7717/peerj.4119 (2017).
López-Londoño, T. et al. Physiological and ecological consequences of the water optical properties degradation on reef corals. Coral Reefs 40, 1243–1256. https://doi.org/10.1007/s00338-021-02133-7 (2021).
Todd, P. A. et al. Towards an urban marine ecology: Characterizing the drivers, patterns and processes of marine ecosystems in coastal cities. Oikos 128, 1215–1242. https://doi.org/10.1111/oik.05946 (2019).
Tagliafico, A., Baker, P., Kelaher, B., Ellis, S. & Harrison, D. The effects of shade and light on corals in the context of coral bleaching and shading technologies. Front. Mar. Sci. 9, 919382. https://doi.org/10.3389/fmars.2022.919382 (2022).
Houlbrèque, F. & Ferrier-Pagès, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17. https://doi.org/10.1111/j.1469-185X.2008.00058.x (2009).
Towle, E. K., Enochs, I. C. & Langdon, C. Threatened Caribbean coral is able to mitigate the adverse effects of ocean acidification on calcification by increasing feeding rate. PLoS ONE 10, e0123394. https://doi.org/10.1371/journal.pone.0123394 (2015).
Grottoli, A. G., Rodrigues, L. J. & Palardy, J. E. Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189. https://doi.org/10.1038/nature04565 (2006).
Conti-Jerpe, I. E. et al. Trophic strategy and bleaching resistance in reef-building corals. Sci. Adv. 6, eaaz5443. https://doi.org/10.1126/sciadv.aaz5443 (2020).
Rubin, E. T. et al. Molecular mechanisms of coral persistence within highly urbanized locations in the Port of Miami, Florida. Front. Mar. Sci. 8, 695236. https://doi.org/10.3389/fmars.2021.695236 (2021).
Rivest, E. B., Comeau, S. & Cornwall, C. E. The role of natural variability in shaping the response of coral reef organisms to climate change. Curr Clim. Change Rep. 3, 271–281. https://doi.org/10.1007/s40641-017-0082-x (2017).
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N. & Bay, R. A. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898. https://doi.org/10.1126/science.1251336 (2014).
DeMerlis, A. et al. Pre-exposure to a variable temperature treatment improves the response of Acropora cervicornis to acute thermal stress. Coral Reefs 41, 435–445. https://doi.org/10.1007/s00338-022-02232-z (2022).
IPCC. in Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds S. Solomon et al.) (Cambridge University Press, 2007).
Enochs, I. C. et al. Coral persistence despite extreme periodic pH fluctuations at a volcanically acidified Caribbean reef. Coral Reefs 39, 523–528. https://doi.org/10.1007/s00338-020-01927-5 (2020).
Poquita-Du, R. C., Huang, D., Chou, L. M. & Todd, P. A. The contribution of stress-tolerant endosymbiotic dinoflagellate Durusdinium to Pocillopora acuta survival in a highly urbanized reef system. Coral Reefs 39, 745–755. https://doi.org/10.1007/s00338-020-01902-0 (2020).
Jain, S. S. et al. Endosymbiont communities in Pachyseris speciosa highlight geographical and methodological variations. Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.759744 (2021).
Burt, J. A. et al. Insights from extreme coral reefs in a changing world. Coral Reefs 39, 495–507. https://doi.org/10.1007/s00338-020-01966-y (2020).
Deignan, L. K. & McDougald, D. Differential response of the microbiome of Pocillopora acuta to reciprocal transplantation within Singapore. Microb. Ecol. 83, 608–618. https://doi.org/10.1007/s00248-021-01793-w (2022).
Rosenberg, Y. et al. Urbanization comprehensively impairs biological rhythms in coral holobionts. Glob. Chang. Biol. 28, 3349–3364. https://doi.org/10.1111/gcb.16144 (2022).
Parkinson, J. E. et al. Molecular tools for coral reef restoration: Beyond biomarker discovery. Conserv. Lett. https://doi.org/10.1111/conl.12687 (2019).
Todd, P. A. Morphological plasticity in scleractinian corals. Biol. Rev. 83, 315–337. https://doi.org/10.1111/j.1469-185X.2008.00045.x (2008).
Fine, M., Gildor, H. & Genin, A. A coral reef refuge in the Red Sea. Glob. Chang. Biol. 19, 3640–3647. https://doi.org/10.1111/gcb.12356 (2013).
Walton, C. J., Hayes, N. K. & Gilliam, D. S. Impacts of a regional, multi-year, multi-species coral disease outbreak in Southeast Florida. Front. Mar. Sci. https://doi.org/10.3389/fmars.2018.00323 (2018).
Collins, M. K., Magle, S. B. & Gallo, T. Global trends in urban wildlife ecology and conservation. Biol. Conserv. 261, 109236. https://doi.org/10.1016/j.biocon.2021.109236 (2021).
Fabos, J. G. Introduction and overview: The greenway movement, uses and potentials of greenways. Landsc. Urban Plan 33, 1–13 (1995).
Burt, J. A. & Bartholomew, A. Towards more sustainable coastal development in the Arabian Gulf: Opportunities for ecological engineering in an urbanized seascape. Mar. Pollut. Bull. 142, 93–102. https://doi.org/10.1016/j.marpolbul.2019.03.024 (2019).
Studivan, M. S. & Voss, J. D. Population connectivity among shallow and mesophotic Montastraea cavernosa corals in the Gulf of Mexico identifies potential for refugia. Coral Reefs 37, 1183–1196. https://doi.org/10.1007/s00338-018-1733-7 (2018).
Afiq-Rosli, L. et al. Barriers and corridors of gene flow in an urbanized tropical reef system. Evol. Appl. 14, 2502–2515. https://doi.org/10.1111/eva.13276 (2021).
Enochs, I. C. et al. Subsurface automated samplers (SAS) for ocean acidification research. Bull. Mar. Sci 96, 735–752. https://doi.org/10.5343/bms.2020.0018 (2020).
Dickson, A. G., Sabine, C. L. & Christian, J. R. Guide to best practices for ocean CO2 measurements. PICES Spec. Publ. 3, 191 (2007).
Gattuso, J.-P. et al. Seacarb: Seawater Carbonate Chemistry, 2016.
Friis, K., Körtzinger, A. & Wallace, D. W. R. The salinity normalization of marine inorganic carbon chemistry data. Geophys. Res. Lett. 30, 1085. https://doi.org/10.1029/2002gl015898 (2003).
Zhang, J. Z., Ortner, P. B. & Fischer, C. J. Method 353.4 Determination of nitrate and nitrite in estuarine and coastal waters by gas segmented continuous flow colorimetric analysis. National Exposure Research Laboratory Office of Research and Development US Environmental Protection Agency. Cincinatti. (1997).
Zhang, J.-Z., Fischer, C. J. & Ortner, P. B. Comparison of open tubular cadmium reactor and packed cadmium column in automated gas-segmented continuous flow nitrate analysis. Int. J. Environ. Analyt. Chem. 76, 99–113. https://doi.org/10.1080/03067310008034123 (2000).
Zhang, J. & Berberian, G. EPA Method 366.0: Determination of dissolved silicate in estuarine and coastal waters by gas segmented flow colorimetric analysis. US Environmental Protection Agency, Washington, DC. (EPA-600-R-97-072, 1997).
Zhang, J.-Z., Ortner, P., Fischer, C. & Moore, L. Determination of ammonia in estuarine and coastal waters by gas segmented continuous flow colorimetric analysis. EPA Method 349 (1997).
Beijbom, O. et al. Towards automated annotation of benthic survey images: Variability of human experts and operational modes of automation. PLoS ONE 10, e0130312. https://doi.org/10.1371/journal.pone.0130312 (2015).
RStudio: Integrated development for R (RStudio, Inc., Boston, MA, 2015).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016).
Carslaw, D. C. & Ropkins, K. Openair—An R package for air quality data analysis. Environ. Model Softw. 27–28, 52–61. https://doi.org/10.1016/j.envsoft.2011.09.008 (2012).
vegan: Community Ecology Package v. R package version 2.5–6 (2019).
Acknowledgements
Funding for this research was provided by the NOAA OAR’s Omics initiative. Analytical equipment for the determination of carbonate chemistry was purchased with funds from NOAA’s Ocean Acidification Program. Additional field-based instrumentation was purchased with support from NOAA’s Coral Reef Conservation Program. We are grateful to Lisa Gregg at the Florida Fish and Wildlife Conservation Commission, Jocelyn Karazsia at NOAA’s Southeast Regional Office Habitat Conservation Division, and to the Miami-Dade County Department of Regulatory and Economic Resources for advice and support during this project. The Coral City Camera was made possible with financial support from the Bridge Initiative, as well as site access from the Biscayne Bay Pilots and Port Miami.
Author information
Authors and Affiliations
Contributions
I.E., C.F. and D.M. designed the study. I.E., M.S., G.K., C.F., I.B., A.B., N.F., A.K., E.R., M.J., I.S. and C.K. collected and processed the data. I.E. and M.S. analyzed the data. All authors were involved with manuscript development.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Enochs, I.C., Studivan, M.S., Kolodziej, G. et al. Coral persistence despite marginal conditions in the Port of Miami. Sci Rep 13, 6759 (2023). https://doi.org/10.1038/s41598-023-33467-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33467-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.