Abstract
Maize (Zea mays L.) is one of the most widely distributed and important crops in China. Maize ear differentiation plays an important role grain yield formation. However, it is unclear if ear and root morphophysiology status affects yield formation by altering ear differentiation and development under different nitrogen (N) conditions. The aim of this study is to understand how the ear differentiation and development are affected by ear and root morphophysiology traits, as affected by the N rate. The experiment consisted of two N rates: high nitrogen (180 kg ha−1), and low nitrogen (60 kg ha−1). Two N-efficient varieties (NEVs) and two N-inefficient varieties (NIVs) were grown in the field. The results showed higher nitrogen accumulation and grain yield in NEVs than in NIVs, which was mainly attributed to the increased N uptake by the larger root system under both N conditions. Under high N conditions, among ear differentiation-related traits, only FR was significantly positively correlated with grain yield, and NEVs ensure FR through higher N concentration and ZR content in ear at the fertilization stage. Under low N conditions, NEVs obtained higher FP, SR and FR through higher N concentration and IAA in ear at the early stage of ear differentiation, maintained lower AR and BTL by higher RA, R-ZR and E-ZR at the late stage of ear growth. These results suggest that NEVs have a more complex mechanism for obtaining higher grain yield under low N conditions than N sufficiency, and that phytohormones play an important role in this process.
Similar content being viewed by others
Introduction
It is expected that by 2050, the global demand for food will increase by 70%1. Nitrogen (N), a key limiting element for the growth of most crops, plays an important role in maximizing crop yields on a global scale, leading to a significant increase in the consumption of N fertilizers2,3,4. Global N fertilizer consumption has increased nine fold from 11 to 106 tonnes over the past 40 years (1960–2009), while grain yield has only increased by 164%5. During this period, maize production in China stagnated or even declined significantly6,7. Larger nitrogen input and lower nitrogen use efficiency will not only increase production costs and waste resources8,9, but also lead to soil acidification, decreased soil microbial activity, and adversely affect the environment. Sustainability presents challenges10,11. Therefore, future crop production should pay more attention to the efficient use of resources12. Further increases in grain yield must be achieved through increased nitrogen efficiency rather than higher nitrogen inputs13,14.
Developing the most suitable N fertilizer strategy for high-yielding crop systems is a critical step in achieving food security and environmental protection. Efficient use of N fertilizers is an effective strategy for both yield and profit. Studies have shown that crop rotation, no-tillage measures, the use of controlled-release nitrogen fertilizers, the deep application of urea, and the cultivation of NEVs (varieties with higher yields under both N conditions) can be achieved15,16,17,18, to overcome the depletion effects of excessive N application, thereby improving crop yield and agro-ecosystem sustainability. In general, genetic improvement of NEVs is an effective way to increase yield19,20,21,22,23.
Studies have also been conducted around N application levels, grain yield and N use efficiency, but the results vary widely due to differences in varieties, fertilizer levels, and climatic conditions15,24. Due to the continuous renewal of varieties, the production process puts forward higher requirements for nutrient input and efficiency improvement. The results of experiments on multiple corn varieties at multiple sites show that the selection of high-yield and high-efficient varieties can increase yield by 8–10%, while reducing N fertilizer input by 16–21%9. With the improvement of breeding technology, more maize varieties are used in production, but the nitrogen absorption and utilization among varieties show great differences. Nitrogen Use Efficiency (NUE) can be divided into Nitrogen Uptake Efficiency (NUpE) and Nitrogen Use Efficiency (NUtE). The former is mainly the nitrogen absorption capacity of plants, which is expressed as the nitrogen accumulation of plants; the latter is mainly the assimilation and redistribution capacity of plants to nitrogen, which is expressed as biomass or yield production capacity. Therefore, how to select varieties and combine their nitrogen utilization to meet the goals of high yield and resource efficiency is still a major problem to be solved by researchers.
Phytohormones are a class of organic substances produced by plants’ own metabolism, and participate in the regulation of various plant physiology and developmental processes. Some studies have shown that phytohormones play an important regulatory role in the process of seed growth and development25. The higher cytokinin (CTK) concentrations in seeds have been observed to be closely associated with rapid endosperm development during the early stages of seed development in cereals, peas, and soybeans26,27,28. Although CTKs are generally considered to be a class of hormones that regulate endosperm development, there is still insufficient evidence for the correlation between cytokinins and endosperm development. Auxin (IAA), gibberellin (GA) and abscisic acid (ABA) are also thought to be involved in the regulation of grain development29. Eeuwens et al.30 observed that the content of GA in endosperm cells was the highest during the elongation phase of pea pods. Some studies have shown that the content of GA in young panicles is higher at the flowering stage of rice and before flowering31. Lur et al.32 found that the concentration of IAA in endosperm cells increased rapidly on the tenth day after pollination of maize, which was consistent with the increase of deoxyribonucleic acid content in the nucleus. There are many research reports on the relationship between ABA content and seed growth and development, but the claim that it is involved in regulating the transport of assimilates to grains is still controversial33,34,35,36.
In this study, through a 2-year field experiment, four main summer maize varieties in Heilongjiang Province with similar growth periods were selected, and two fertilization levels of high (180 kg ha−1) and low (60 kg ha−1) N were set. To analyze the main reasons for the difference in yield of maize varieties with different N-efficiency under low and high nitrogen conditions. The root morphysiological characteristics of NEVs were systematically analyzed. Root and ear hormones were contained in ear differentiation and development stages, and their relationship with traits related to ear differentiation and development. This study improves our understanding of the roles of phytohormones in the regulation of morphological and physiological basis in N utilization and grain yield formation. This information could be used for research focused on improving N utilization and grain yield of maize.
Materials and methods
Plant materials and site description
Field experiments were conducted at the Qiqihar maize experiment base of heilongjiang academy of agricultural sciences in Heilongjiang Province, China (46° 52′ N, 123° 46′ E) during the rice growing season (May to October) in 2018 and 2019. The test area belongs to the mid-temperate continental monsoon climate, which is dry and windy in spring and warm and rainy in summer. The annual precipitation is 477 mm, and the frost-free period is about 130 days. The soil type was dark brown forest soil. The physicochemical properties of composite topsoil samples (0–20 cm) were determined, and the average values are shown in Table 1. Two N-efficient varieties (NEVs), Nengdan19 (ND19, N8924 × N7923) and Nengdan29 (ND29, N7923 × 1064), and two N-inefficient varieties (NIVs), Nengdan17 (ND17, N788411 × N1503) and Neng19022 (N19022, NH75121 × NY18), were used. The four maize varieties have similar growth periods. A crop rotation system was applied with continuous cropping of maize.
Experimental design
The experiments were laid out in a complete randomized block design with three replicates. The N fertilizer rate was the main plot treatment, and the maize varieties formed the sub-plot treatments. Each plot was 30-m in length and 9.6-m in width with 60 cm row spacing. Maize was planted by hand and the planting density was 65,000 plant ha−1. Nitrogen rates were 180 kg ha−1 (high N conditions) and 60 kg ha−1 (low N conditions). Phosphorus (P2O5) rates were 90 kg ha−1 and potassium (K2O) rates were 120 kg ha−1. N, P, and K fertilizers were used urea, Ca(H2PO4)2 and K2SO4, respectively. All fertilizers were broadcasted before sowing. With the exception of the different N fertilizer rates, the other cultivation requirements were identical for all plots in both years. Chemicals were used to control diseases, pests and weeds to prevent yield loss during the experiment. Other field management is the same as conventional maize cultivation.
Sampling and measurements
Grain yield and its components
Grain yield and its components were measured in 2018 and 2019. At R6 stage, two middle rows of plants (20-m length) per plot were harvested, excluding border plants. Grain yield was standardized to a moisture content of 0.14 g H2O g−1 and its components, i.e. grain number per ear (GNE) and thousand-kernel weight (TKW) were determined.
N accumulation and remobilization
In 2018 and 2019, five representative plants were taken from each plot in the R1 and R6 stages. The plants were divided into leaves, stems, cobs, bracts and grains. All parts were fixed at 105 °C for 30 min, and then dried at 80 °C until constant weight, weighed separately, and recorded the total mass of leaves, stems and plants. After crushing through a 100-mesh sieve, an 80 mg sample was weighed, and the nitrogen concentration of each part was measured using an elemental analyzer (EA1110, Thermo Electon SPA., Italy). The following indexes were calculated to investigate nitrogen accumulation and partitioning:
-
(1)
Straw N content at R1 (kg ha−1) = straw N concentration at R1 × straw dry weight at R1;
-
(2)
Straw N content at R6 (kg ha−1) = straw N concentration at R6 × straw dry weight at R6;
-
(3)
Grain N content at R6 (kg ha−1) = grain N concentration at R6 × grain dry weight at R6;
-
(4)
Nitrogen accumulation before silking (kg ha−1) = Straw N content at R1;
-
(5)
Nitrogen accumulation after silking (kg ha−1) = Straw and grain N content at R6 (kg ha−1) − Nitrogen accumulation before silking (kg ha−1);
-
(6)
Nitrogen remobilization (kg ha−1) = Straw N content at R1 − Straw N content at R6;
-
(7)
Cotibution of nitrogen remobilization (%) = NT/Grain N content at R6 × 100.
Ear traits and ear development
In 2018 and 2019, five representative ears per plot were sampled to determine row number per ear (RNE), grain number per row (GNR), ear length (EL) and barren tip length (BTL) at R6 stage. The RNE was the number of grains in the cross section in the middle of the ear. The GNR was the ratio of the number of grains per ear to the RNE. The EL was the axial distance from bottom to apex of ear. The BTL was the axial distance from the topmost grain to apex of ear.
At the R1 stage in 2018 and 2019, plants that entered the silking stage on the same day were marked. When maize silks withered, five representative ears per plot were sampled for determine the floret primordia (FP), silking number (SN), fertilized floret number (FFN) and aborted grain number (AGN). The FP was the number of all visible protrusions on the maize cob. After gently shaking the ear, the number of shedding filaments and unshedding filaments was the SN, where the number of shedding filaments was the FFN, and the AGN was the FFN minus the number of terminal grains. The following ratios were calculated to evaluate ear development:
-
(1)
Silking ratio (SR) = SN/FP × 100%;
-
(2)
Fertilized ratio (FR) = FFN/SN × 100%;
-
(3)
Aborted ratio (AR) = AGN/FFN × 100%.
Young ear biomass and nitrogen concentration during ear differentiation
In order to clarify the effect of nitrogen fertilizer on the ear differentiation of maize varieties, this study was based on the previous investigation of the silking period of the tested materials, and sampling was carried out around the silking period to determine the ear nitrogen concentration, ear dry weight, root morphysiological characteristics, ear and root phytohormone content. The stages were S1 (R1 − 14d), S2 (R1 − 7d), S3 (R1), S4 (R1 + 7d) and S5 (R1 + 14d). Five representative ears per plot were sampled from S1 to S5 stages. All ears were fixed at 105 °C for 30 min, and then dried at 80 °C until constant weight, weighed separately, and recorded the total mass of ears. After crushing through a 100-mesh sieve, an 80 mg sample was weighed, and the nitrogen concentration of each part was measured using an elemental analyzer (EA1110, Thermo Electon SPA., Italy).
Root morphological and physiological traits during ear differentiation
Root samples were taken from S1 to S5, and the whole plant was excavated for sampling. Five representative plants with no marginal effect were excavated from each plot. The sampling depth was 60 cm, with the plant as the center, and the sampling area was 0.15 m2 (length 0.6 m, width 0.25 m). The excavated roots were washed with clean water and packed into ziplock bags, brought back to the laboratory, and scanned and analyzed with Win RHIZO Pro 2007d (Regent Instruments Inc., Quebec, Canada) to obtain the total root length. The treated roots were dried in an oven at 80 °C to constant weight, and the dry weight of the roots was recorded. The root activity (RA) was measured using the method described by Ramasamy et al.37.
Ear and root phytohormones content during ear differentiation
The ear and root samples were freeze dried with liquid N and stored at − 80 °C refrigerator for phytohormone analysis. The IAA, ABA, GA and ZR were extracted by enzyme-linked immunosorbent assay (ELIAS) with reference to the operation guide of China Agricultural University ELIAS kit38,39. The IAA, ABA, GA and ZR content were determined using a Multiskan™ FC Microplate Photometer (Thermo Fisher Scientific (China) Co., Ltd., Shanghai, China).
Statistical analysis
For experimental variables, one-way of variance (ANOVA) was applied to assess differences among treatments with SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). Significant differences between treatments are indicated by different letters at P < 0.05 level according to Fisher’s LSD. Graphs were drawn with Origin 2018 software (OriginLab, Northampton, MA, USA) R software (Available online: http://www.r-project.org/) and Adobe Illustrator CS6 (Adobe Systems Inc., CA, USA).
Statement
The authors ensure that all maize seeds used in this study originated from Qiqihar Branch of Heilongjiang Academy of Agricultural Sciences in Heilongjiang Province, China. The legality of these seeds complies with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. The maize seeds collected in the study are all cultivated maize in China rather than endangered and wild species. These varieties have passed the legal variety certification procedures in China and are licensed for production, planting, and market operations. The authors declare that the cultivation of plants and carrying out study in the Qiqihar maize experiment base of Heilongjiang academy of agricultural sciences complies with all relevant institutional, national and international guidelines and treaties.
Results
Post-silking N accumulation and remobilization
As shown in Table 2, the nitrogen accumulation before silking and after silking under low nitrogen condition was significantly lower than that under high nitrogen condition. The nitrogen accumulation before silking and after silking in NEVs were significantly higher than that in NIVs under both N conditions. The nitrogen remobilization and contribution of nitrogen remobilization under low nitrogen condition was significantly higher than that under high nitrogen condition. The nitrogen remobilization and contribution of nitrogen remobilization in NEVs were significantly lower than that in NIVs under both N conditions.
Grain yield and its components
The grain yield (GY) under high nitrogen conditions was significantly higher than that under low nitrogen conditions in all varieties. The GY was significantly higher for NEVs than for NIVs under both N conditions (except for under high nitrogen conditions in 2019) (Table 3). As shown in Table 3, compared with high nitrogen conditions, the grain number per ear and thousand-kernel weight were significantly decreased under low nitrogen conditions, the grain number per ear in NEVs and NIVs were reduced 8.63–15.51% and 19.67–20.50%, respectively. The grain number per ear were significantly higher for NEVs than for NIVs under both N conditions. Compared with high nitrogen conditions, the thousand-kernel weight in NEVs and NIVs were reduced 5.97–11.59% and 9.44–11.62%, respectively. This result indicates that the grain number per ear is the main reason for the difference in yield of different nitrogen-efficiency varieties under both nitrogen conditions. As shown in Fig. 1, compared with high nitrogen conditions, the row number per ear, grain number per row, and ear length were significantly decreased, and the barren tip length were significantly increased under low nitrogen conditions. The ear length of NEVs were significantly higher than that of NIVs under low nitrogen conditions, the barren tip length of NEVs were significantly lower than that of NIVs under both nitrogen conditions.
Row number per ear, grain number per row, ear and barren tip length of the four varieties under different nitrogen rate. Result is the average from 2018 to 2019. Error bars are given as S.E. HN high nitrogen conditions, LN low nitrogen conditions. The different small letters above the column indicate significant difference among cultivars in the same N condition during the same growth stage at P < 0.05.
Floret and grain development
As shown in Table 4, the floret and grain development were affected by varieties and nitrogen rates. Compared with high nitrogen conditions, the floret primordia were significantly decreased under low nitrogen conditions, the floret primordia in NEVs and NIVs were reduced 2.22–4.15% and 5.09–10.33%, respectively. The silking ratio and fertilized ratio of NEVs were significantly higher than that of NIVs under both nitrogen conditions, and nitrogen availability has less effect on silking ratio and fertilized ratio. Compared with high nitrogen conditions, the abortion ratio was significantly increased under low nitrogen conditions, the abortion ratio in NEVs and NIVs were increased 54.40–87.41% and 92.13–106.31%, respectively. In addition, the abortion ratio of NEVs were significantly lower than NIVs under low nitrogen conditions.
The relationships among grain yield and the ear and grain development
The correlations between GY and the ear and grain development were shown in Fig. 2. Under low N conditions, GNE, RNE, GNR, EL, FP, SR and FR were strongly and positively, and BTL and AR was strongly and negatively correlated with GY. Under high N conditions, GNE, RNR, GNR and FR were strongly and positively, and BRL was strongly and negatively correlated with GY. Under low N conditions, GNR, EL, FP, SR and FR were strongly and positively, and BTL, and AR were strongly and negatively correlated with GNE. Under high N conditions, FP, SR and FR was strongly and positively, BTL was strongly and negatively correlated with GNE.
The relationship between Grain yield and its components and ear differentiation traits under both N conditions. The * and **indicate that at the level of 0.05 and 0.01, respectively. GN grain yield, GNE grain number per ear, TKW thousand-kernel weight, RNR row number per ear, GNR grain number per row, EL ear length, BTL barren tip length, FP floret primordia, SR silking ratio, FR fertilized ratio, AR aborted ratio.
Young ear biomass and nitrogen concentration during ear differentiation
As shown in Fig. 3, during the main period of ear development, the responses of different nitrogen-efficiency varieties to nitrogen fertilizer were quite different. With the development of ear, the biomass of young ear increased and the nitrogen concentration decreased. There were significant differences in ear nitrogen concentration among different nitrogen rate and nitrogen efficiency varieties during S1 to S3 period, the nitrogen concentration under high nitrogen condition were significantly higher than that under low nitrogen condition, and the nitrogen concentration of NEVs were significantly higher than NIVs under both nitrogen conditions. During the period from S3 to S5, there were significant differences in ear biomass among different nitrogen fertilizer treatments and nitrogen efficiency varieties. The ear dry matter mass under high nitrogen conditions were significantly higher than that under low nitrogen conditions, and the ear biomass mass of NEVs were significantly higher than NIVs under both nitrogen conditions.
Dynamics of ear dry weight and nitrogen concentration during critical ear differentiation stages. Each point represents a replicate deriving from 2018 to 2019. HN high nitrogen conditions, LN low nitrogen conditions. The letter as indicates the difference between varieties at P < 0.05. From top to bottom, represent NEV-HN, NIV-HN, NEV-LN and NIV-LN.
The morphological and physiological characteristics of the root during spike differentiation
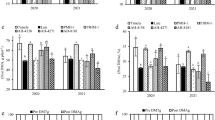
The RLD of all varieties showed a trend of increasing first and then decreasing from the S1 to S5 stage, the maximum value occurs at the S2 or S3 stage under both N conditions (Fig. 4). The RLD were significantly higher for NEVs than for NIVs from the S1 to S5 under both N conditions. The RWD of all varieties showed a trend of increasing first and then decreasing from the S1 to S5 stage, the maximum value occurs at the S3 stage under both N conditions. The RWD were significantly higher for NEVs than for NIVs from the S1 to S5 under both N conditions. The RA of all varieties was not significantly different from S1 to S3 stage, and gradually decreased from S3 to S5 under low nitrogen conditions. While the RA of all varieties increased and then decreased from S1 to S5 stage, the maximum value occurs of NEVs at the S3 stage, the maximum value occurs of NIVs at the S2 stage under high nitrogen conditions. Overall, NEVs can expand root distribution in the growing environment by promoting root elongation and maintain higher root activity.
The phytohormone content in young ear during ear differentiation
The young ear phytohormones content are presented in Fig. 5. The IAA content in the ear of all varieties decreased from the S1 to S5 stage under both N conditions, and was significantly higher in NEVs than in NIVs from the S1 to S3 stage under both N conditions. The ABA content in the ear of all varieties increased from the S1 to S5 stage under both N conditions, was significantly higher in NEVs than in NIVs at the S4 and S5 stage under both N conditions. The GA content in the ear of all varieties decreased from the S1 to S5 stage under both N conditions, and was significantly higher in NEVs than in NIVs from the S1and S2 stage under both N conditions. The ZR content in the ear of all varieties decreased from the S1 to S5 stage under both N conditions, and was significantly higher in NEVs than in NIVs from the S2 to S5 stage under both N conditions.
The IA, ABA, GA and ZR of ear during critical ear differentiation stages. Result is the average from 2018 to 2019. NEV-HN NEVs under high nitrogen conditions, NIV-HN NIVs under high nitrogen conditions, NEV-LN NEVs under low nitrogen conditions, NIV-LN NIVs under low nitrogen conditions. The different small letters above the box indicate significant difference among the same ear differentiation stage at P < 0.05.
The phytohormone content in root during ear differentiation
The root phytohormones content are presented in Fig. 6. There was no significant difference in IAA content in roots at different ear development stages under both N conditions, and was significantly higher in NEVs than in NIVs from the S2 to S5 stage under both N conditions. The ABA content in the root of all varieties increased from the S1 to S5 stage under both N conditions, and was significantly higher in NEVs than in NIVs from the S3 to S5 stage under high N conditions, and at the S5 stage under low N conditions. The GA content in the root of all varieties decreased from the S1 to S5 stage under high N conditions, and was significantly higher in NEVs than in NIVs at the S1, S4 and S5 stage under high N conditions. The ZR content in the root of all varieties decreased from the S1 to S5 stage under both N conditions, and was was significantly higher in NEVs than in NIVs from the S2 to S5 stage under both N conditions.
The IA, ABA, GA and ZR of root during critical ear differentiation stages. Result is the average from 2018 to 2019. NEV-HN NEVs under high nitrogen conditions, NIV-HN NIVs under high nitrogen conditions, NEV-LN NEVs under low nitrogen conditions, NIV-LN NIVs under low nitrogen conditions. The different small letters above the box indicate significant difference among the same ear differentiation stage at P < 0.05.
Correlations between ear and grain development, and leaf and root phytohormones
The results of correlation analysis (Fig. 2) and principal component analysis (Fig. 7) showed that FR was the main factor causing the GY and GNE difference under high nitrogen conditions, while FP, SR, FR and AR were the main factors affecting GY and GNE under low nitrogen conditions. Under high N conditions, the N concentration of ear and E-IAA content were significantly positively correlated with FR from the S1 to S4 stage, while under low N conditions, the N concentration of ear and E-IAA content were significantly positively correlated with FR and SR Correlated from S1 to S3 stages, AR was significantly negatively correlated with RA, R-ZR and E-ZR from S3 to S5.
Discussion
There is great genetic variability in both nitrogen uptake efficiency and nitrogen physiological use efficiency in maize. In many cases, maize varieties that perform best under high nitrogen fertilizer inputs do not necessarily perform well when nitrogen supplies are reduced40, which is related to nitrogen management strategies and environmental factors41. Nitrogen uptake and nitrogen utilization efficiency of different maize varieties tend to be opposite at different nitrogen supply levels. Due to the complex and diverse indicators related to nitrogen absorption and utilization efficiency, and the grain yield directly reflects the economic value of maize. Therefore, it is simple and feasible to use maize grain yield under different nitrogen supply levels as a criterion for evaluating nitrogen-efficient maize varieties. In this study, the grain yield of maize under high and low N conditions was used to evaluate nitrogen-efficient corn varieties. Two NEVs, ND19 and ND29, and two NIVs, ND17 and N19022, were obtained. The results of nitrogen accumulation and transport showed that, compared with the NIVs, the NEVs had higher nitrogen uptake capacity, while the nitrogen remobilization was relatively lower in NEVs. The lower nitrogen remobilization is mainly due to the large amount of nitrogen accumulation in the NEVs, which does not require a large amount of leaf nitrogen to be transferred, while the higher leaf nitrogen accumulation is beneficial to maintain the carbon assimilation and transport capacity of leaves in the later stages of growth. This result suggests that higher nitrogen uptake is beneficial for higher maize yields regardless of low or high N supply.
The grain yield decreased with the reduction of nitrogen application rate, and the grain number per ear is an important factor reflecting the storage capacity of the ear, which is the main reason for the difference in grain yield of maize varieties with different nitrogen efficiency. Compared with NEVs, low nitrogen had a greater effect on the grain number per ear of NIVs. The formation time of grain number per ear is from the early stage of spinning (the first visible filament is exposed) to about two weeks after spinning. The ear differentiation is the key to determine grain number per ear42. The ear differentiation stage is from the formation of florets in the early stage of silking to about two weeks after silking43. The relevant indicators of grain number per ear formation period are regulated by genetic and environmental factors, including the floret primordia, silking ratio, fertilized ratio and abortion ratio. Previous studies have shown that nutrient stress has no significant effect on floret primordia44,45,46, while the present study found that low nitrogen stress significantly decreased floret primordia. Nitrogen deficiency can trigger asynchronous pollination47, thus affecting the number of fertilized florets, or it may also reduce the supply of NSCs at the onset of grain filling, which may in turn affect grain abortion (especially in the apical ones grains) have an effect. Previous studies have shown that filament growth dynamics under genotypic differences may be related to the ability of varieties to maintain pollination, which plays a crucial role in determining the grain number per ear48,49. Low nitrogen stress significantly increased aborted grains and unpollinated filaments, resulting in a decrease in grain number. Rossini et al.50 pointed out that the grain number per ear due to N-deficient grain abortion can be reduced by up to 41%, and it is also reduced by 20% under sufficient N conditions. In the present study, low N significantly decreased silking ratio, fertilized ratio and increased abortion ratio compared with high N conditions. Under the low N conditions, the genotypic differences in the grains number per ear were related to floret differentiation, the lower proportion of grain abortion in unfertilized and fertilized florets, and NEVs were less affected by low N conditions.
In order to clarify the physiological and biochemical basis of the response of grain number formation-related traits to nitrogen supply levels, this study measured the ear nitrogen concentration and phytohormone content, root morphophysiological characteristics and root phytohormone content at the main stages of young ear development. There are few studies on the relationship between the shoots and roots of different varieties under field conditions, and most of the previous related studies were carried out under potted conditions. The morphology of the underground part is closely related to the growth status of the above-ground part of the plant, and plants with larger root biomass are usually more conducive to obtaining soil nutrients51,52, corresponding to The aboveground biomass is also larger. In the present study, the RLD, RWD and RA of NEVs were significantly larger than those of NIVs under both nitrogen conditions. The RLD, RWD and RA were significantly reduced by low N, and NEVs were relatively less affected. In this study, it is believed that the excellent root index of NEVs ensures the high ear nitrogen concentration in the early stage of ear development. These results indicated that NEVs can coordinate root morphophysiological to obtain more N and produce higher grains number per ear under both N conditions.
Phytohormones play an important role in synergistically regulating the growth and development of rice organs, nutrient absorption, carbon and N assimilation, transport and distribution as well as inducing defensive adaptation to stress53. IAA is the earliest phytohormone discovered, and its content is very low in plants, but it plays an important role in crop organogenesis and morphogenesis, tissue differentiation tropism and apical dominance54,55. Higher N concentration in differentiated organs is beneficial to promote IAA synthesis to promote organ differentiation56,57. ABA is known as the senescence hormone, which can promote the senescence of plants, but the role of abscisic acid in the senescence process is contradictory. Some studies have shown that ABA can coordinate the senescence process to ensure grain yield in the later stages of crop growth58,59,60. ZR is a plant endogenous hormone, which belongs to cytokinin, which can promote cell division and is mainly synthesized in roots61, the ZR content of ear was significantly correlated with the ZR content of root in the middle and late stages of ear development under both N conditions. In this study, compare with high N conditions, the IAA, GA, and ZR levels increased and ABA levels decreased under low N conditions, which is consistent with previous studies62,63. The results of the PCA showed that the IAA content of the ear was significantly correlated with the N concentration in the ear, especially under low nitrogen conditions, they were significantly positively correlated with the FP, SR and FR at the floret differentiation stage (S1 to S3). Furthermore, under low N conditions, the AR and BTL was significantly negatively correlated with RA, R-ZR and E-ZR from S3 to S5 stage. These results suggest that NEVs can coordinate the phytohormone content of ears and roots at different stages of ear differentiation, resulting in higher grain numbers per ear under low N conditions.
Conclusions
Compared with NIVs, NEVs showed a stronger tolerance to low N stress, and a higher yield potential under high N conditions. Under both N conditions, compared with NIVs, NEVs showed greater RLD, RWD, and RA in the ear growth stages, which brought higher ear N concentration and laid a favorable nutritional foundation for ear differentiation and growth. Under high N conditions, among ear differentiation-related traits, only FR was significantly positively correlated with grain yield, and NEVs ensure FR through higher N concentration and ZR content in ear at the fertilization stage. Under low N conditions, NEVs obtained higher FP, SR and FR through higher N concentration and IAA in ear at the early stage of ear differentiation, maintained lower AR and BTL by higher RA, R-ZR and E-ZR at the late stage of ear growth. These results suggest that NEHs have a more complex mechanism for obtaining higher grain yield under low N conditions than N sufficiency, and that phytohormones play an important role in this process. Further research is needed to understand the mechanism of exogenous phytohormones in the regulation of ear differentiation and development, and to improve the grain yield in maize under different N supply levels.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request (C.Q., qianjianyi318@163.com).
References
Tester, M. & Langridge, P. Breeding technologies to increase crop production in a changing world. Science 327(5967), 818–822 (2010).
Hu, B. et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47(7), 834–838 (2015).
Li, H., Hu, B. & Chu, C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 68, 2477 (2017).
Liu, X., Hu, B. & Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genomics 49, 394 (2021).
FAO. FAOSTAT Online Database. Food and Agriculture Organization of the United Nations. Accessed Apr 2018. http://faostat3.fao.org/browse/Q/QC/E (2018).
Jia, X. C. et al. Effect of different nitrogen and irrigation treatments on yield and nitrate leaching of summer maize (Zea may L.) under lysimeter conditions. Agric. Water Manage. 137, 92–103 (2014).
Liu, Z. et al. Effects of integrated agronomic practices management on root growth and development of summer maize. Eur. J. Agron. 84, 140–151 (2017).
Chen, F. J. et al. Evaluation of the yield and nitrogen use efficiency of the dominant maize hybrids grown in North and Northeast China. Sci. China-Life Sci. 56(6), 552–560 (2013).
Guo, J. M., Wang, Y. H., Fan, T. L., Chen, X. P. & Cui, Z. L. Designing corn management strategies for high yield and high nitrogen use efficiency. Agron. J. 108, 922 (2016).
Galloway, J. N. et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 320, 889–892 (2008).
Ju, X. T. Direct pathway of nitrate produced from surplus nitrogen inputs to the hydrosphere. Proc. Natl. Acad. Sci. 11(4), 416 (2014).
Robertson, G. P. & Vitousek, P. M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 34(1), 97–125 (2009).
Tilman, D., Cassman, K., Matson, P., Naylor, R. & Polasky, S. Agricultural sustainability and intensive production practices. Nature 418(6898), 671–677 (2002).
Chen, K., Kumudini, S., Tollenaar, M. & Vyn, T. Plant biomass and nitrogen partitioning changes between silking and maturity in newer versus older maize hybrids. Field Crop Res. 183, 315–328 (2015).
Wu, Y. et al. Low-nitrogen stress tolerance and nitrogen agronomic efficiency among maize inbreds: Comparison of multiple indices and evaluation of genetic variation. Euphytica 180(2), 281 (2011).
Zhao, B., Dong, S. T., Zhang, J. W. & Liu, P. Effects of controlled-release fertiliser on nitrogen use efficiency in summer maize. PLoS ONE 8, e70569 (2013).
Mu, X. H. et al. Genetic improvement of root growth increases maize yield via enhanced post-silking nitrogen uptake. Eur. J. Agron. 63, 55–61 (2015).
Reetz, H. F., Heffer, P. & Bruulsema, T. W. 4R nutrient stewardship: A global framework for sustainable fertilizer management. In Managing Water and Fertilizer for Sustainable Agricultural Intensification (eds Drechsel, P. et al.) 65 (International Fertilizer Industry Association (IFA), International Water Management Institute (IWMI), International Plant Nutrition Institute (IPNI), and International Potash Institute (IPI), 2015).
Kant, S., Bi, Y. M. & Rothstein, S. J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 62, 1499–1509 (2011).
Haegele, J. W., Cook, K. A., Nichols, D. M. & Below, F. E. Changes in nitrogen use traits associated with genetic improvement for grain yield of maize hybrids released in different decades. Crop Sci. 53, 1256–1268 (2013).
Li, X. X. et al. Kernel number as a positive target trait for prediction of hybrid performance under low-nitrogen stress as revealed by diallel analysis under contrasting nitrogen conditions. Breed. Sci. 64, 389–398 (2014).
Ceccarelli, S. GM crops, organic agriculture and breeding for sustainability. Sustainability 6, 4273–4286 (2014).
Swain, E. et al. Optimizing nitrogen use efficiency in wheat and potatoes: Interaction between genotypes and agronomic practices. Euphytica 199, 119–136 (2014).
Cui, Z. L. et al. Interaction between genotypic difference and nitrogen management strategy in determining nitrogen use efficiency of summer maize. Plant Soil. 317, 267–276 (2009).
Yang, J. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 127(1), 315–323 (2001).
Morris, R. O. et al. Cytokinins in plant pathogenic bacteria and developing cereal grains. Funct. Plant Biol. 20, 621 (1993).
Yang, J. et al. Grain filling pattern and cytokinin content in the grains and roots of rice plants. Plant Growth Regul. 30, 261–270 (2002).
Yang, J. et al. Correlations of cytokinin levels in the endosperms and roots with cell number and cell division activity during endosperm development in rice. Ann. Bot. 90, 369–437 (2002).
Hansen, H. & Grossmann, K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 124, 1437–1448 (2000).
Eeuwens, C. & Schwabe, W. Seed and pod wall development in Pisunz sativum L. in relation to extracted and applied hormones. J. Exp. Bot. 26, 1–14 (1995).
Suzuki, Y., Kurogoch, S., Murofushi, N., Ota, Y. & Takahashi, N. Seasonal changes of GAS, GAIL and abscisic acid in three rice eultivars. Plant Cell Physiol. 22, 1085–1093 (1981).
Lur, H. S. & Setter, T. L. Role of auxin in maize endosperm development: Timing of nuclear DNA endoreduplication, zein expression, and cytokinins. Plant Physiol. 103, 273–280 (1993).
De, S. M. & Vreugdenhil, D. Abscisic acid and assimilate partitioning to develop seeds, I. Dose abscisic acid influence the growth rate of pea seeds? J. Plant Physiol. 140, 201–206 (1992).
Kato, T., Sakurai, N. & Kuraishi, S. The changes of endogenous abscisic acid in developing grains of two rice cultivars with different grain size. Jpn. J. Crop Sci. 62, 456–461 (1993).
Schussler, J. R., Brenner, M. L. & Brun, W. A. Relationship of endogenous abscisic acid to sucrose level and seed growth rate of soybeans. Plant Physiol. 96, 1308–1313 (1991).
Yang, J., Zhang, J., Liu, K., Wang, Z. & Liu, L. Abscisic acid and ethylene interact in wheat grains in response to soil drying during grain filling. New Phytol. 171, 293–303 (2006).
Ramasamy, S., Berge, H. & Purushothaman, S. Yield formation in rice in response to drainage and nitrogen application. Field Crop Res. 51, 65–82 (1997).
Liu, Y., Ding, Y., Wang, Q., Meng, D. & Wang, S. Effects of nitrogen and 6-benzylaminopurine on rice tiller bud growth and changes in endogenous hormones and nitrogen. Crop Sci. 51, 786–792 (2011).
Yu, N., Zhang, J., Liu, P., Zhao, B. & Ren, B. Integrated agronomic practices management improved grain formation and regulated endogenous hormone balance in summer maize (Zea may L.). J. Integr. Agric. 19(7), 1768–1776 (2020).
Gallais, A. & Coque, M. Genetic variation and selection for nitrogen use efficiency in maize: A synthesis. Maydica 50(3), 531–547 (2005).
Fixen, P. et al. Nutrient/fertilizer use efficiency: Measurement. Curr. Situat. Trends 33, 8–38 (2015).
Zheng, Y., Persson, S. & Zhang, D. B. Molecular and genetic pathways for optimizing spikelet development and grain yield. aBIOTECH 2, 276 (2020).
Gonzalez, V. H., Lee, E. A., Lewis, L. L. & Swanton, C. J. The relationship between floret number and plant dry matter accumulation varies with early season stress in maize (Zea may L.). Field Crops Res. 238, 129–138 (2019).
Lemcoff, J. H. & Loomis, R. S. Nitrogen and density influences on silk emergence, endosperm development, and grain yield in maize (Zea may L.). Field Crops Res. 38, 63–72 (1994).
Ciampitti, I. A. et al. Physiological dynamics of maize nitrogen uptake and partitioning in response to plant density and nitrogen stress factors: II. Reproductive phase. Crop Sci. 53(6), 2588–2602 (2013).
Oury, V., Tardieu, F. & Turc, O. Ovary apical abortion under water deficit is caused by changes in sequential development of ovaries and in silk growth rate in maize. Plant Physiol. 171, 986–996 (2015).
Oury, V. et al. Is change in ovary carbon status a cause or a consequence of maize ovary abortion in water deficit during flowering? Plant Physiol. 171(2), 997–1008 (2016).
Otegui, M. E. & Bonhomme, R. Grain yield components in maize: I. Ear growth and kernel set. Field Crops Res. 56(3), 247–256 (1998).
Yan, P., Chen, Y. Q., Sui, P., Vogel, A. & Zhang, X. P. Effect of maize plant morphology on the formation of apical kernels at different sowing dates and under different plant densities. Field Crop Res. 223, 83–92 (2018).
Rossini, M. A., Maddonni, G. A. & Otegui, M. E. Inter-plant variability in maize crops grown under contrasting N× stand density combinations: Links between development, growth and kernel set. Field Crop Res. 133, 90–100 (2012).
Worku, M. et al. Nitrogen efficiency as related to dry matter partitioning and root system size in tropical mid-altitude maize hybrids under different levels of nitrogen stress. Field Crop Res 130, 57–67 (2012).
Zhang, F. L. et al. Studies on the root characteristics of maize varieties of different eras. J. Integr. Agric. 12(3), 426–435 (2013).
Xin, W. et al. An integrated analysis of the rice transcriptome and metabolome reveals root growth regulation mechanisms in response to nitrogen availability. Int. J. Mol. Sci. 20, 5893 (2019).
Benková, E., Michniewicz, M. & Sauer, M. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115(5), 591–602 (2003).
Reinhardt, D. Vascular patterning: More than just auxin? Curr. Biol. 13(12), 485–487 (2003).
Kepinski, S. & Leyser, O. Plant development: Auxin in loops. Curr. Biol. 15(6), 208–210 (2005).
Bohn, I. Auxin: A major regulator of organogenesis. C. R. Biol. 333(4), 290–296 (2010).
Kato, T. Effect of spikelet removal on the grain filling of Akenohoshi, a rice cultivar with numerous spikelets in a panicle. J. Agric. Sci. 142, 177–181 (2004).
Peng, S. et al. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop Res 96, 37–47 (2006).
Dong, M. et al. Comparative proteomics analysis of superior and inferior spikelets in hybrid rice during grain filling and response of inferior spikelets to drought stress using isobaric tags for relative and absolute quantification. J. Proteomics 1, 51–54 (2014).
Kiba, T., Kudo, T., Kojima, M. & Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 62, 1399–1409 (2011).
Krouk, G. et al. A framework integrating plant growth with hormones and nutrients. Trends Plant Sci. 16, 178–182 (2011).
Luo, J. et al. Global poplar root and leaf transcriptomes reveal links between growth and stress responses under nitrogen starvation and excess. Tree Physiol. 35, 091 (2015).
Funding
This work was supported by grants from the Special project for the construction of modern agricultural industry technology system (CARS-02-43).
Author information
Authors and Affiliations
Contributions
B.M. and C.Q. designed the study and provided experimental materials. J.W. analyzed the results and prepared the figures and tables. B.M. wrote the paper. All authors discussed the results and commented on the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, B., Wang, J., Han, Y. et al. The response of grain yield and ear differentiation related traits to nitrogen levels in maize varieties with different nitrogen efficiency. Sci Rep 12, 14620 (2022). https://doi.org/10.1038/s41598-022-18835-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18835-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.