Abstract
Fusarium crown rot and wheat sharp eyespot are major soil-borne diseases of wheat, causing serious losses to wheat yield in China. We applied high-throughput sequencing combined with qPCR to determine the effect of winter wheat seed dressing, with either Trichoderma atroviride HB20111 spore suspension or a chemical fungicide consisting of 6% tebuconazole, on the fungal community composition and absolute content of pathogens Fusarium pseudograminearum and Rhizoctonia cerealis in the rhizosphere at 180 days after planting. The results showed that the Trichoderma and chemical fungicide significantly reduced the amount of F. pseudograminearum in the rhizosphere soil (p < 0.05), and also changed the composition and structure of the fungal community. In addition, field disease investigation and yield measurement showed that T. atroviride HB20111 treatment reduced the whiteheads with an average control effect of 60.1%, 14.9% higher than the chemical treatment; T. atroviride HB20111 increased yield by 7.7%, which was slightly more than the chemical treatment. Therefore, T. atroviride HB20111 was found to have the potential to replace chemical fungicides to control an extended range of soil-borne diseases of wheat and to improve wheat yield.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum L.) production is challenged by soil-borne fungal diseases such as Fusarium crown rot and wheat sharp eyespot. Among these, Fusarium crown rot is a disease of global significance that occurs in the major winter wheat growing regions in China1,2. It rapidly spread throughout the country in recent years, especially in the HuangHuai area (the main wheat production in China), posing a major threat to the safety and security of wheat production3,4,5,6. Fusarium crown rot is caused by Fusarium spp., amongst which Fusarium pseudograminearum is the dominant pathogen7,8,9. Studies have shown that the infection by some Fusarium pathogens may directly affect food safety by leading to mycotoxin contamination of grains10. In addition to Fusarium crown rot, wheat sharp eyespot caused by Rhizoctonia cerealis, is also a major soil-borne fungal disease of wheat11,12,13,14. The main circumstances that drive infection by the above-mentioned diseases are the annual return of straw to the field, resulting in the accumulation of fungal sources in the soil, and the use of susceptible wheat varieties5. The main control methodsare crop rotation, the use of tolerant varieties and biological or chemical agents.
Most of the chemical preparations for controlling wheat diseases use tebuconazole, propiconazole, difenoconazole and/or kresoxim-methyl as active substances, which are fungicides that inhibit the biosynthesis of ergosterol and lead to excessive electrolyte loss15,16. Biological agents Streptomyces, Bacillus subtilis, Pseudomonas, Actinomyces, Trichoderma, etc.17,18,19,20,21,22,23,24 can reduce the colonization of pathogenic fungi, produce antibiotics and organic compounds in the soil, and promote plant growth25.
Seed dressing is a cost-effective method that has the potential for large-scale application in the prevention and control of crop diseases26. Seed dressing with biological agents is widely used to prevent or mitigate fungal diseases caused by pathogenic fungi such as Fusarium sp.27,28,29. As a biocontrol agent, Trichoderma seed dressing has been increasingly used to control plant fungal diseases. Common pathogens controlled by Trichoderma include Bipolaris sorokiniana, F. pseudograminearum, F. oxysporum, R. solani, R. cerealis and many other plant pathogens30,31. Internationally, more than 60% of registered biofungicide formulations contain at least one strain of Trichoderma32, which is due to the frequent ability of Trichoderma spp. to detect, invade and destroy pathogenic fungi33. Research has shown that when fungi colonize plant roots, and when different pathogens are detected, the abundance of Trichoderma changes34. Trichoderma spp. can also promote plant absorption of nutrients, plant height, dry and fresh weight of roots and stems, and grain weight35,36,37. At present, commonly used Trichoderma species include T. harzianum, T. atroviride and T. aculeatus38.
In recent years, the regulation of the structure and interaction of plant microbiomes as a biological control strategy for pathogens has become a focus for research39,40. The rhizosphere is a significant plant microbiome for beneficial and harmful microorganisms. Understanding the community compositions of rhizosphere microorganisms and how these changes in response to treatments with biological controls is particularly important in the process of pathogen control. The composition of soil microbial communities can be better understood, and information about pathogens is not required before sequencing with the application of high-throughput sequencing technology41,42,43,44. Fluorescent quantitative PCR (qPCR) technology can quickly and quantitatively detect target pathogens45,46,47,48. The combination of the two methods can accurately analyze the changes in rhizosphere microorganism communities and populations of pathogenic fungi.
In this study, high-throughput sequencing and qPCR technology were used to evaluate the biological control effect of T. atroviride HB20111 upon the major wheat soil-borne diseases. The work is mainly to quantify (1) the impact of Trichoderma seed dressing treatment on wheat fungal communities, and (2) the biological effect of Trichoderma in the control of Fusarium crown rot and wheat sharp eyespot.
Results
The abundance of fungal pathogens
qPCR results showed that different treatments had different effects on the abundance of targeted fungal pathogens (Fig. 1, Supplementary Fig. S1). The copy number of total fungi in T. atroviride HB20111 treatment was lower 47.2% than that of the control, in the meanwhile, the chemical fungicide was slightly higher than that of the control, but the differences were not significant (p < 0.05, Fig. 1A). With the chemical fungicide and Trichoderma seed dressing, the copy number of F. pseudograminearum in the rhizosphere of wheat was significantly lower than that of the control with values of 32.5% and 28.9% respectively (p < 0.05, Fig. 1B). For R. cerealis, the chemical treatment was significantly lower than that of the control by 38.5%, and there was no significant difference in Trichoderma treatment (p < 0.05, Fig. 1C). The F. pseudograminearum and R. cerealis levels in the Trichoderma treatment were not significantly different from the chemical treatment (p < 0.05).
OTU composition
OTU composition analysis was shown in Fig. 2. The first two principal coordinates signified 44.57% (PCo1) and 22.88% (PCo2) of the soil fungal community variation. The X-axis distance between the three different treatment groups were not significant (p < 0.05), indicating that Trichoderma and chemical treatment did not have a significant impact on the beta diversity of the microbial community. The Venn diagram showed the overlap of OTUs in the microbial community between the different treatments. The total number of OTUs for the three treatments did not change significantly. Among the three treatments, the number of unique OTUs detected by the Trichoderma treatment was the most, at 78, accounting for 49.1% of the total, followed by chemical treatment with 74, accounting for 46.8%.
Abundance and diversity
Alpha diversity analyses targeting the fungal community of the wheat rhizosphere showed that both T. atroviride HB20111 and chemical fungicide seed dressing treatments affected the structure and composition (Fig. 3). The treated group had slightly less fungal OTUs than that of the control (Fig. 3A), and Shannon index were slighter lower under the chemical and Trichoderma treatments (Fig. 3B).
The diversity and richness of the fungal community in wheat rhizosphere soil under different treatments i.e. control seed dressing (Control), chemical fungicide seed dressing (Chemical) and T. atroviride HB20111 seed dressing (Trichoderma). Observed OTUs of Richness of different treatments (A) and Shannon index of different treatments (B).
The structure of the fungal community
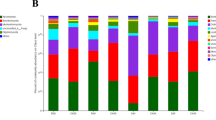
The relative abundance of fungi at the phylum level for each treatment was shown in Fig. 4A. Multiplying the total amount of fungi by the relative abundance to get the absolute abundance (Fig. 4B). The relative abundance of fungi at the genus level for each treatment was shown in Fig. 4C. It can be seen from the relative abundance that different treatments affected the composition of the fungal community (Fig. 4A). Ascomycota was the most dominant phylum, accounting for about 50% in each treatment. The proportion of Ascomycota in untreated rhizosphere soil was 6% higher than Trichoderma treatment and 13% lower than that of chemical treatment. The proportion of Mucoromycota and Olpidiomycota in Trichoderma treatment were 8% and 3% higher than the other two groups, respectively. In addition, the content of Basidiomycota in the chemical treatment was 5% higher than that in the other two groups (Fig. 4A). And the absolute abundance calculations that the 3 seed dressing treatments had different impacts on wheat rhizosphere soil fungal community composition (Fig. 4B). Compared with the control group, most of fungi decreased in different degrees in Trichoderma treatment. Ascomycota and Olpidiomycota were changed significantly in the rhizosphere fungal community following Trichoderma seed dressing, compared with the control, among which, the content of Ascomycetes decreased by 54.2%, and the Olpidiomycota increased by 54.8% (p < 0.05, Fig. 4B). The dominant genera differed according to treatments (Fig. 4C), except the genera with the highest relative abundance in different treatments were all Alternaria spp. and Cladosporium spp. Trichoderma and chemical treatments both reduced the relative abundance of Fusarium spp. The relative abundance of Alternaria spp. in the Trichoderma treatment decreased by 3.26% and 11.28% compared with the control and chemical treatments, respectively. These decreases were accompanied by notable increases in the relative abundance of Olpidium and Botryotrichum in the rhizosphere soil. Inoculation caused the inoculant, Trichoderma, to have the relative abundance (< 1%), whereas it was not found in other groups.
Rhizosphere fungal community structure at the phylum and genus level as affected by control seed dressing (Control), T. atroviride HB20111 seed dressing (Trichoderma), and chemical fungicide seed dressing (Chemical). Relative fungal abundance at the phylum level (A), absolute fungal abundance at the phylum level (B) and relative fungal abundance at the genus level (C). ns, not significant at p < 0.05; *Indicates significance at p < 0.05; **Indicates significance at p < 0.01 based on Games–Howell as the post hoc test and Benjamini–Hochberg FDR as the multiple test correction method.
Correlation between Trichoderma treatment and fungal pathogens in the wheat rhizosphere
Co-occurrence Network Analysis showed that Trichoderma had a linear relationship with 18 plant pathogens, of which 13 were negatively related (Fig. 5). The pathogenic fungi that could cause Fusarium crown rot was negatively correlated with the occurrence of Trichoderma. In addition to Fusarium, Trichoderma also had inhibitory effects on many other pathogens.
Impact of Trichoderma on the targeted wheat soil-borne diseases
The survey results of wheat sharp eyespot and Fusarium crown rot disease at 220 days after sowing showed that both T. atroviride HB20111 and chemical fungicide seed dressing treatments significantly reduced the disease index of them (p < 0.05, Fig. 6A,B, Supplementary Fig. S2). For Fusarium crown rot, Trichoderma and chemical treatments reduced the disease index by 64.3% and 39.3%, respectively. We observed that the stalk bases of whiteheads appeared fragile and were mostly covered with brown spots, matching the typical symptoms caused by Fusarium crown rot. The survey results showed that both T. atroviride HB20111 and chemical fungicide seed dressing treatments significantly reduced the whiteheads rate (p < 0.05). The control efficiency of T. atroviride HB20111 seed dressing was 60.1%, compared to the 45.2% control obtained with chemical seed dressing (p < 0.05, Fig. 6C). Compared with the control, the yield of wheat treated with T. atroviride HB20111 seed dressing increased by 7.7% and with chemical fungicide seed dressing increased by 2.4% (p < 0.05, Fig. 6D, Supplementary Fig. S3).
Impact of T. atroviride HB20111 seed dressing (Trichoderma) and chemical fungicide seed dressing (Chemical) on the targeted wheat soil-borne diseases. The disease index of wheat sharp eyespot (A); the disease index of Fusarium crown rot (B); the whiteheads rate and control efficiency of different treatments (C); the yield of different treatments (D).
Discussion
It had been reported that Trichoderma could control Fusarium crown rot, most of which were T. harzianum49,50,51. We believed that the different effects of Trichoderma on the control of wheat diseases may be related to pathogenic fungi, Trichoderma strains, ecological environment and rhizosphere soil microbial communities. Studies had shown that T. harzianum reduced the disease incidence by 51.79% against Fusarium crown rot which caused by F. culmorum52. The experimental results showed that T. atroviride HB20111 could effectively reduce the occurrence or severity of wheat diseases, including Fusarium crown rot and wheat sharp eyespot, when used to coat wheat seeds (Fig. 6A,B). The control efficiency was as high as 60.1% (Fig. 6C), which was higher than that of a chemical fungicide (45.2%), and may had the added benefit of being able to promote the growth and disease resistance of plants. Seed coating has been widely used in agriculture, and can promote plant health in the soil through early disease pressure and trigger plant defense responses to prolong the effect of biological control of strains53,54,55. This was confirmed by the control efficiency and final output of the field experiment of this work.
In this study, we explored the effect of seed dressing with T. atroviride HB20111 on the fungal community in the rhizosphere soil of wheat. OTU composition analysis showed that different treatments did not change the beta diversity of the fungal community, but Trichoderma treatment impacted its composition (Fig. 2A). Compared with the control group, alpha diversity analysis showed that both Trichoderma and chemical treatment slightly reduced the community abundance, yet the diversity of Trichoderma treatment not changing significantly (Fig. 2B). Treatment with Trichoderma spp. had been reported to show higher abundance of fungi56. Ideally, T. atroviride HB20111 inoculation would activate soil microbes and increase their diversity. However, combined with qPCR results analysis (Fig. 1), it indicated that Trichoderma treatment reduced the content of some wheat pathogenic fungi so that the species richness decreased, but the community uniformity improved, which was similar to the results of previous studies57,58. Owing to the advantages of vigorous vitality and rapid growth, Trichoderma spp. can inhibit the growth space of pathogenic fungi by altering the soil microbiome to absorb the necessary nutrients and induce pathogen suppressiveness. Conversely, both the richness and diversity of the fungal microbial community in the rhizosphere of wheat in the chemical seed dressing treatment were both significantly reduced (Fig. 2B). The richness and diversity of microbial populations in the rhizosphere of plants were closely related to plant health59. Therefore it can be seen that the Trichoderma treatment has multiple advantages in that it does not cause chemical pollution and has less impact on the community of microorganisms compared to chemical treatment.
The results of ITS sequencing at the phylum level showed that Ascomycota was the dominant fungus in the fungal community (Fig. 4A), which was consistent with the previous reports60,61. The colonization of Trichoderma in the rhizosphere changed the abundance of fungi at phylum level, such as increasing the content of Olpidiomycota and Mucoromycota. Trichoderma seed dressing affected the structure and diversity of the rhizosphere microbial community62,63,64, these changes may created a more suitable micro-ecological environment for wheat growth. Interestingly, when we combined the qPCR total fungi results with the relative content, converting the relative abundance of fungal communities in different treatment groups into absolute abundance, we found that the changes of Ascomycota and Olpidiomycota were significant different compared with their relative abundance (p < 0.05, Fig. 4B). In particular, the relative abundance of Ascomycota in Trichoderma treatment was higher than that in chemical treatment, but the absolute content was lower, which could better reflect the content of pathogenic fungi in soil. This provides an idea for future accurate analysis of high-throughput sequencing data. Alternaria spp. as a pathogen, an endophyte and a saprophyte, could cause various plant diseases such as wheat leaf spot, pumpkin seed rot and cumin blight, etc.65. As the most dominant genus, the relative abundance of Alternaria spp. was reduced in Trichoderma treatment (Fig. 4C), indicating that T. atroviride HB20111 may also be effective in controlling plant diseases caused by it. Moreover, the content of the main pathogen, F. pseudograminearum, was also decreased. The relative abundance of Olpidium spp. and Botryotrichum spp. increased, but whether the reason for the increase was related to Trichoderma regulation was unknown. Chemical treatment significantly reduced the content of R. cerealis in the rhizosphere, but there was no significant difference between it and the Trichoderma treatment (p < 0.05). In terms of the pathogen abundance in the rhizosphere soil of different treatments (Fig. 1C), the content of R. cerealis was low (0.9%). The whiteheads were mainly caused by Fusarium crown rot in the 220-day disease investigation, which showed that the occurrence of disease was related to the content of pathogenic fungi in the soil. In addition, we found that Rhizoctonia spp. was almost absent in high-throughput sequencing (Fig. 4C), indicating that high-throughput sequencing has inconsistent amplification efficiency for each fungus. Therefore, it is necessary to combine the results of qPCR and high-throughput sequencing for analysis.
As previously reported, Trichoderma inoculants to colonize and persisted in the rhizosphere soil for 8 weeks, up to 112 days66,67, which explained why the content of Trichoderma in the rhizosphere soil obtained at 180 days was very low. T. atroviride HB20111 increased yield by 7.7% (Fig. 6D). It was consistent with the previous experimental results that Trichoderma could increase wheat yield by 6–11%68. Given that the wheat yield of the Trichoderma treatment was higher than that of the chemical treatment and the blank control, it can be seen that there is a great potential for the use of T. atroviride for the prevention and control of Fusarium crown rot in the future.
There are many plant pathogenic fungi in the rhizosphere fungal community of wheat. We suggested that the presence of these plant pathogenic genera that were positively related to Trichoderma was limited, and not enough to cause plant disease, despite being present at elevated levels. Furthermore, there were no reports indicating that these genera of fungi caused serious disease on wheat specifically. By contrast, the main pathogenic fungi that caused plant symptoms in this work were Fusarium species. It was evident that Trichoderma treatment was negatively correlated with the abundance of a variety of fungal genera that were well-known as pathogens of wheat rhizosphere, especially Fusarium. qPCR and high-throughput sequencing results both showed that the coating of wheat seeds with T. atroviride HB20111 decreases Fusarium spp. content in the rhizosphere. This is consistent with previous studies that Trichoderma had an obvious suppression on F. pseudograminearum until the wheat matured69. It is a further evidence to support the biological control effect of Trichoderma on wheat diseases. In addition, Trichoderma was negatively correlated with a range of pathogens such as Gibberella, Bulmeria, Cladosporium and Setosphaeria (Fig. 5), which may cause plant diseases such as Fusarium head blight, wheat powdery mildew, tomato leaf mildew and maize leaf spot70,71,72. This indicates that Trichoderma has more potential and development space in the application of biocontrol.
During the jointing stage, the disease symptoms were not significant, but the symptoms appeared at flowering and filling period. The content of F. pseudograminearum in wheat rhizosphere soil of different treatments was determined by qPCR at 180 days, indicating that Trichoderma and chemical seed treatment reduced the content of pathogenic fungi. It was associated with reduced incidence of disease, and consistent with the 220-day disease index survey. This method can monitor the pathogenic fungi and realize early warning of disease occurrence to take preventive measures in advance.
Conclusions
This study indicated that T. atroviride HB20111 seed dressing may create a healthier soil micro-ecological environment for the growth of wheat by reducing the content of plant pathogens in the fungal community of wheat rhizosphere, thereby providing evidence for the prevention and control of important diseases and the improvement of wheat yield.
The use of qPCR in this work further explored the effectiveness of T. atroviride HB20111 treatment and chemical treatment in reducing the rhizosphere content of pathogenic fungi that cause wheat diseases, including Fusarium crown rot and wheat sharp eyespot. Among them, the rhizosphere populations of F. pseudograminearum and R. cerealis in the T. atroviride treatment and chemical treatment were lower than in the control. This illustrates that Trichoderma has the potential to replace chemical fungicides in the control of plant fungal diseases, providing theoretical basis for the future research on the biological control of wheat by Trichoderma and the development of green agriculture.
Materials and methods
Overview of the test field
The experiment was conducted at the research station of Shandong Luyan Agricultural Seed Co., Ltd. (37°4′ N,116°7′ E; Xixinzhuang Village, Shentou Town, Lingcheng District, Dezhou City, Shandong Province) between October 8th 2019 and June 16th 2020. The experimental field was level, with a soil pH of 7.5 in CaCl2, hydrolyzable N content of 31.15 mg/kg, available P content of 38.41 mg/kg, available K content of 168.5 mg/kg, organic matter content of approximately 1.57% and a moderate soil water content. The site was recently used to produce maize, with all plant residues crushed and returned to the field after harvest. Before sowing wheat, potassium sulfate compound fertilizer (N: P2O5: K2O = 17:17:17, Jinzhengda Ecological Engineering Group Co., Ltd., Linyi, China) was applied to the field as the base fertilizer at a rate of 0.6 t/hm2, and urea was added as a topdressing at a rate of 0.23 t/hm2 (Luxi Chemical Industry Group Co., Ltd., Liaocheng, China). The field was sown with wheat cultivar Jimai 44 (Shandong Luyan Seed Industry Co., Ltd., Jinan, China) at a rate of 0.11 t/hm2, using a mechanical seeder. The cultivar Jimai 44 is a high-yielding and high-quality cultivar, yet is sensitive to Fusarium crown rot. The experimental field was divided into plots of 8 × 1.5 m2.
Trichoderma strain
Trichoderma atroviride HB20111 isolated by Shandong Provincial Key Laboratory of Applied Microbiology was used in the wheat seed dressing experiment. It was deposited in the China General Microorganism Culture Collection and Management Center (CGMCC) under the registration no. CGMCC16963.
Seed dressing treatment
Seeds were surface-sterilized, soaked for 5 min in an aqueous solution of 0.5% sodium hypochlorite, then rinsed three times with sterile distilled water and air-dried. The T. atroviride HB20111 was grown on Potato dextrose agar (PDA, Dingguo, Beijing, China) for ten days and a conidia suspension was obtained as described in a previous study73. Briefly, under sterile conditions, Trichoderma culture plates were flooded with sterile water and the resulting conidia suspension was filtered through filter paper to separate the conidia from the mycelium. The conidia suspension concentration was adjusted to 2 × 108 CFU/mL. Trichoderma spores were applied gradually to continuously rotating wheat seeds at a rate of 100 mL per 10 kg seeds until complete adhesion and absorption to ensure even distribution of Trichoderma among the seeds. Chemical fungicide Raxil® seed dressing consisting of 6% tebuconazole (Bayer Crop Science Co., Ltd.; Leverkusen, Germany) applied at the rate of 30 mL per 10 kg seeds, according to manufacturer's recommendation. The blank group was controlled with water at the rate of 10 mL/kg seed.
Experimental design
The experiment was designed with three seed-dressing treatments and three replications for each treatment. Treatment 1 was a T. atroviride HB20111 seed dressing. Treatment 2 was a chemical fungicide. Treatment 3 was a control. The experimental field was divided into 9 blocks, with each treatment randomly distributed with three blocks.
The effectiveness of Trichoderma seed treatment assessed
20 Trichoderma-treated wheat seeds were transferred into 500 mL Erlenmeyer flask place with 100 mL sterile water and placed on shaker (120 r/min) for 30 min. The resulting suspensions and their serial dilutions (10–3, 10–4, 10–5) plated onto PDA agar. Colonies of T. atroviride HB20111 were counted after 5 days at 25 °C. Finally, we calculated and got the result with the number of effective coats of 5 × 105 CFU/seed.
Soil sample collection
At 180 days after sowing (jointing stage), 5 randomly selected wheat plants were harvested from each plot, including their whole root systems. The soil sample of each plot was obtained by removing the attached rhizosphere soils from each plant and combined. Each soil sample was then sieved with a 20 mm mesh and packed in a sterile ziplock bag. The test procedure of DNeasy PowerSoil Kit (QIAGEN, Valencia, CA, USA) was applied to soil samples (0.3 g each) to extract the total soil microorganism DNA for subsequent qPCR and high-throughput sequencing analysis.
Evaluation of the seed dressing treatments
Effects of the T. atroviride HB20111 and chemical fungicide seed dressing treatments against R. cerealis (wheat sharp eyespot) and F. pseudograminearum (Fusarium crown rot) were evaluated in 3 aspects; disease index and percent of whiteheads at 220 days, and wheat yield at 240 days after sowing.
For disease index, 5 randomly selected wheat plants per plot were assessed for each disease. Assessment of wheat sharp eyespot was performed as per the section “Fungicides control wheat sharp eyespot” in GB/T 17,980.108–2004 pesticide field efficacy test guidelines74, as Grade 0 = Asymptomatic; Grade 1: brown or obvious sharp eyespot on the outer leaf sheath and the lesion diameter less than 1/2 of the sheath circumference; Grade 2: obvious sharp eyespot on the outer leaf sheath and the lesion diameter greater than 1/2 of the sheath circumference, with the asymptomatic inner sheath; Grade 3: brown or obvious sheath blight spots on the inner sheath and the lesion diameter less than 1/2 of the sheath circumference; Grade 4: obvious sharp eyespot on the inner sheath and the lesion diameter greater than 1/2 of the sheath circumference; and Grade 5: dead.
Fusarium crown rot was assessed using disease classification standards75 on the size of browning on the first internode as Grade 0: no browning; Grade 1; 1–25%; Grade 2: 26–50%; Grade 3: 51–75%; and Grade 4: 76–100%.
Disease indices were calculated as follows:
where “stage” means the level of disease severity, and “relative value” means the grade numbers.
We assessed the presence of whiteheads, which is a common symptom shared by wheat sharp eyespot and Fusarium crown rot, manifested as whitish wheat ears, and slender grains or empty husks. The occurrence of whiteheads was investigated in 5 wheat plants for each plot.
The efficiency of control whiteheads by seed dressing treatments T. atroviride HB20111 and chemical fungicide was calculated as follows:
Analysis of wheat yield
For wheat yield, grains were harvested using a small plot wheat harvester (Delta, Wintersteiger, Beijing, China). Yield for each plot was measured upon harvest. The yield of wheat is calculated according to the actual harvest of wheat yield to remove impurities and a fixed grain water content of 13%.
Real-time fluorescent quantitative PCR (qPCR) analysis
The abundances of total fungi in wheat rhizosphere soil were determined using the SYBR Green qPCR method. The ITS1F/ ITS2R primers76 were used to quantify total fungal abundance (Table 1). A tenfold dilution series of a plasmid containing the 18S rRNA gene of Saccharomyces cerevisiae was used to generate a standard curve (the number of copies of the gene ranged from 3.0 × 103 to 3.0 × 107). The qPCR reaction system (20 µL) included: 10 µL 2 × SG Fast qPCR Master Mix (Sangon Biotech Co., Ltd., Shanghai, China), 20 µM forward and reverse primers each 1 µL, 7 µL molecular biology Grade water and 1 µL of soil sample DNA. qPCR was carried out using a three-step protocol: Pre-denaturation was at 95 °C for 10 min; denaturation was at 95 °C for 30 s, annealing was at 50 °C for 30 s, extension was at 72 °C for 1 min, for 40 cycles. Fluorescence was acquired multiple times in the extended phase of each cycle (72 °C). The dissolution profile was analyzed after PCR amplification to verify the specificity of the amplification. The procedure for the dissolution curve was: 95 °C, 1 min; 56 °C, 1 min; from 56 °C for every 0.5 °C for 10 s, and then continuous increase 89 times (to 95 °C).
The abundances of R. cerealis in wheat rhizosphere soil were determined using the SYBR Green I fluorescent dye method. The RctubF4/RctubR4 primers77 were used to quantify R. cerealis (Table 1). A standard curve (gene copy number range) was generated with a tenfold dilution series of a plasmid containing the β-tubulin gene fragment, from 3.2 × 102 to 3.2 × 107. The qPCR reaction system (20 µL) included: 10 µL 2 × SG Fast qPCR Master Mix (Sangon Biotech Co., Ltd., Shanghai, China), 20 µM forward and reverse primers each 1 µL, 7 µL molecular biology Grade water and 1 µL of soil sample DNA. The qPCR was carried out using a two-step protocol: Pre-denaturation was at 95 °C for 180 s, denaturation was at 94 °C for 15 s, annealing and extension were at 60 °C for 30 s, with 40 cycles. The procedure for the dissolution curve was: 95 °C, 1 min; 56 °C, 1 min; from 56 °C for every 0.5 °C for 10 s, and then continuous increase 89 times (to 95 °C).
The abundances of F. pseudograminearum was measured using probe-based qPCR. The Fp_TEF1α.2F/2R primers and Fp_TEF1α.2P probe78 were used to quantify F. pseudograminearum (Table 1). The probe used was double-labeled with 6-carboxyfluorescein (6-FAM) fluorescent reporter dye and Black Hole Quencher® (BHQ) fluorescence quencher. A tenfold dilution series of a plasmid containing the F. pseudograminearum tri5 gene was used to generate a standard curve (the number of copies of the gene ranged from 3.3 × 103 to 3.3 × 107). The qPCR reaction system (20 µL) included 10 µL universal TaqMan premix (Sangon Biotech Co., Ltd., Shanghai, China), 2 µM TaqMan probe 2 µL, 20 µM forward and reverse primers each 1 µL, 2 µL DNF buffer, 3 µL molecular biology grade water and 1 µL of soil sample DNA. The qPCR was carried out using a two-step protocol: Pre-denaturation was at 95 °C for 180 s, denaturation was at 94 °C for 15 s, annealing and extension were at 60 °C for 30 s, with 40 cycles. The procedure for the dissolution curve was: 95 °C, 1 min; 56 °C, 1 min; from 56 °C for every 0.5 °C for 10 s, and then continuous increase 89 times (to 95 °C).
The qPCR reaction procedure was performed using an iCycler iQ5 (Bio-Rad, California, USA) real-time PCR instrument. Each sample was repeated three times, and water was used as a negative control to assess contamination during operation. The copy number of DNA was calculated according to the standard curve.
Standard plasmids were obtained by ligating specific fragments into the pMD18-T vector (Sangon Biotech Co., Ltd., Shanghai, China). A standard curve, based on threshold cycles (Cq), was created for each of the three recombinant plasmids using the corresponding primer pairs (Table 1).
Plasmid copy number calculation:
All primers used in this study were synthesized by Sangon Biotech Co., Ltd., Shanghai, China.
Illumina MiSeq sequencing of fungal ITS genes
We selected 9 DNA samples (3 treatments × 3 replicate samples) for fungal community analysis. In the first amplification reaction, primers with ITS3 (CCAGCASCYGCGGTAATWCC) and ITS4 (ACTTTCGTTCTTGATYRA)79 were used for ITS rDNA amplification, at 0.2 µmol/L primer. This reaction was prepared in a final volume of 30 µL, containing 15 µL of 2 × Taq master Mix (Thermo, New York, NY, USA), 1 µL each of primers (10 µmol/L), and 20 ng of template DNA. Amplification conditions were: 94 °C for 3 min, 5 cycles of amplification of 94 °C for 30 s, 45 °C for 20 s, 65 °C for 30 s, and 20 cycles of amplification of 94 °C for 20 s, 55 °C for 20 s, 72 °C for 30 s, and 20 cycles of amplification, followed by extension at 72 °C for 300 s. The second round of amplification used Illumina bridge PCR compatible primers, with the first round of PCR products as templates. The reaction system was the same as above. Amplification conditions were: 95 °C for 30 s, 95 °C for 15 s, 55 °C for 15 s, 72 °C for 30 s, 5 cycles of amplification, and 72 °C extension for 300 s. After the amplification was completed, the product was purified and quantified with Qubit2.0. According to the measured DNA concentration, the samples were mixed in equal proportions and homogenized to form a sequencing library, which was sequenced on an Illumina MiSeq sequencer by Sangon Biotech Co., Ltd., Shanghai,China.
Bioinformatics analysis
The original data were uploaded to the GSA (Genome Sequence Archive) database to submit and save the original information for sequencing (Login number: CRA003983). The ITS sequences were divided into operational taxonomic units (OTUs) based on the similarity between the sequences, and performs bioinformatics statistical analysis at a similarity level of 97%. Unite80 database was used was used to obtain OTU table with species classification information by blast comparison.
We deleted plant-derived sequences, resulting in 431,514 readings from 9 samples (average of 47,946 readings per sample). In order to obtain an equivalent sequencing depth for later analysis, all samples in the OTU table were parsed into 42,376 sequences.
Statistical analysis
All statistical analyses were performed using the IBM SPSS 20.0 software program (IBM Corporation, New York, USA). Data were analyzed using one-way analysis of variance (ANOVA) with Duncan’s multiple range test, and significant differences were identified with the least significant difference test at p < 0.05 (HSD0.05). The FUNGuild81 database was used to identify fungal OTUs belonging to phytopathogenic fungi and to calculate their relative abundance. R’s vegan software package was used to calculate the alpha diversity index (Shannon index and Observed Richness of OTUs). R’s ggplot2 software package was used to plot principal coordinate (PCoA) analysis. The species annotation results at the phylum level of the OTU table are classified and summed, and the average value is calculated to obtain the community composition map of the fungal community. The number of OTUs associated with predicted phytopathogenic fungi in wheat rhizosphere soil under different treatments was analyzed using MUNA82,83. Cytoscape (3.6.1) was used to generate co-occurrence networks.
Ethics approval
The experimental research and field studies on plants, including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation. The appropriate permissions and/or licenses for collection of plant or seed specimens were obtained for the study.
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Data availability
The datasets generated during and/or analyzed during the current study are available in the GSA (Genome Sequence Archive) database (http://gsa.big.ac.cn/) with the login number of CRA003983.
References
Zhang, X. X. et al. Survey of Fusarium spp. causing wheat crown rot in major winter wheat growing regions of China. Plant Dis. 99, 1610–1615. https://doi.org/10.1094/pdis-04-14-0422-re (2015).
Xu, F. et al. Spatial distribution of root and crown rot fungi associated with winter wheat in the North China plain and its relationship with climate variables. Front. Microbiol. 9, 1054. https://doi.org/10.3389/fmicb.2018.01054 (2018).
Huiyun, Z. et al. Research advances and prospect on wheat sharp eyespot in China. J. Triticeae Crops 027, 1150–1153 (2007).
Haoliang, C. Analysis on occurrence and damage of wheat sheath blight. J. Agric. Catastrophol. 02, 7–12 (2011).
Haifeng, Z., Yun, Y., Yajuan, N., Hongxia, Y. & Honglian, L. Occurrence and control methods of crown rot of wheat. J. Henan Agric. Ences 43, 114–117 (2014).
ShengYing, H. & Gui, Z. Study on the losses of wheat yield and the ELT to wheat root rot. J. Northwest Sci. Tech. Univ. Agric. For. 30, 76–78 (2002).
Bin, W. et al. Identification and pathogenicity of pathogens associated with the wheat crown rot in the southwest of Shandong Province. J. Triticeae Crops 38, 358–365 (2018).
Chengcheng, M., Xiaofengi, S. & Li, Z. Pathogen identification for wheat crown rot in Shandong Province. J. Shandong Agric. .Univ. Nat. Sci. Ed. 50, 753–757 (2019).
Fei, X. et al. Occurrence dynamics and characteristics of Fusarium root and crown rot of wheat in Henan Province during 2013–2016. Plant Prot. 42, 126–132 (2016).
Mudge, A. M. et al. A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol. Mol. Plant Pathol. 69, 73–85 (2006).
Jianrong, S. & Yuzhong, W. Pathogenicity of Rhizoctonia cerealis to Wheat in Jiangsu Province. Jiangsu J. Agric. Sci. 013, 188–190 (1997).
Ying, C. et al. Composition and virulence of pathogen of wheat sharp eyespot in north latitude 33° of China. J. Triticeae Crops 29, 1110–1114 (2009).
Ming, W., Bolin, L., Xiaoping, X. & Hongling, L. Acta Phytopathologica Sinica, 556–560.
Zhengping, L., Lixin, W., Rongxi, L., Caifeng, L. & Yongjie, L. Identification and biological character test of wheat common rot pathogen. Acta Agric. Boreall 17, 44–48 (2002).
Davut Soner, A. & Ali, E. Effect of wheat cultivars, fertilizers, and fungicides on Fusarium foot rot disease of wheat. Turk. J. Agric. For. 40, 101–108 (2016).
Desen, N. International Congress on Engineering and Life Science.
Winter, M., Samuels, P. L., Otto-Hanson, L. K., Dill-Macky, R. & Kinkel, L. L. Biological control of Fusarium crown and root rot of wheat by streptomyces isolates it’s complicated. Phytobiomes J. 3, 52–60. https://doi.org/10.1094/pbiomes-11-18-0052-r (2019).
Mnasri, N. et al. Efficacy of some rhizospheric and endophytic bacteria in vitro and as seed coating for the control of Fusarium culmorum infecting durum wheat in Tunisia. Eur. J. Plant Pathol. 147, 501–515. https://doi.org/10.1007/s10658-016-1018-3 (2017).
El-Gremi, S. M., Draz, I. S. & Youssef, W. A. E. Biological control of pathogens associated with kernel black point disease of wheat. Crop Prot. 91, 13–19. https://doi.org/10.1016/j.cropro.2016.08.034 (2017).
Benderradji, L., Kellou, K., Ghadbane, M., Salmi, M. & Brini, F. Effects of plant growth promoting rhizobacteria (PGPR) on in vitro bread wheat (Triticum aestivum L.) growth parameters and biological control mechanisms. Adv. Microbiol. 6, 677–690 (2016).
Bello, G. M. D., Mónaco, C. I. & Simón, M. R. Biological control of seedling blight of wheat caused by Fusarium graminearum with beneficial rhizosphere microorganisms. World J. Microbiol. Biotechnol. 18, 627–636 (2002).
Dweba, C. C. et al. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 91, 114–122 (2016).
Palazzini, J. M. et al. Biological control of Fusarium graminearum sensu stricto, causal agent of Fusarium head blight of wheat, using formulated antagonists under field conditions in Argentina. Biol. Control 94, 56–61. https://doi.org/10.1016/j.biocontrol.2015.12.009 (2016).
Winter, M., Samuels, P. L., Otto-Hanson, L. K., Dill-Macky, R. & Kinkel, L. L. Biocontrol of Fusarium crown and root rot of wheat by Streptomyces isolates: it’s complicated. Phytobiomes J. 3, 1–52 (2019).
Prasad, V. S. S. K., Davide, G. & Emilio, S. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Ences 19, 952 (2018).
Rocha, I., Ma, Y., Souza-Alonso, P., Vosátka, M. & Oliveira, R. S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 10, 1357 (2019).
Din, G. M. U. et al. Effects of Tilletia foetida on microbial communities in the rhizosphere soil of wheat seeds coated with different concentrations of Jianzhuang. Microb. Ecol. 82, 736–745 (2021).
Sharma, K. K., Singh, U. S., Sharma, P., Kumar, A. & Sharma, L. Seed treatments for sustainable agriculture: A review. J. Appl. Nat. Sci. 7, 521–539 (2015).
Din, G. M. U. et al. Effects of Tilletia foetida on microbial communities in the rhizosphere soil of wheat seeds coated with different concentrations of Jianzhuang. Microb. Ecol. https://doi.org/10.1007/s00248-021-01696-w (2021).
Brunner, K. et al. Improvement of the fungal biocontrol agent Trichoderma atroviride to enhance both antagonism and induction of plant systemic disease resistance. Appl. Environ. Microbiol. 71, 3959–3965 (2005).
Rojo, F. G., Reynoso, M. M., Ferez, M., Chulze, S. N. & Torres, A. M. Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Prot. 26, 549–555 (2007).
Murakami, H., Tsushima, S., Kuroyanagi, Y. & Shishido, Y. Reduction in resting spore density of Plasmodiophora brassicae and clubroot severity by liming. Soil Plant Nutr. 48, 685–691 (2002).
Mukherjee, P. K., Horwitz, B. A., Herrera-Estrella, A., Schmoll, M. & Kenerley, C. M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 51, 105–129 (2013).
Woo, et al. The molecular biology of the interactions between Trichoderma spp. phytopathogenic fungi and plants. Phytopathology 96, 181–185 (2006).
Singh, D., Pande, S. K., Ta, K., Yadav, J. K. & Kumar, S. Bioefficacy of Trichoderma spp. against Bipolaris sorokiniana causing spot blotch disease of wheat and barley. Int. J. Curr. Microbiol. Appl. 7, 2322–2327 (2018).
Rajendraprasad, M., Vidyasagar, B., Devi, G. U., Rao, S. R. K. & Sagar, V. In vitro evaluation of fungicides and biocontrol agents against Rhizoctonia solani in tomato. Int. J. Plant Soil Sci. 17, 1–9 (2017).
Barnett, S., Zhao, S., Ballard, R. & Franco, C. Selection of microbes for control of Rhizoctonia root rot on wheat using a high throughput pathosystem. Biol. Control 113, 45–57 (2017).
Dipietro, A., Lorito, M., Hayes, C., Broadway, R. & Harman, G. Endochitinase from Gliocladium virens: Isolation, characterization, and synergistic antifungal activity in combination with gliotoxin. Phytopathology 83, 308–313 (1993).
Berg, G. et al. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. https://doi.org/10.1093/femsec/fix050 (2017).
Gabriele, B. et al. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 1, 050 (2017).
Liu, C. M. et al. Fluazinam positively affected the microbial communities in clubroot cabbage rhizosphere. Sci. Hortic. https://doi.org/10.1016/j.scienta.2019.05.046 (2019).
Blaalid, R. et al. Changes in the root-associated fungal communities along a primary succession gradient analysed by 454 pyrosequencing. Mol. Ecol. 21, 1897–1908. https://doi.org/10.1111/j.1365-294X.2011.05214.x (2012).
Rakel, B. et al. Changes in the root-associated fungal communities along a primary succession gradient analysed by 454 pyrosequencing. Mol. Ecol. 21, 1897–1908 (2011).
Munir, S. Fluazinam positively affected the microbial communities in clubroot cabbage rhizosphere. Horticulturae 1, 1–10 (2019).
Cheun, H. I., Makino, S. I., Watarai, M., Erdenebaatar, J. & Uchida, I. Rapid and effective detection of anthrax spores in soil by PCR. J. Appl. Microbiol. 95, 728–733 (2010).
Chilvers, M. Development and application of qPCR and RPA genus and species-specific detection of Phytophthora sojae and Phytophthora sansomeana root rot pathogens of soybean. Plant Dis. 101, 1–10 (2017).
Wang, J., Jacobs, J. L., Byrne, J. M. & Chilvers, M. I. Improved diagnoses and quantification of Fusarium virguliforme, causal agent of soybean sudden death syndrome. Phytopathology 105, 378 (2015).
Abdullah, A. S. et al. Real-time PCR for diagnosing and quantifying co-infection by two globally distributed fungal pathogens of wheat. Front. Plant Sci. 9, 1086 (2018).
Cai, X. Y., Zhao, H. H., Liang, C., Li, M. & Liu, R. J. Effects and mechanisms of symbiotic microbial combination agents to control tomato fusarium crown and root rot disease. Front. Microbiol. 12, 973. https://doi.org/10.3389/fmicb.2021.629793 (2021).
Lakhesar, D. P. S., Backhouse, D. & Kristiansen, P. Nutritional constraints on displacement of Fusarium pseudograminearum from cereal straw by antagonists. Biol. Control 55, 241–247. https://doi.org/10.1016/j.biocontrol.2010.09.002 (2010).
Alvindia, D. G. Sodium bicarbonate enhances efficacy of Trichoderma harzianum DGA01 in controlling crown rot of banana. J. Gen. Plant Pathol. 79, 136–144. https://doi.org/10.1007/s10327-013-0432-z (2013).
Kthiri, Z., Jabeur, M. B., Machraoui, M., Gargouri, S. & Hamada, W. Coating seeds with Trichoderma strains promotes plant growth and enhance the systemic resistance against Fusarium crown rot in durum wheat. Egypt. J. Biol. Pest Control 30, 338 (2020).
Powell, W. A., Klingeman, W. E., Ownley, B. H. & Gwinn, K. D. Evidence of endophytic Beauveria bassiana in seed-treated tomato plants acting as a systemic entomopathogen to larval Helicoverpa zea (lepidoptera: noctuidae). J. Entomol. Sci. 44, 391–396. https://doi.org/10.18474/0749-8004-44.4.391 (2009).
Perello, A. E. & Dal Bello, G. M. Suppression of tan spot and plant growth promotion of wheat by synthetic and biological inducers under field conditions. Ann. Appl. Biol. 158, 267–274. https://doi.org/10.1111/j.1744-7348.2011.00460.x (2011).
Ma, Y. et al. Delivery of Inoculum of Rhizophagus irregularis via seed coating in combination with Pseudomonas libanensis for cowpea production. Agronomy https://doi.org/10.3390/agronomy9010033 (2019).
Ye, L. et al. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. https://doi.org/10.1038/s41598-019-56954-2 (2020).
Xiong, W. et al. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol. Biochem. 114, 238–247. https://doi.org/10.1016/j.soilbio.2017.07.016 (2017).
Asghar, W. & Kataoka, R. Effect of co-application of Trichoderma spp. with organic composts on plant growth enhancement, soil enzymes and fungal community in soil. Arch. Microbiol. 203, 4281–4291. https://doi.org/10.1007/s00203-021-02413-4 (2021).
Huang, X. Q. et al. Plant pathological condition is associated with fungal community succession triggered by root exudates in the plant-soil system. Soil Biol. Biochem. https://doi.org/10.1016/j.soilbio.2020.108046 (2020).
Rossmann, M. et al. Multitrophic interactions in the rhizosphere microbiome of wheat: From bacteria and fungi to protists. FEMS Microbiol. Ecol. https://doi.org/10.1093/femsec/fiaa032 (2020).
Illescas, M. et al. Effect of inorganic N top dressing and Trichoderma harzianum seed-inoculation on crop yield and the shaping of root microbial communities of wheat plants cultivated under high basal N fertilization. Front. Plant Sci. https://doi.org/10.3389/fpls.2020.575861 (2020).
Zhou, X., Jia, H., Xin, G. & Wu, F. Effects of vanillin on the community structures and abundances of Fusarium and Trichoderma spp. in cucumber seedling rhizosphere. J. Plant Interact. 13, 45–50 (2018).
Yu, X. X., Zhao, Y. T., Cheng, J. & Wang, W. Biocontrol effect of Trichoderma harzianum T4 on brassica clubroot and analysis of rhizosphere microbial communities based on T-RFLP. Biocontrol Sci. Technol. 25, 1493–1505 (2015).
Li, J., Philp, J., Li, J., Wei, Y. & Wang, Y. Trichoderma harzianum inoculation reduces the incidence of clubroot disease in Chinese cabbage by regulating the rhizosphere microbial community. Microorganisms 8, 1325 (2020).
Ji, S. D., An, Y. B., Zhang, H. F., Wang, Y. C. & Liu, Z. H. Trichoderma biofertilizer (mixTroTha) mediates Malus sieversii resistance to Alternaria alternata. Biol. Control https://doi.org/10.1016/j.biocontrol.2021.104539 (2021).
Conn, V. M. & Franco, C. Effect of Microbial Inoculants on the Indigenous Actinobacterial Endophyte Population in the Roots of Wheat as Determined by Terminal Restriction Fragment Length Polymorphism (Springer, 2021).
Stummer, B. E., Zhang, X. J., Yang, H. T. & Harvey, P. R. Co-inoculation of Trichoderma gamsii A5MH and Trichoderma harzianum Tr906 in wheat suppresses in planta abundance of the crown rot pathogen Fusarium pseudograminearum and impacts the rhizosphere soil fungal microbiome. Biol. Control https://doi.org/10.1016/j.biocontrol.2021.104809 (2022).
Xue, A. G. et al. Effect of seed treatment with novel strains of Trichoderma spp. on establishment and yield of spring wheat. Crop Protect. 96, 97–102. https://doi.org/10.1016/j.cropro.2017.02.003 (2017).
Stummer, B. E., Zhang, Q., Zhang, X., Warren, R. A. & Harvey, P. R. Quantification of Trichoderma afroharzianum, Trichoderma harzianum and Trichoderma gamsii inoculants in soil, the wheat rhizosphere and in planta suppression of the crown rot pathogen Fusarium pseudograminearum. J. Appl. Microbiol. 129, 971–990. https://doi.org/10.1111/jam.14670 (2020).
Ramirez, M. L. et al. Population genetic structure of Gibberella zeae isolated from wheat in Argentina. Food Addit. Contam. A 24, 1115–1120. https://doi.org/10.1080/02652030701546487 (2007).
Lahoz, E., Carrieri, R., Crescenzi, A. & Fanigliulo, A. in 4th International Symposium on Tomato Diseases, 99–105 (2015).
Wang, J. J. et al. qNCLB702, a novel QTL for resistance to northern corn leaf blight in maize. Mol. Breed. https://doi.org/10.1007/s11032-017-0770-1 (2018).
Steinkellner, S., Mammerler, R. & Vierheilig, H. J. Microconidia germination of the tomato pathogen Fusarium oxysporum in the presence of root exudates. Mol. Breed. 1, 23–30 (2005).
Quarantine. GB/T 17980.108-2004 (Beijing: Chinese Standard Publishing House, 2004).
Moya-Elizondo, E. A., Jacobsen, B. J., Hogg, A. C. & Dyer, A. T. Population dynamics between Fusarium pseudograminearum and Bipolaris sorokiniana in wheat stems using real-time qPCR. Plant Dis. 95, 1089–1098 (2011).
White, T. J., Bruns, S., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1, 315–322 (1990).
Zitnick-Anderson, K., Simons, K. & Pasche, J. S. Detection and qPCR quantification of seven Fusarium species associated with the root rot complex in field pea. Can. J. Plant Path. 40, 261–271. https://doi.org/10.1080/07060661.2018.1429494 (2018).
Knight, N. L., Sutherland, M. W., Martin, A. & Herde, D. J. Assessment of infection by Fusarium pseudograminearum in wheat seedling tissues using quantitative PCR and a visual discoloration scale. Plant Dis. 96, 1661–1669 (2012).
Okubo, A. & Sugiyama, S. I. Comparison of molecular fingerprinting methods for analysis of soil microbial community structure. Ecol. Res. 24, 1399–1405 (2009).
Uroz, S. et al. Functional assays and metagenomic analyses reveals differences between the microbial communities inhabiting the soil horizons of a Norway spruce plantation. PLoS ONE https://doi.org/10.1371/journal.pone.0055929 (2013).
Nguyen, N. H. et al. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248. https://doi.org/10.1016/j.funeco.2015.06.006 (2016).
Deng, Y. et al. Molecular ecological network analyses. BMC Bioinform. https://doi.org/10.1186/1471-2105-13-113 (2012).
Deng, Y. et al. Molecular ecological network analyses. BMC Bioinform. 13, 113. https://doi.org/10.1186/1471-2105-13-113 (2012).
Acknowledgements
This work was supported by the Grains Research and Development Corporation, the Department of Trade, Tourism and Investment of the South Australian Government, and The University of Adelaide.
Funding
This research was funded by the Provincial Key Research and Development Project (major scientific and technological innovation) from Shandong Province (Project ID:2019JZZY010718, 2020CXGC010803), the National Key Research and Development Program of China (Grant Number: 2017YFD0201700, 2017YFD0201102), the International Technology Cooperation Project from Shandong Academy of Sciences (Grant Number: 2019GHZD11), Natural Science Foundation of Shandong Province of China (Grant Number: ZR2020QC044).
Author information
Authors and Affiliations
Contributions
Y.W. (Yanli Wei)and J.H. designed the experiment; L.S., J.L. (Junhui Li) and J.H. analyzed data and wrote the first draft; K.Y., H.L., L.L., J.L. (Jishun Li), M.R., R.T., Y.Z. and M.D. were involved in formal analysis; J.L. (Jishun Li) gave the funding support; J.P. and Y.W. (Yan Wang) reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sui, L., Li, J., Philp, J. et al. Trichoderma atroviride seed dressing influenced the fungal community and pathogenic fungi in the wheat rhizosphere. Sci Rep 12, 9677 (2022). https://doi.org/10.1038/s41598-022-13669-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13669-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.