Abstract
To avoid brain damage in newborn infants, effective tools for prevention of excessive neonatal hyperbilirubinemia are needed. The objective of this study was to evaluate a new transcutaneous bilirubinometer (JAISY). For this purpose, 930 bilirubin measurements were performed in 141 newborn infants born near-term or at term (gestational age 35–41 weeks; postnatal age 1–6 days; 71 boys; including 29 infants with darker skin) and compared to those of a previously validated instrument (JM105). In each infant, the mean of three repeated measurements in the forehead was calculated for each instrument, followed by a similar measurement on the chest. The bilirubin values varied between 0 and 320 µmol/l (0–18.8 mg/dl). There was a high degree of agreement with significant correlations between bilirubin values measured with the two devices on the forehead (Pearson’s r = 0.94, p < 0.001) and the chest (r = 0.94, p < 0.001). The correlations remained after stratifying the data by gestational age, postnatal age and skin color. The coefficient of variation for repeated bilirubin measurements was 8.8% for JAISY and 8.0% for JM105 (p = 0.79). In conclusion, JAISY provides accurate and reproducible information on low to moderately high bilirubin levels in newborn infants born near-term or at term.
Similar content being viewed by others
Introduction
Neonatal jaundice is common and results in the clear majority from a transient and physiological accumulation of bilirubin. In a small proportion of infants, however, neonatal hyperbilirubinemia may become excessive which ultimately could cause brain damage1,2,3,4. Although a preventable condition, there have been some reports on increasing numbers of disabling neonatal hyperbilirubinemia5,6 and this condition is still a major cause of lifelong neurodevelopmental impairment with high costs for the affected family and the society4,7. While failed prevention may have different causes, inability to measure bilirubin, untimely measurements, and absence of repeated bilirubin measurements have been identified as important safety flaws5.
Guidelines for care of healthy newborn infants include screening and management of neonatal hyperbilirubinemia8,9,10,11,12,13,14. Several guidelines recommend that all infants have at least one bilirubin determination early after birth, and that the prognostic information of that bilirubin value in addition to known risk factors for neonatal hyperbilirubinemia15,16 should be used to determine when the next bilirubin measurement should take place. To avoid painful blood sampling and to have a quick result bedside, non-invasive, transcutaneous (t.c.) bilirubin determinations have reached widespread use as first-line screening tools in high-resource settings17,18,19.
Although judged as cost-effective20, t.c. bilirubin measurements in newborn infants are expensive21 which limits availability outside high-resource settings. Even within hospitals, there are usually only a few instruments. Given that many families nowadays are discharged home by the time neonatal hyperbilirubinemia peaks, there is an emerging need for accurate bilirubinometers for outpatient care and home monitoring. For this purpose, a new and simple t.c. bilirubinometer was developed. The objective of this study was to evaluate this device. The hypothesis tested was that the new bilirubinometer would be feasible for an intended use in near-term or term neonates, and that the test-device would perform as accurately as established standard instruments.
Methods
The protocol of this study and an application was submitted and scrutinized by the Ethics Review Authority in Sweden before the study was executed. Because no personal information (no personal identities, dates or names) was collected and because t.c. bilirubin measurements were considered as part of the routine neonatal management, the Ethics Review Authority did not find that the project involved research according to §§3–4 of the Swedish Ethical Review Act, and that the study could be executed without a formal approval by the Authority. Informed consent for the measurements and data collection was obtained from all parents.
Participants and setting
This study was executed at the delivery, maternity (the well-baby nursery) and neonatal units (in family rooms, no intensive care admissions were included) at Karolinska university hospital, Stockholm, Sweden between October and December 2020. Inclusion criteria were a gestational age of 35 weeks or more, a postnatal age less than 10 days, and newborn infants who had not received phototherapy or exchange transfusion for hyperbilirubinemia. Infants with major malformations or ongoing neonatal morbidity were not considered eligible for this study. All infants included in this study were tested for bilirubin levels as part of the clinical routine following the national Swedish guidelines issued in 201922. Clinical characteristics of the mothers and their infants (n = 141) were collected to describe the study sample.
Study protocol
The t.c. bilirubin measurements were performed by tester 1, a specialist neonatal nurse (n = 45) and by tester 2, a midwife (n = 96). Both testers were familiar with the DRÄGER jaundice meter (JM) 105 (Drägerwerk AG & Co, Lübeck, Germany) used in clinical routine care at Karolinska university hospital for t.c. bilirubin determinations. Tester 1 had a more thorough introduction to JAISY and was assisted by one of the authors (HBC) while using it, whereas tester 2 was more briefly introduced to JAISY after which she did all measurements on her own. Tester 1 performed measurements without any time pressure in the maternity units whereas tester 2 worked with families having scheduled follow-up appointments in which bilirubin screening was one of several items to attend to. The performance of tester 2—i.e., her ability to achieve reproducible measurements—was assessed after 70 measurements. This assessment was then presented to tester 2 before she continued the protocol.
Each infant had t.c. bilirubin levels determined at the forehead and at the chest. At each site, the mean of three subsequent t.c. bilirubin determinations was calculated for each instrument. In 14 of the 141 infants, a second test was performed.
The JM105 was considered gold standard and used according to the manufacturer's manual. The measuring principle of the JM105 has been described elsewhere23 and the instrument had been calibrated at the factory before use. According to the product information, the measurement range was 0–340 μmol/L (0.0–20.0 mg/dL) and the accuracy was ± 25.5 μmol/L (± 1.5 mg/dL) for infants > 35 weeks of gestational age24. The JM103—a precursor to the JM105—has shown good–excellent agreement and correlation (correlation coefficients varying between 0.77 and 0.96) with laboratory instruments for determination of serum bilirubin in newborn infants25,26,27,28.
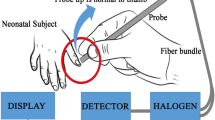
A prototype of the new device JAISY was constructed. It consisted of a measuring probe with a size of 40 × 15 mm, powered by and connected to an ordinary cell phone. The measuring probe was held against the infant’s skin and a light flash was activated by a command on the cellphone screen. The light source was selected based on its wavelength range. Light absorption in bilirubin, hemoglobin, and melanin are wavelength dependent with characteristic variations in the visible spectrum range between 400 and 700 nm. Therefore, white and blue LEDs were chosen considering that they have suitable spectra. The emitted light was absorbed and reflected in the skin. The reflected light was detected and the spectrum of the reflected light was measured by photosensors placed at the tip of the probe. The reflected light was converted into electronic signals by the photosensor. JAISY determined the t.c. bilirubin level of the infant by photospectroscopy. The result could be displayed on the cellphone screen in μmol/L (or in mg/dl) but for the purpose of this study the measuring result was not displayed. Both devices are shown in Fig. 1.
The JAISY device was used in the same way as described for the JM105 with three consecutive measurements at each skin site. The forehead was in all infants measured before the chest, whereas the order of the measurements was randomly altered between the two instruments. The testers were blinded to the test result of JAISY and the instrument displayed only a 6-digit number which was noted in the study protocol. Every measured t.c. bilirubin value and the linked 6-digit number were automatically transmitted from the cellphone to a central database.
Statistical analyses
For descriptive purposes, mean (standard deviation [SD]) values and numbers (proportions [%]) were used. To assess the validity of the new instrument, a mean bilirubin value was calculated from each set of three consecutive t.c. measurements per skin site and instrument. Based on these mean values, Pearsons´s correlation coefficients were calculated and Bland–Altman plots were constructed for t.c. bilirubin values using both methods. To assess reproducibility, the coefficient of variation (CV) for repeated measurements were calculated for each instrument and skin site, and for each tester. Chi-square test was used to test for differences between CVs. To describe associations between t.c. bilirubin measurements and gestational age, postnatal age, skin color and investigator, a series of stratified analyses were performed. All calculations were performed with the software Excel (Google drive) and Matlab R2020a.
Results
Overall, 930 t.c. bilirubin measurements were performed in 141 infants. In 14 infants, a second measurement was performed. Clinical characteristics of the participants and their mothers are presented in Table 1.
The t.c. bilirubin measured with JM105 at the forehead varied between 0 and 302 μmol/L (0–17.8 mg/dL), and at the chest it varied between 0 and 310 μmol/L (0–18.2 mg/dL), i.e., all bilirubin values were within the measurement range of the instrument. There was a high correlation between t.c. bilirubin values assessed with JM105 and JAISY at the forehead (r = 0.94, p < 0.001) and the chest (r = 0.94, p < 0.001) with high degree of agreement (β = 0.97 at the forehead and 0.92 at the chest), Fig. 2. The Bland–Altman plot confirmed the agreement between the two instruments over the full range of t.c. bilirubin values, Fig. 3.
Correlation between t.c. bilirubin values (μmol/L) measured with JM105 and JAISY in the chest (β = 0.92, r = 0.94, p < 0.001) and forehead (β = 0.97, r = 0.94, p < 0.001) of 141 newborn infants (gestational age 35–41 weeks, postnatal age 1–6 days). Filled circles in blue = tester 1 and unfilled circles = tester 2.
The overall mean (± SD) t.c. bilirubin concentrations using JM105 were 165 (± 79) μmol/L at the forehead and 161 (± 80) μmol/L at the chest. The corresponding bilirubin values for JAISY were 168 (± 81) and 153 (± 82) μmol/L, respectively. The mean differences (JM105 minus JAISY) in t.c bilirubin were − 3.1 (95% CI: − 7.2 to + 1.0) μmol/L at the forehead and 7.8 (95% CI 3.7–11.9) μmol/L at the chest.
The overall coefficient of variation (SD for within subject variation/overall mean value) for repeated measurements with JM105 was 12.8/165 = 7.7% at the forehead and 11.1/161 = 6.9% at the chest (p = 0.78 for difference between measuring site). The corresponding overall CVs for JAISY were 20/168 (11.9%) at the forehead and 21/153 (13.6%) at the chest (p = 0.67 for difference between sites).
Since there was no difference in CVs related to measuring site, measurements from forehead and chest were lumped together in a sub-analysis of reproducibility related to tester. The CVs for tester 1 (n = 45 babies, longer introduction for JAISY, assisted during measurements and no time pressure) were 9.9/126 = 7.8% for JM105 and 8.8/126 = 7.0% for JAISY (p = 0.81 for difference between instruments). The corresponding CVs for tester 2 working alone and under clinical conditions were for her first 70 babies 9.9/173 = 5.8% for JM105 and 25.7/167 = 15.3% for JAISY (p = 0.009 for difference). After discussing the performance with tester 2 and instructing her more carefully on how to hold the probe during the measurements to avoid inlet of ambient light, the CVs for her last measurements (n = 26 babies) were 15.4/177 = 8.7% for JM105 and 18.9/184 = 10.3% for JAISY (p = 0.64 for difference). Including only measurements obtained after proper education and instruction (n = 45 infants performed by tester 1 and last 26 infants by tester 2), there were no significant difference in reproducibility between the two instruments—the CV for repeated measurements was 12.3/154 = 8.0% for JM105 and the corresponding CV for JAISY was 13.6/154 = 8.8% (p = 0.79).
The correlation coefficients between t.c. bilirubin values measured with JM105 and JAISY stratified by gestational age varied between 0.88 and 1.0, Fig. 4. The corresponding correlation coefficients between the two methods stratified by postnatal age varied between 0.84 and 0.98, Fig. 5. The correlation coefficient between t.c. bilirubin values measured with JM105 and JAISY in infants with white skin color was 0.95 at the forehead and 0.96 at the chest, and in infants with darker skin it was 0.92 at the forehead and 0.93 at the chest.
There were no adverse events during the measurements and a clear majority (n = 138/141 infants) were calm or sleeping during the measurements. Irrespective of device used, it took approximately 1 min to achieve three repeated measurements.
Discussion
This study demonstrated that the JAISY device exhibited excellent agreement with a validated method used for determination of t.c. bilirubin in newborn infants. The reproducibility of JAISY was high and did not differ from that of JM105. JAISY was simple to use and feasible in neonates.
Irrespective of gestational age, the correlation coefficients between JM105 and JAISY were high. This suggests that JAISY can provide reliable information about bilirubin levels in term and near-term infants25,26,28 in line with national recommendations for use of t.c. bilirubinometers8,14.
The present findings support that t.c. bilirubin measurements are reliable for screening purposes on the first day of postnatal life29. Postnatal age did not significantly influence the methods agreement although on the first day of life, the correlation coefficients between bilirubin levels assessed with JM105 and JAISY were somewhat lower (r = 0.84–0.88) than in older infants. The most likely explanation for this finding is that bilirubin values were significantly lower on day 1 than in older infants with less accurate precision in both instruments at lower bilirubin values.
T.c. bilirubinometers—including JM103—has previously been reported to provide reliable estimates of bilirubin in infants with dark or brown skin30,31 although one report found lower agreement between serum and t.c. bilirubin values in people with darker skin than in those with lighter skin color26. Given the findings reported herein, JAISY can also be used in infants with different skin colors.
The methods agreement and reproducibility were unrelated to skin site, the forehead or the chest. This was expected because the two methods should provide a valid estimate of the same variable, i.e., the total serum-bilirubin, irrespective of skin site. Comparisons with total serum-bilirubin determinations have suggested that t.c. measurements at the chest are more reliable than at the forehead, especially 1–2 days after birth31,32,33. This study did not include serum-bilirubin values and cannot refute nor support those reports.
Similar to other t.c. bilirubin instruments, JAISY was shown to provide a simple and rapid way of obtaining an immediate result for bilirubin levels in neonates. The small size of the instrument makes wearing and disinfection easy and the connection to a standard cell phone enables data transfer to internal or external decision aids. These features should facilitate in- and outpatient bilirubin monitoring.
In order to estimate an impact of handling errors in a clinical situation in which staff in neonatal care would be introduced to the new instrument without much training, we introduced tester 2 more briefly than tester 1 (both testers were blinded to their reproducibility during measurements). We found that tester 2 initially performed less reproducible measurements than tester 1. Therefore and for proper use and reliable test results, users need clear handling instructions and some basic practice.
Although rare, 10/282 (3.5%) t.c. bilirubin values estimated by JAISY deviated 2SD (50 μmol/L) or more from JM105, and in 9 out of 10 of these deviations the bilirubin assessed with JAISY was lower than that assessed by JM105. The JAISY t.c. bilirubin values that were more than 2 SD below the mean were in 9/10 measurements assessed by tester 2 and before she had had feedback on performance. The most likely explanation was that ambient light had been diluting the reflected light from the skin because of suboptimal skin contact with the probe. After instruction on how to handle the probe, recommending tester 2 a closer skin contact to exclude incoming ambient light, the accuracy and reproducibility of tester 2 improved and became comparable to tester 1.
The strengths of this study include application of the JAISY instrument in a context of its intended use, i.e., in clinical practice with different users, including infants of different gestational and postnatal ages and with different skin colors. The users were blinded to the JAISY test-result which excluded assessment bias. A sufficiently large number of measurements was performed to cover the range of bilirubin levels commonly seen in newborn infants and to assess associations to gestational and postnatal age.
Limitations include selection of near-term and term infants only, whereas preterm infants were excluded. Moreover, the JAISY device was not evaluated in infants undergoing phototherapy for neonatal hyperbilirubinemia or with bilirubin values exceeding 310 μmol/L (18.2 mg/dL). The comparison of bilirubin values did not include blood test for determination of serum-bilirubin. Transcutaneous bilirubinometers should be considered as screening tools and critically high bilirubin values, as well as decisions on treatment of hyperbilirubinemia should always be validated by determinations of the concentration of total bilirubin in serum. However, determinations of t.c. bilirubin with JM103 have repeatedly been reported to exhibit good agreement with laboratory analyses of bilirubin from blood tests, at least up to 250 μmol/L (14.7 mg/dL)25,26,27,28. JAISY is not yet commercially available and pricing remains to be established after regulatory approvals, production and marketing expenditures have been taken into account.
In conclusion, JAISY provides accurate and reproducible information on low to moderate bilirubin levels in newborn infants born near-term or at term.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ebbesen, F. et al. Extreme hyperbilirubinaemia in term and near-term infants in Denmark. Acta Paediatr. 94, 59–64 (2005).
Johnson, L., Bhutani, V. K., Karp, K., Sivieri, E. M. & Shapiro, S. M. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J. Perinatol. Off. J. Calif. Perinat. Assoc. 29(Suppl 1), S25-45. https://doi.org/10.1038/jp.2008.211 (2009).
Kuzniewicz, M. W. et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics 134, 504–509. https://doi.org/10.1542/peds.2014-0987 (2014).
Slusher, T. M. et al. Burden of severe neonatal jaundice: A systematic review and meta-analysis. BMJ Paediatr. Open 1, e000105. https://doi.org/10.1136/bmjpo-2017-000105 (2017).
Alken, J., Hakansson, S., Ekeus, C., Gustafson, P. & Norman, M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw. Open 2, e190858. https://doi.org/10.1001/jamanetworkopen.2019.0858 (2019).
Ebbesen, F. Recurrence of kernicterus in term and near-term infants in Denmark. Acta Paediatr. 89, 1213–1217 (2000).
Olusanya, B. O., Kaplan, M. & Hansen, T. W. R. Neonatal hyperbilirubinaemia: A global perspective. Lancet Child Adolesc. Health 2, 610–620. https://doi.org/10.1016/S2352-4642(18)30139-1 (2018).
American Academy of Pediatrics Subcommittee on, H. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316 (2004).
Bhutani, V. K. et al. Management of jaundice and prevention of severe neonatal hyperbilirubinemia in infants >or=35 weeks gestation. Neonatology 94, 63–67. https://doi.org/10.1159/000113463 (2008).
Bratlid, D., Nakstad, B. & Hansen, T. W. National guidelines for treatment of jaundice in the newborn. Acta Paediatr. 100, 499–505. https://doi.org/10.1111/j.1651-2227.2010.02104.x (2011).
Bhutani, V. K. et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J. Pediatr. 162, 477–482.e471. https://doi.org/10.1016/j.jpeds.2012.08.022 (2013).
Newman, J. Re: Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks' gestation)—Summary. Paediatr Child Health 2007;12(5):401–7. Paediatr Child Health 12, 613. https://doi.org/10.1093/pch/12.7.613 (2007).
Olusanya, B. O. et al. Management of late-preterm and term infants with hyperbilirubinaemia in resource-constrained settings. BMC Pediatr. 15, 39. https://doi.org/10.1186/s12887-015-0358-z (2015).
(NICE). National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. (2016).
Lee, B. K. et al. Haemolytic and nonhaemolytic neonatal jaundice have different risk factor profiles. Acta Paediatr. 105, 1444–1450. https://doi.org/10.1111/apa.13470 (2016).
Norman, M., Aberg, K., Holmsten, K., Weibel, V. & Ekeus, C. Predicting nonhemolytic neonatal hyperbilirubinemia. Pediatrics 136, 1087–1094. https://doi.org/10.1542/peds.2015-2001 (2015).
De Luca, D., Romagnoli, C., Tiberi, E., Zuppa, A. A. & Zecca, E. Skin bilirubin nomogram for the first 96 h of life in a European normal healthy newborn population, obtained with multiwavelength transcutaneous bilirubinometry. Acta Paediatr. 97, 146–150. https://doi.org/10.1111/j.1651-2227.2007.00622.x (2008).
Engle, W. D., Jackson, G. L. & Engle, N. G. Transcutaneous bilirubinometry. Semin. Perinatol. 38, 438–451. https://doi.org/10.1053/j.semperi.2014.08.007 (2014).
Jain, M., Bang, A., Tiwari, A. & Jain, S. Prediction of significant hyperbilirubinemia in term neonates by early non-invasive bilirubin measurement. World J. Pediatr. 13, 222–227. https://doi.org/10.1007/s12519-016-0067-1 (2017).
McClean, S., Baerg, K., Smith-Fehr, J. & Szafron, M. Cost savings with transcutaneous screening versus total serum bilirubin measurement for newborn jaundice in hospital and community settings: A cost-minimization analysis. CMAJ Open 6, E285–E291. https://doi.org/10.9778/cmajo.20170158 (2018).
De Luca, D. et al. Using BiliCheck for preterm neonates in a sub-intensive unit: Diagnostic usefulness and suitability. Early Hum. Dev. 83, 313–317. https://doi.org/10.1016/j.earlhumdev.2006.06.006 (2007).
Alkén, J., A. F., Andersson, O., Odelberg-Johnson, P., Bohlin Blennow, K. & Norman, M. Vårdprogram Neonatal Hyperbilirubinemi (Swedish National Guideline on Hyperbilirubinemia). (2019).
el-Beshbishi, S. N., Shattuck, K. E., Mohammad, A. A. & Petersen, J. R. Hyperbilirubinemia and transcutaneous bilirubinometry. Clin Chem 55, 1280–1287. https://doi.org/10.1373/clinchem.2008.121889 (2009).
DRÄGER Jaundice Meter JM105, Product information.
Engle, W. D., Jackson, G. L., Stehel, E. K., Sendelbach, D. M. & Manning, M. D. Evaluation of a transcutaneous jaundice meter following hospital discharge in term and near-term neonates. J. Perinatol. Off. J. Calif. Perinat. Assoc. 25, 486–490. https://doi.org/10.1038/sj.jp.7211333 (2005).
Maisels, M. J. et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics 113, 1628–1635. https://doi.org/10.1542/peds.113.6.1628 (2004).
Grohmann, K. et al. Bilirubin measurement for neonates: Comparison of 9 frequently used methods. Pediatrics 117, 1174–1183. https://doi.org/10.1542/peds.2005-0590 (2006).
Raimondi, F. et al. Measuring transcutaneous bilirubin: A comparative analysis of three devices on a multiracial population. BMC Pediatr. 12, 70. https://doi.org/10.1186/1471-2431-12-70 (2012).
Samanta, S. et al. The value of Bilicheck as a screening tool for neonatal jaundice in term and near-term babies. Acta Paediatr. 93, 1486–1490. https://doi.org/10.1080/08035250410033042 (2004).
Bhutani, V. K. et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 106, E17. https://doi.org/10.1542/peds.106.2.e17 (2000).
Chimhini, G. L. T., Chimhuya, S. & Chikwasha, V. Evaluation of transcutaneous bilirubinometer (DRAEGER JM 103) use in Zimbabwean newborn babies. Matern. Health Neonatol. Perinatol. 4, 1. https://doi.org/10.1186/s40748-017-0070-0 (2018).
Holland, L. & Blick, K. Implementing and validating transcutaneous bilirubinometry for neonates. Am. J. Clin. Pathol. 132, 555–561. https://doi.org/10.1309/AJCPN9BMFW8COTWP (2009).
Yamauchi, Y. & Yamanouchi, I. Transcutaneous bilirubinometry: Effect of postnatal age. Acta Paediatr. Jpn. 33, 663–667. https://doi.org/10.1111/j.1442-200x.1991.tb01883.x (1991).
Acknowledgements
We thank neonatal specialist nurse Kristina Jonsson and midwife Helena Rissanen for valuable contributions and collecting the data, and Yunus Karamavus, Ph.D., for design and production of the prototype of JAISY in collaboration with Hasan Basri Celebi.
Funding
Open access funding provided by Karolinska Institute. This study was supported by grants from Sweden’s innovation agency Vinnova (JAISY Health AB, Grant Number: 2019-03826). The funding bodies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
H.B.C. had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and study design: all authors. Acquisition of data: H.B.C. and M.N.. Statistical analysis: all authors. Interpretation: all authors. Drafting of the manuscript: M.N. Critical revision of the manuscript for important intellectual content: all authors. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
All three authors have shares in Jaisy Health AB, a small start-up innovation company.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Norman, M., Aytug, H. & Celebi, H.B. Evaluation of a new transcutaneous bilirubinometer in newborn infants. Sci Rep 12, 5835 (2022). https://doi.org/10.1038/s41598-022-09788-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09788-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.