Abstract
The role of β-catenin and Dickkopf-1 (DKK1) is dependent on the specific immunobiology of T cell inflammation in biliary tract cancer (BTC). We aimed to analyze the role of DKK1 or β-catenin as a prognostic factor in BTC, and determine the clinical associations of ß-catenin and DKK1 with CD8+ tumor-infiltrating lymphocytes (TIL). We used data from The Cancer Genome Atlas Research Network and the clinicopathological data of 145 patients with BTC who had undergone primary radical resection between 2006 and 2016. CD8+ TIL expression was a significant predictor of favorable overall survival (OS) and relapse-free survival (RFS) (median OS, 34.9 months in high-TIL, 16.7 months in low-TIL, P < 0.0001 respectively; median RFS, 27.1 months in high-TIL, 10.0 months in low-TIL, P < 0.0001 respectively). In the high-CD8+ TIL BTC group, the tumor expression of β-catenin and DKK1 had a significant negative impact on either OS or RFS. In the low-TIL BTC group, there were no differences according to ß-catenin and DKK1 expression. Cox regression multivariate analysis demonstrated that CD8+ TIL and β-catenin retained significant association with OS. Among patients with resected BTC, the β-catenin and DKK1 protein and high CD8+ TIL levels were associated with poor and good clinical outcomes, respectively.
Similar content being viewed by others
Introduction
Biliary tract cancer (BTC) accounts for approximately 3% of all gastrointestinal malignancies and is the most common hepatobiliary cancer after hepatocellular carcinoma1. BTC is a fatal and rare adenocarcinoma that arises in the biliary tree, including the intrahepatic (ICC) and extrahepatic (ECC) bile duct and gallbladder (GBC)2,3. Surgical resection is the primary treatment modality for early-stage BTC, but the majority of patients still develop a recurrence4. Further, the mortality rate is high primarily because patients are diagnosed at a late stage5. Despite recent advancements in our understanding of the molecular biology of BTC, limited progress has been made in cytotoxic systemic therapy for this disease. Agents targeting IDH1, fibroblast growth factor receptor, and EGFR have demonstrated only limited efficacy5,6,7. In addition, immunotherapy has minimal usefulness in BTC, showing only modest benefits in advanced BTC patients with deficient mismatch repair and high microsatellite instability status8. Therefore, new therapeutic molecular studies are required to improve the survival of patients by overcoming the heterogeneity of BTC.
The Wnt/β-catenin signaling cascade controls cell proliferation, cell polarity, and cell fate during embryonic development and homeostasis in human tissues9. The mutational status of this pathway’s components is highly relevant to the pathogenesis of BTC2. Particularly, activation of the Wnt pathway has been shown to be associated with chemoresistance and metastatic spread in ECC and ICC10,11. Different mutations found in ECC and ICC sequencing are known to act on Wnt/β-catenin to induce chromosomal instability and oncogenesis12,13. Some genetic variants in the Wnt/β-catenin pathway are associated with decreased apoptosis of GBC and influenced susceptibilities2.
Dickkopf-related protein1 (DKK1), the most well-known Wnt antagonist of the canonical Wnt/β-catenin pathway, is a 35-kDa protein that contains a secreted signal peptide sequence. DKK1 can inhibit Wnt signaling by interacting with the Wnt receptors of the Frizzled family14. DKK1 plays a role in cancer proliferation, invasion, and growth via the modulation of Wnt signaling14,15. DKK1 is expressed in multiple tumor types, including BTC, but its impact and the clinical role of this pathway’s components are not completely understood9,16. Recent studies have suggested that high DKK1 expression in BTC is associated with immunosuppressive conditioning through myeloid-derived suppressor cells(MDSC) and tumor-associated macrophages17. It is also associated with high expression of matrix metalloproteinase 9 proteins, which play a role in tumor cell invasion, angiogenesis, and lymph node metastasis in BTC and are correlated with poor prognosis15,16,18.
Given that BTC is constantly exposed to intestinal microbial products, it has both the immune tolerance capability to suppress inappropriate inflammatory responses and the immune capability to protect against harmful stimuli, such as infection and tumor19,20,21. Several studies have demonstrated that BTC contains abundant tumor-infiltrating lymphocytes (TILs) and cancer-associated fibroblasts in the tumor microenvironment (TME)19,21. In addition, Wnt/β-catenin signaling and DKK1 expression are associated with immunosuppressive modulation and protumoral conditioning in BTC through several mechanisms19, including activation of innate immune cells such as tumor-associated macrophages or MDSCs21,22,23 or loss of effector functions by T cell de-differentiation24. However, the clinical implications for β-catenin according to tumor infiltrative immune cells and DKK1, as an antagonist of Wnt/β-catenin signaling, remains controversial. Therefore, this study aimed to investigate the role of DKK1 and β-catenin as prognostic factors in BTC with reference to CD8+ TILs.
Materials and methods
Wnt/β-catenin gene expression according to CD8 in The Cancer Genome Atlas BTC database manuscript formatting
This retrospective study analyzed data from The Cancer Genome Atlas (TCGA) Research Network to identify the associations of Wnt/β-catenin gene expression in CD8 cells and clinical outcomes in BTC. mRNA expression data and clinical data were downloaded from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/). All mRNA expression data were log2-transformed. The expressions of CD8A, DKK1, and CTNNB1 genes were analyzed in 45 samples.
Study population. To validate the findings from TCGA BTC datasets, we analyzed the clinicopathological data of 145 patients with BTC who had undergone primary radical resection between 2006 and 2016 at Uijeongbu St. Mary’s Hospital of the Catholic University of Korea. BTC was classified into ICC; ECC; and GBC, including perihilar cholangiocarcinoma and distal common bile duct cancer. The inclusion criteria were (1) presence of pathologically confirmed biliary tract adenocarcinoma, (2) treatment by radical resection without preoperative radiation or chemotherapy, and (3) availability of paraffin-embedded tumor specimens. Postoperative pathological staging was based on the American Joint Committee on Cancer staging criteria, 8th edition. Patient data, including age, sex, date of diagnosis, recurrence, and death, were retrieved from the electronic medical records.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections. Whole tissue sections of representative tumor samples were used for antigen retrieval and incubated with human-specific antibodies against DKK1 (1: 200, Abcam, Cambridge, UK) and β-catenin (1:100, Cell Signaling, Danvers, MA, USA). The intensity of DKK1 staining was scored as follows: 0, no staining; 1, weakly positive; 2, moderately positive; and 3, strongly positive. The proportion of DKK1-stained cells was calculated as the percentage of positive tumor cells. H scores, ranging from 0 to 300, were determined by multiplying the intensity score by the proportion of DKK1-stained cells. For the characterization of the inflammatory components, sections were incubated with primary antibodies: CD8 (1:100, C8/144B, Dako, Cambridge, UK) following the manufacturer’s instructions. ß-catenin protein immunoreaction was considered positive if more than 10% of tumor cells showed nuclear and cytoplasmic staining9. The cut-off H score for high DKK1 expression was set at 80 using maximally selected rank statistics (quartile) calculated using R statistical programming, version 3.2.3 (https://www.r-project.org/), and high CD8 positivity was set at 32 (median), following previous reference articles9,25.
Statistical analysis
Categorical variables were compared using the chi-square test and Fisher’s exact test, as needed. Overall survival (OS) was defined as the time from diagnosis to any-cause death or the last follow-up. Relapse-free survival (RFS) was calculated from the date of diagnosis to the date of the first distant or local disease recurrence or the last follow-up. Survival curves were generated using the Kaplan–Meier method and compared using the log-rank test by GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA, USA). Cox proportional hazards regression models were used to identify the significance of prognostic factors. Survival rates and hazard ratios (HRs) were shown with their respective 95% confidence intervals (CIs). All statistical analyses were performed using R statistical programming (version 3.4.1) and the SPSS software package (version 23, SPSS, Chicago, IL, USA). A two-sided P-value of < 0.05 was considered statistically significant in all tests and models.
Ethics approval and consent to participate
This study was approved by the Institutional Research Ethics Board of Uijeongbu St. Mary’s Hospital of the Catholic University of Korea (UC15SISI0155) and adhered to the Declaration of Helsinki. Written informed consent was obtained from all subjects involved in the study. Patient anonymity was preserved.
Results
Gene expression in TCGA
Neither DKK1 nor CTNNB1 expression was associated with CD8A gene expression (Supplementary Fig. S1). There was no significant relationship between DKK1 and CTNNB1 gene expression, even in the analysis stratified by CD8A expression (Supplementary Fig. S2). However, high CD8A and CTNNB1 gene expressions were correlated with clinical outcomes, as expected (Supplementary Fig. S3). Interestingly, the clinical role of the CTNNB1 gene was only observed in patients with high CD8A gene expression (Supplementary Fig. S4). This showed that low β-catenin expression tends to have a good prognostic role only in high CD8+ T cell conditioning. β-catenin signaling and DKK1 expression indicated mutual and exclusively poor clinical outcomes, and that immunobiology, such as CD8+ T cells, was involved in the process.
Patient characteristics
Baseline characteristics of the 145 patients are summarized in Table 1. The median age was 67.1 years (range 31–87 years), and 46.9% of the patients were male. Among them, 64.8% (n = 94), 22.8% (n = 33), and 12.4% (n = 18) had GBC, ECC, and ICC, respectively. Pathologically, 52 (35.9%) patients had stage T1 or T2 disease; 28 (19.3%) patients, ≥ N1 and 72 (49.7%) patients, pathological stage III or IV. The median follow-up period after curative surgical resection was 32.1 months (range 2.4 to 138.3 months). Overall, 86 (59.3%) patients were dead, while 59 (40.7%) patients were alive at the last follow-up. Disease recurrence was observed in 101 patients (69.7%).
Marker expression and its relationships with clinicopathological findings
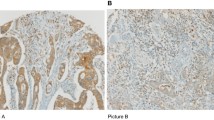
In the non-neoplastic biliary epithelium, only scant or weak cytoplasmic staining of DKK1 and ß-catenin was detected (Fig. 1A,B). High expression of DKK1 and β-catenin positivity were observed in 37 (25.5%) and 40 (27.6%) tumors, respectively (Fig. 1C–F). Based on the median cut-off score for CD8 expression, the patients were classified into high (n = 73) and low (n = 72) CD8+ TIL groups (Fig. 1G,H). Low CD8 expression was significantly associated only with advanced T stage (P = 0.007), advanced TNM stage (P = 0.002), and BTC other than GBC (P = 0.040) (Table 1).
Immunohistochemical staining of DKK1, β-catenin, and CD8. Representative images (C, E, and G), (D, F, and H) are derived from the same tissue blocks. (A) DKK1 and (B) β-catenin expression in the normal bile duct mucosa, (C) low and (D) high expression of DKK1, (E) low and (F) high expression of β-catenin, (G) low and (H) high density of CD8+ tumor-infiltrating lymphocytes. (Original magnification, × 100; scale bar, 100 mm).
With respect to marker expression, β-catenin or DKK1 positivity was not related to CD8 expression (Supplementary Fig. S5). High DKK1 expression was significantly associated with advanced T stage regardless of CD8 expression (low-CD8+ TIL, P = 0.029; high-CD8+ TIL, P = 0.036, respectively). However, there was no association between ß-catenin positivity and any clinicopathological factor based on CD8 expression (Table 2).
Prognostic value of CD8, β-catenin, and DKK1 expression in bile duct cancer. The overall 5-year OS rate was 16.5% and the median OS time was 24.7 months (range 1.3–138.3 months). Meanwhile, the overall 5-year RFS rate was 14.4% and the median RFS time was 18.2 months (range, 0.9–138.3 months). Analysis of the correlation between marker expression and prognosis showed that TIL expression was a significant predictor of favorable OS and RFS, as expected (median OS, 34.9 months in high-TIL, 16.7 months in low-TIL, P < 0.0001, respectively; median RFS, 27.1 months in high-TIL, 10.0 months in low-TIL, P < 0.0001, respectively) (Fig. 2A, B). Patients with positive ß-catenin expression tended to have a shorter OS (median OS, 23.95 months in positive ß-catenin, 26.1 months in negative ß-catenin, P = 0.1009, respectively) than those with negative expression, but the difference was not significant (Supplementary Fig. S6A,B). Meanwhile, patients with high DKK1 expression showed significantly shorter OS (median OS, 19.4 months in high DKK1, 31.7 months in low DKK1, P = 0.0093 respectively), but not RFS (P = 0.2924) (Supplementary Fig. S4C,D).
In the univariate analyses, patients who were older (P = 0.009), had poorly differentiated grade (P < 0.001), non-GBC primary site (P = 0.036), positive margin (P = 0.015), advanced stage (P < 0.001), lymphatic invasion status (P < 0.001), and perineural invasion status (P < 0.001) predicted poor RFS (Table 3). Of these, age (HR 1.727, 95% CI 1.092–2.733; P = 0.020), positive margin (HR 1.686, 95% CI 1.083–2.623; P = 0.021), advanced stage (HR 1.816, 95% CI 1.106–2.982; P = 0.018) and perineural invasion status (HR 1.960, 95% CI 1.187–3.237; P = 0.009) were independent prognostic factors of poor RFS in the multivariate analysis. A shorter OS was found to be significantly associated with older age (P = 0.006), poorly differentiated grade (P < 0.001), positive margin (P = 0.022), advanced stage (P < 0.001), lymphatic invasion status (P = 0.001), and perineural invasion status (P < 0.001) in the univariate analysis. Risk factors that were significantly associated with shortening OS included older age (HR 1.956, 95% CI 1.198–3.194; P = 0.007) and advanced stage (HR 1.751, 95% CI 1.042–2.942; P = 0.035) in the multivariate analysis. High CD8+ TIL level was associated with good prognostic values in both RFS (HR 0.579, 95% CI 0.375–0.895; P = 0.014 in multivariate analysis) and OS (HR 0.477, 95% CI 0.296–0.769; P = 0.002 in multivariate analysis). Although, positive β-catenin and DKK1 expression did not reveal statistical significance in RFS, these were correlated with poor prognostic values in OS (HR 1.611, 95% CI 1.011–2.568; P = 0.045 in β-catenin; HR, 1.616, 95% CI 0.997–2.620; P = 0.051 in DKK1).
The Wnt/β-catenin pathway contributes to immune evasion in tumors by suppressing the function of immune cells and attenuating CD8+ T cell infiltration24,26. TIL levels have prognostic values in various cancers. Therefore, to eliminate the influence of TIL expression on prognosis, we analyzed the independent prognostic values of β-catenin and DKK1 stratified by CD8+ TIL expression. Notably, the expression of β-catenin and DKK1 in tumors had a significant negative impact on OS and RFS in the CD8+ TIL-high BTC group (β-catenin: P = 0.0146 for OS and P = 0.0112 for RFS, respectively; DKK1: P = 0.0950 for OS and P = 0.3904 for RFS, respectively) (Fig. 3A,B). However, there were no differences in the OS or RFS according to β-catenin expression (OS, P = 0.5108 and RFS, P = 0.8431, respectively) and DKK1 expression (OS, P = 0.1127 and RFS, P = 0.1095, respectively) in the TIL-low BTC group (Fig. 3A–D). These findings confirmed the differential clinical role of Wnt/β-catenin proteins according to TIL expression.
Survival curves according to ß-catenin and DKK1 expression stratified by CD8 expression (A, B). There was a significant difference in prognosis according to the presence or absence of β-catenin in the high CD8 group. Survival curves according to the difference in DKK1 level in the high and low expression CD8 groups (C, D). DKK1 was a significant predictor of prognosis in both the high and low CD8 expression groups.
The above results confirmed the difference in the extent of CD8+ T cell infiltration according to the primary tumor site, with these differences primarily being due to the immunobiological characteristics of BTC (Table 1). Therefore, we further investigated if the clinical outcomes (OS and RFS) differ according to the primary tumor site in patients with positive DKK1 and ß-catenin expression. In GBC and ECC, DKK1 and ß-catenin confirmed the good prognostic role of high CD8+ T cells, but ICC, known as immune exclusive TME, with strong immunosuppressive tendency, revealed the different roles of each marker (Supplementary Fig. S7A–F).
Discussion
The impact of the Wnt/β-catenin pathway on cancer progression is well known. This pathway mediates immune exclusion in cancer tissue, including BTC. However, BTC has a distinct immunobiology, characterized by the presence of abundant tumor-infiltrating immune cells, owing to the constant exposure to intestinal microbes19,20. Furthermore, the relationship of β-catenin and DKK1 with the clinical outcomes of BTC remains unclear. In this study, a combined analysis of β-catenin and DKK1 protein expression, and CD8+ TILs showed that the expression of the β-catenin and DKK1 proteins was an independent adverse prognostic factor for BTC. In contrast, a high CD8+ TIL count was a favorable prognostic factor. Notably, the favorable clinical effect of high CD8+ TIL was observed only in BTC patients with low β-catenin or DKK1 protein expression19,27. These findings suggest that CD8+ T cells have a more profound prognostic impact in tumors with low Wnt/β-catenin activation.
In general, cytotoxic CD8+ T lymphocytes can recognize tumors and induce tumor cell death via the release of cytotoxic granules28. Thus, the degree of TIL infiltration is considered to reflect the growth, progression, and metastasis of cancer. Moreover, it is predictive of the response to cytotoxic treatments, such as chemotherapy and radiotherapy. Previous studies have demonstrated the importance of CD8+ T cell specificity and cytotoxicity27,28. CD8+ T cells are the most functionally representative and most abundant TILs to be found within the tumor27,28,29 High CD8+ TIL infiltration was recently shown to be correlated with better OS in patients with BTC29,30. Similarly, this study found that TIL expression was a strong favorable prognostic factor for OS and RFS in BTC.
The activation of the Wnt/β-catenin pathway is a hallmark of disease progression, which is associated with poor clinical outcomes in various malignancies2,31. In the current study, ß-catenin expression was also associated with a shorter duration of survival. The COSMIC database shows that Wnt/β-catenin activation occurs in 4.2–7.5% of cholangiocarcinoma tissue samples32 and in up to 57.1% of GBC tissue samples33. Exome sequencing analysis of 209 BTC samples from Asia and Europe showed that mutations at the CTNNB1 and APC loci are rare, unlike other gastrointestinal cancers34. This suggests that aberrant activation of the Wnt signaling pathway is mainly modulated by Wnt ligands and negative regulators in BTC. For example, the loss of activation of RNF43, an inhibitor of Wnt signaling, could increase Wnt signaling in the absence of mutations at the APC or CTNNB1 locus12. Loss of PEG3, an imprinted gene that regulates apoptosis, activates Wnt signaling, eventually leading to chromosomal instability in BTC13. Additionally, inflammatory macrophages are required to establish this Wnt-high state via the production of Wnt ligands35.
DKK1 is known to inhibit Wnt signaling by interacting with the Wnt receptors of the Frizzled family14,36. Moreover, DKK1 is associated with the so-called immune-desert microenvironment that results from the activation of MDSC and downregulation of natural killer cells in the cancer milieu17,18. However, the role of DKK1 as a tumor suppressor or oncogene varies across various malignant tumors. DKK1 overexpression has been correlated with adverse prognosis in patients with gastric, lung, and breast cancers9,16. We found that high DKK1 expression is associated with a poor prognosis, in accordance with these findings. Given that the Wnt signaling pathway does not serve as a binary on/off switch during tumorigenesis, it is difficult to define the role of DKK1, the modulator of the Wnt/β-catenin pathway36. In addition to the disruption in the negative feedback loop between DKK1 and the Wnt/β-catenin pathway18,36, DKK1 promotes malignancy via non-canonical Wnt pathway mechanisms37. DKK1 also plays a role in creating an immunosuppressive TME by activating MDSC, which downregulate the T-cell response17,38, as demonstrated by several in vitro studies. D'Amico et al. observed that downregulated DKK1 affects the MDSC count by rescuing β-catenin in these cells, and restores T cell recruitment at the tumor site17. Other studies found that DKK1 is associated with the so-called immune-desert microenvironment via the activation of MDSCs, downregulation of natural killer cells18 and contribution to the recruitment of Foxp3+ Treg cells, in order to restore cancer-immunological homeostasis39. Therefore, our clinical data on the differential roles of β-catenin and DKK1 depending on the TIL status were congruent with these experimental data.
The complex prognostic role of DKK1 remains unclear. Data from recent studies have demonstrated that DKK1 acts not only as a tumor suppressor protein, but also as an oncogene9,18. The influence of DKK1 on the clinical outcomes differs according to the location and TME components40, which is also interpreted as a dynamic effect depending on the TIL background in connection with the action of β-catenin24. The regulation of the Wnt/β-catenin signaling pathway is a complex process with a highly regulated hierarchy, which is influenced by both auto-regulatory and para-regulatory signals. As noted above, BTC is considered to be a heterogeneous cancer2,30,42. The role of Wnt/β-catenin signaling and its antagonist, DKK1, is dynamic and can vary according to the type of tumor (i.e., high-density TIL-infiltrated immune-inflamed “hot tumors” and immune-exclusive “cold tumors” with low TIL density)19,22,23. Wnt/β-catenin signaling is essential for T cell differentiation, effector functions, proliferation, and migration via the T-cell factor (TCF), which is the effector transcription factor of the Wnt signaling pathway41. As such, “hot tumors" present with more profound Wnt/β-catenin signaling and TCF modulation. Meanwhile, Wnt/β-catenin signaling and TCF modulation do not have a significant influence on the prognosis of “cold tumors” because of the insufficient substrates available for alteration in these tumors24,26. The differences in the resulting clinical changes in the protumoral and immunosuppressive status, according to β-catenin activation, were more prominent in the high TIL inflamed subgroup, whose clinical prognosis was comparable to that of the immune exclusive subgroup.
Notably, we found no significant differences between the OS and RFS of patients with high β-catenin/DKK1 and high TIL expression and those with low TIL expression. These results suggest that despite the high CD8 TIL levels in the tumor tissues, the favorable clinical effect of CD8 T cells could be expected from low Wnt/β-catenin signaling activation. Several modulators are associated with T cell infiltration in the TME, and the Wnt/β-catenin signaling molecule is one of the best characterized factors19,24. It has been widely accepted that Wnt/β-catenin signaling affects cancer immunosurveillance across various tumor types19,43. Tumor-intrinsic Wnt/β-catenin pathway activation impedes antitumor immunity via various mechanisms, including the release of immunosuppressive chemokines, tumor exclusion of dendritic cells, downregulation of innate immune sensors on the tumor cell surface, and suppression of dendritic cell maturation44. Interestingly, Wnt/β-catenin signaling is involved in T cell differentiation, effector function, and migration. The differentiation of naïve CD8+ T cells into CD8+ T effector cells is inhibited by the activation of TCF-1/β-catenin signaling43, in accordance with the findings of this study. The mechanisms discussed above indicate that the increased expression of β-catenin in tumors is inversely correlated with intratumoral T-cell infiltration. However, we did not find an inverse correlation between tumoral β-catenin or DKK1 expression and the CD8+ TILs. This was similar to the findings of Saleh et al., who reported that Wnt signaling- and β-catenin/TCF complex-associated genes were among the significantly upregulated genes in CD8+ TILs among patients with advanced colorectal cancer45. This suggests that the cytotoxic functions of CD8+ effector T cells could be compromised by Wnt/β-catenin signaling. β-catenin or DKK1 protein expression in patients with a high-density of CD8+ TILs in the intra-tumoral area was correlated with poor histological differentiation.
Our data confirmed that the distribution of TILs and the roles of DKK1 and β-catenin differ among GBC, ECC, and ICC. Although several studies have shown similar prognoses among the three BTC subtypes using a traditional merged analysis2,30,46, a stratified molecular study found a difference in prognosis among these subtypes. There are several possible explanations for this result. First, the immune escape mechanisms vary according to the type of biliary malignancy. In general, BTC is constantly stimulated by intestinal microbes and toxic materials and possesses an immune tolerance ability that can suppress inappropriate inflammatory responses, in addition to an immune ability that protects against harmful stimuli, such as infection and tumor19,20,21. Therefore, the immunobiology, including the immune escape mechanism, depends on the location of exposure47. T-cell receptor clonality, the immune gene signature/RNA repertoire, and major histocompatibility complex class polymorphisms have been extensively investigated in various human cancers, including BTC47,48. Goeppert et al. reported that downregulated MHC 1 expression in cancer cells may be associated with a low density of anti-tumor inflammatory cells, such as TILs30. This mechanism might underlie the poor prognostic role of DKK1 and β-catenin in GBC and ECC, and the opposite result in ICC.
The present study has some limitations. First, this retrospective analysis was performed with a small sample size, which may be attributed to the complexity of the biological function of the molecules and the different cut-off values used to evaluate the significance of these molecules. However, considering the rarity of BTC, the sample size is adequate to derive significant findings and establish the prognostic impact of the expression of the β-catenin and DKK1 proteins. Second, it is difficult to draw widely applicable conclusions from the results, owing to insufficient numbers of patients in some subgroups classified on the basis of the tumor response patterns. Third, the association between the expression levels of the Wnt/β-catenin gene and DKK1 and distribution of CD8+ TIL were not confirmed by other methods. The amount of archival tissue available for this retrospective study was insufficient to conduct other procedures, such as whole-genome and RNA sequencing. Since the immune signatures and molecular profiling of ICC, ECC, and GBC are different, we focused on identifying the clinical implications at the protein level and conducted analyses using the immunohistochemistry method. To the best of our knowledge, no study has investigated the dynamicity and differential effects of the two molecules (β-catenin and DKK1) in BTC depending on the infiltration of CD8+ TILs. Further research is needed to validate the mechanism and prognostic function of DKK1 and β-catenin according to the heterogeneous immune status in BTC.
Conclusions
β-catenin or DKK1 protein expression is associated with poor clinical outcomes in patients with resected BTC, whereas high CD8+ TIL levels are associated with good clinical outcomes. Notably, a high CD8+ TIL level with high β-catenin or DKK1 protein expression is associated with an unfavorable prognosis. These findings confirm the differential clinical implications of Wnt/β-catenin proteins according to TIL expression in BTC.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions e.g., privacy or ethical.
References
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England) 388, 1545–1602. https://doi.org/10.1016/s0140-6736(16)31678-6 (2016).
Valle, J. W., Lamarca, A., Goyal, L., Barriuso, J. & Zhu, A. X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 7, 943–962. https://doi.org/10.1158/2159-8290.Cd-17-0245 (2017).
Charbel, H. & Al-Kawas, F. H. Cholangiocarcinoma: Epidemiology, risk factors, pathogenesis, and diagnosis. Curr. Gastroenterol. Rep. 13, 182–187. https://doi.org/10.1007/s11894-011-0178-8 (2011).
Valle, J. W. et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27, v28–v37. https://doi.org/10.1093/annonc/mdw324 (2016).
Marin, J. J. G. et al. Current and novel therapeutic opportunities for systemic therapy in biliary cancer. Br. J. Cancer 123, 1047–1059. https://doi.org/10.1038/s41416-020-0987-3 (2020).
Yoo, K. H. et al. Genomic alterations in biliary tract cancer using targeted sequencing. Transl. Oncol. 9, 173–178. https://doi.org/10.1016/j.tranon.2016.01.007 (2016).
Banales, J. M. et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 17, 557–588. https://doi.org/10.1038/s41575-020-0310-z (2020).
Marabelle, A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10. https://doi.org/10.1200/jco.19.02105 (2020).
Hong, S. A. et al. Prognostic value of Dickkopf-1 and ß-catenin expression in advanced gastric cancer. BMC Cancer 18, 506. https://doi.org/10.1186/s12885-018-4420-8 (2018).
Wang, W. et al. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget 6, 42276–42289. https://doi.org/10.18632/oncotarget.5514 (2015).
Shen, D.-Y., Zhang, W., Zeng, X. & Liu, C.-Q. Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 104, 1303–1308. https://doi.org/10.1111/cas.12223 (2013).
Loregger, A. et al. The E3 ligase RNF43 inhibits Wnt signaling downstream of mutated beta-catenin by sequestering TCF4 to the nuclear membrane. Sci. Signal. 8, ra90. https://doi.org/10.1126/scisignal.aac6757 (2015).
Deng, Y. & Wu, X. Peg3/Pw1 promotes p53-mediated apoptosis by inducing Bax translocation from cytosol to mitochondria. Proc. Natl. Acad. Sci. U.S.A. 97, 12050–12055. https://doi.org/10.1073/pnas.97.22.12050 (2000).
Goyal, L. et al. Phase I and biomarker study of the Wnt pathway modulator DKN-01 in combination with gemcitabine/cisplatin in advanced biliary tract cancer. Clin. Cancer Res. 26, 6158–6167. https://doi.org/10.1158/1078-0432.Ccr-20-1310 (2020).
Kagey, M. H. & He, X. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br. J. Pharmacol. 174, 4637–4650. https://doi.org/10.1111/bph.13894 (2017).
Shi, R. Y. et al. High expression of Dickkopf-related protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer 119, 993–1003. https://doi.org/10.1002/cncr.27788 (2013).
D’Amico, L. et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J. Exp. Med. 213, 827–840. https://doi.org/10.1084/jem.20150950 (2016).
Betella, I. et al. Wnt signaling modulator DKK1 as an immunotherapeutic target in ovarian cancer. Gynecol. Oncol. 157, 765–774. https://doi.org/10.1016/j.ygyno.2020.03.010 (2020).
Loeuillard, E., Conboy, C. B., Gores, G. J. & Rizvi, S. Immunobiology of cholangiocarcinoma. JHEP Rep. Innov. Hepatol. 1, 297–311. https://doi.org/10.1016/j.jhepr.2019.06.003 (2019).
Robinson, M. W., Harmon, C. & O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 13, 267–276. https://doi.org/10.1038/cmi.2016.3 (2016).
Franklin, R. A. et al. The cellular and molecular origin of tumor-associated macrophages. Science (New York, N.Y.) 344, 921–925. https://doi.org/10.1126/science.1252510 (2014).
Raggi, C. et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J. Hepatol. 66, 102–115. https://doi.org/10.1016/j.jhep.2016.08.012 (2017).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550. https://doi.org/10.1038/s41591-018-0014-x (2018).
Li, X. et al. WNT/β-catenin signaling pathway regulating T cell-inflammation in the tumor microenvironment. Front. Immunol. 10, 2293. https://doi.org/10.3389/fimmu.2019.02293 (2019).
Kim, S. W., Roh, J. & Park, C. S. Immunohistochemistry for pathologists: Protocols, pitfalls, and tips. J. Pathol. Transl. Med. 50, 411–418. https://doi.org/10.4132/jptm.2016.08.08 (2016).
Gattinoni, L., Ji, Y. & Restifo, N. P. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin. Cancer Res. 16, 4695–4701. https://doi.org/10.1158/1078-0432.Ccr-10-0356 (2010).
Malenica, I., Donadon, M. & Lleo, A. Molecular and immunological characterization of biliary tract cancers: A paradigm shift towards a personalized medicine. Cancers https://doi.org/10.3390/cancers12082190 (2020).
Krummel, M. F., Bartumeus, F. & Gérard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 16, 193–201. https://doi.org/10.1038/nri.2015.16 (2016).
Kim, R. et al. Prognostic value of CD8CD45RO tumor infiltrating lymphocytes in patients with extrahepatic cholangiocarcinoma. Oncotarget 9, 23366–23372. https://doi.org/10.18632/oncotarget.25163 (2018).
Goeppert, B. et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br. J. Cancer 109, 2665–2674. https://doi.org/10.1038/bjc.2013.610 (2013).
Rosenbluh, J., Wang, X. & Hahn, W. C. Genomic insights into WNT/β-catenin signaling. Trends Pharmacol. Sci. 35, 103–109. https://doi.org/10.1016/j.tips.2013.11.007 (2014).
Tate, J. G. et al. COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941-d947. https://doi.org/10.1093/nar/gky1015 (2019).
Rashid, A. et al. Beta-catenin mutations in biliary tract cancers: A population-based study in China. Can. Res. 61, 3406–3409 (2001).
Chan-On, W. et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat. Genet. 45, 1474–1478. https://doi.org/10.1038/ng.2806 (2013).
Boulter, L. et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J. Clin. Investig. 125, 1269–1285. https://doi.org/10.1172/jci76452 (2015).
Flanagan, D. J., Vincan, E. & Phesse, T. J. Wnt signaling in cancer: Not a binary ON:OFF switch. Can. Res. 79, 5901–5906. https://doi.org/10.1158/0008-5472.Can-19-1362 (2019).
Kikuchi, A., Yamamoto, H., Sato, A. & Matsumoto, S. New insights into the mechanism of Wnt signaling pathway activation. Int. Rev. Cell Mol. Biol. 291, 21–71. https://doi.org/10.1016/b978-0-12-386035-4.00002-1 (2011).
Malladi, S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60. https://doi.org/10.1016/j.cell.2016.02.025 (2016).
Chae, W. J. et al. Membrane-bound Dickkopf-1 in Foxp3(+) regulatory T cells suppresses T-cell-mediated autoimmune colitis. Immunology 152, 265–275. https://doi.org/10.1111/imm.12766 (2017).
Menezes, M. E., Devine, D. J., Shevde, L. A. & Samant, R. S. Dickkopf1: A tumor suppressor or metastasis promoter?. Int. J. Cancer 130, 1477–1483. https://doi.org/10.1002/ijc.26449 (2012).
Luke, J. J., Bao, R., Sweis, R. F., Spranger, S. & Gajewski, T. F. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin. Cancer Res. 25, 3074–3083. https://doi.org/10.1158/1078-0432.Ccr-18-1942 (2019).
Kitano, Y. et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br. J. Cancer 118, 171–180. https://doi.org/10.1038/bjc.2017.401 (2018).
Gattinoni, L. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813. https://doi.org/10.1038/nm.1982 (2009).
Swafford, D. & Manicassamy, S. Wnt signaling in dendritic cells: Its role in regulation of immunity and tolerance. Discov. Med. 19, 303–310 (2015).
Saleh, R. et al. Differential gene expression of tumor-infiltrating CD8(+) T cells in advanced versus early-stage colorectal cancer and identification of a gene signature of poor prognosis. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-001294 (2020).
Komuta, M. et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 55, 1876–1888. https://doi.org/10.1002/hep.25595 (2012).
Du, C. & Wang, Y. The immunoregulatory mechanisms of carcinoma for its survival and development. J. Exp. Clin. Cancer Res. 30, 12–12. https://doi.org/10.1186/1756-9966-30-12 (2011).
Seliger, B. Novel insights into the molecular mechanisms of HLA class I abnormalities. Cancer Immunol. Immunother. CII 61, 249–254. https://doi.org/10.1007/s00262-011-1153-9 (2012).
Funding
This study was supported by a grant from an industry–academic co-operation research fund from the Catholic University of Korea (5-2016-D0174-00001 to Y.H.K.), and The Catholic University of Korea Uijeongbu St. Mary’s Hospital Clinical Research Laboratory Foundation made in the program year of 2018 (UJBCRL201801 to Y.H.K.). The funder did not play any role in the study design, collection, analysis, interpretation of data, or in the drafting of this paper.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: S.R., H.S., K.H. and Y.H.; Collection and assembly of data: S.R., H.S., J.H., D.S., K.I., M.E., S.A., K.H. and Y.H.; Data analysis and interpretation: S.R., K.I., S.A., J.S., S.H. and Y.H.; Manuscript writing: All authors; Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S.R., Won, H.S., Yang, J.H. et al. Prognostic value of Dickkopf-1 and ß-catenin expression according to the antitumor immunity of CD8-positive tumor-infiltrating lymphocytes in biliary tract cancer. Sci Rep 12, 1931 (2022). https://doi.org/10.1038/s41598-022-05914-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05914-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.