Abstract
Pembrolizumab appears promising for patients with programmed cell death ligand-1 (PD-L1)-positive solid tumors. However, data on immunotherapy for biliary tract cancers (BTC) are limited. We aimed to investigate the predictive value of PD-L1 expression as an immunotherapeutic biomarker in BTC. Patients with advanced BTC (n = 175) were screened for PD-L1 expression using PharmDx assay and microsatellite instability (MSI) status. Of the total of 175 patients, 125 (71%) showed tumoral PD-L1 positivity (≥ 1%) while only two (2/142, 1.4%) showed MSI-High. Among 175 patients, 26 patients were treated with pembrolizumab as a second-line therapy, and tumor response was evaluated. Separating these patients into two groups by PD-L1 expression (high [≥ 50%] vs. low [< 50%]), overall response rate was 23% (56% [5/9] in high PD-L1 group vs. 6% [1/17] in low PD-L1 group, P = 0.004). Disease control rate was also higher in high PD-L1 group (78% vs. 35%, P = 0.019). The six responders showed median progression-free survival of 5.8 months after starting pembrolizumab, and none of them was MSI-High. High PD-L1 expression was associated with a better response to pembrolizumab. PD-L1 expression can potentially serve as an alternative predictive biomarker for pembrolizumab therapy in advanced BTC.
Similar content being viewed by others
Introduction
Biliary tract cancers encompass a heterogeneous group of cancers including those of the intrahepatic and extrahepatic bile duct as well as the gallbladder, and they show dismal clinical outcomes with 5-year survival rates of 20–30%1. Most patients are diagnosed at an advanced stage making palliative chemotherapy the only option1. Gemcitabine-cisplatin combination is the standard first-line treatment for advanced biliary tract cancer with no standard second-line therapy2,3. Several anti-programmed cell death protein 1 (PD-1)/programmed cell death ligand-1 (PD-L1) agents have demonstrated long-lasting anti-tumoral effects in various solid tumors and are now approved for standard treatment in several solid cancers including lung cancer4,5. PD-L1 expression has been reported in 9.1–72.2% patients with biliary tract cancer6,7,8; thus, anti-PD-1 or PD-L1 agents can be a promising treatment modality in certain patients with biliary tract cancers. However, immunotherapeutic data on biliary tract cancer are limited compared to other tumors9,10. Nevertheless, interim results of an ongoing trial (KEYNOTE-028, NCT02054806) showed promising outcomes of pembrolizumab (anti-PD-1 antibody) in patients with PD-L1-positive advanced biliary tract cancers9.
Biomarker development is essential in enhancing efficacy of immunotherapy. For instance, in several tumors including lung and gastric cancers, PD-L1 expression evaluated by immunohistochemistry (IHC) is used as a crucial biomarker predicting response to anti-PD-1/PD-L1 agents11,12. PD-L1 IHC (PharmDx assay) has been approved as a companion diagnostic test to select lung cancer patients eligible for pembrolizumab treatment5. The approval was based on the observation that patients with high PD-L1 expression showed improved response rates13. However, predictive biomarkers remain unknown for the vast majority of other tumors; hence, microsatellite instability (MSI) status, tumor-infiltrating lymphocytes (TIL), tumor mutational burden, and several other factors are being investigated as candidate immunotherapeutic biomarkers14. Pembrolizumab was recently approved by the Food and Drug Administration for treatment of patients with metastatic or unresectable mismatch repair (MMR)-deficient and/or MSI-High (MSI-H) solid tumors that progressed after prior therapy regardless of tumor type15. However, MSI-H biliary tract cancers are rare16. To date, studies on immunotherapeutic biomarkers in biliary tract cancers are limited. In this study, we evaluated therapeutic response of advanced biliary tract cancer patients to pembrolizumab, and correlated treatment response with biomarkers including PD-L1 IHC.
Results
PD-L1 and MSI screening
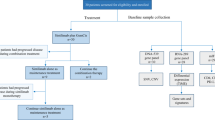
In the 175 patients, the primary tumor site was the intrahepatic bile duct in 55 (31%) patients, hilar bile duct in 39 (22%), distal extrahepatic bile duct in 18 (10%), gallbladder in 56 (32%), and ampulla of Vater in 7 (4%) patients. Among them, 71.4% (125 of 175) of patients showed tumoral PD-L1 positivity at 1% cut off. High PD-L1 expression (tumor proportion score [TPS] ≧ 50) was present in 8.6% (15 of 175) of patients. MSI status was evaluated in 142 patients, and only 2 (1.4%) out of 142 had MSI-H status as revealed by polymerase chain reaction (PCR) assay. These two MSI-H tumors showed low PD-L1 expression (TPS = 1 and 5). One patient received pembrolizumab and the other did not. The one who received pembrolizumab showed progressive disease. The PD-L1 IHC and MSI status are summarized in Table 1. Overall patient flowchart is shown in Fig. 1.
Treatment outcome
Between Feb 2018 and June 2019, 26 patients among PD-L1 positive patients (≥ 1%) were treated with pembrolizumab as a second-line therapy and they were evaluated the tumor response by computed tomography (CT) or magnetic resonance imaging (MRI). The median age was 67 years with an age range of 41 to 86 years. The ratio of sex was equal, and all were of Asian ethnicity. All patients had received prior standard chemotherapy and had been found refractory. The median number of cycles was 4 as of Dec 2019. The median follow-up duration was 13.1 months. Patient information is summarized in Table 2.
As listed in Table 3, no patient achieved complete remission. A total of six (23%) patients showed partial response. Seven (27%) patients showed stable disease. The overall tumor response rate (ORR) was 23% and was statistically different between the two groups (56% [5/9] in high PD-L1 group vs. 6% [1/17] in low PD-L1 group, P = 0.004). Also, disease control rate (DCR) was 50% and showed a statistically significant difference between the two groups (78% [7/9] high PD-L1 group vs. 35% [6/17] low PD-L1 group, P = 0.019). The computed tomography and IHC images of one of the responders belonging to high PD-L1 group are shown in Fig. 2.
Representative images of abdominopelvic computed tomography and immunohistochemistry from one responder with gallbladder cancer (TPS = 80). (A) Baseline computed tomography scan. (B) Computed tomography scan after 3 cycles of pembrolizumab. (C) Computed tomography scan after 10 cycles of pembrolizumab. (D) PD-L1 immunohistochemistry showing high PD-L1 expression (20 × magnification).
The six responders including five in high PD-L1 group and one in low PD-L1 group showed median progression-free survival of 5.8 months (range 1.8–14.5 months) after starting pembrolizumab, and none of them were MSI-H.
Additional analyses were performed for PD-L1 positive patients who did not receive pembrolizumab. First, in all of the patients with high PD-L1, patients treated with pembrolizumab showed a significant improvement in survival (HR 0.423, 95% CI 0.249–0.952). However, initial patient status such as Eastern Cooperative Oncology Group performance and carbohydrate antigen 19–9 showed different trends in the treated and untreated patients (Supplementary Table 1). Second, in all of the patients without treatment, the high (n = 5) vs low (n = 72) PD-L1 group did not show a significant difference in clinical characteristics (Supplement Table 2).
Toxicity profile
The patients who were evaluated for tumor response (n = 26) and who dropped out early because of toxicity or intolerance (n = 9) were assessed for toxicity profile (Fig. 1 and Table 4). Generally, overall treatment-related adverse events including (1) hematologic, (2) other laboratory, and (3) clinical adverse effects did not show a significant difference between the two groups. Unlike other cytotoxic chemotherapeutic agents, neutropenia was relatively low (grade I-II 6%, grade III-IV 3%). Grade I-II hypercalcemia was present in five (14%) patients. Overall, alanine transferase (ALT) elevation was observed in nine (25%) patients: grade I-II 14% and grade III-IV 11%.
Association of response with biomarkers
As described above, high-PD-L1 group showed ORR of 56% while low PD-L1 group showed ORR of 6% (P = 0.004). DCR was 78% vs. 35% (high PD-L1 group vs. low PD-L1 group, P = 0.019). PD-L1 TPS and combined positive score (CPS) showed the same results as shown in Supplementary Table 3. Only one patient with MSI-H status (in low PD-L1 group) was treated with pembrolizumab and showed progressive disease. Using TIL level as a biomarker, high TIL level (≥ 7.5%, median value) and low TIL level (< 7.5%) showed 36% vs. 13% in ORR (P = 0.251) and 55% vs. 47% in DCR (P = 0.922). Using CD8+ cells level as a biomarker, high CD8+ cells count (≥ 50, median value) and low CD8+ cells count (< 50) showed 36% vs. 17% in ORR (P = 0.355) and 55% vs. 58% in DCR (P = 0.834).
Discussion
Immunotherapy has emerged as a new paradigm in cancer treatment. Pembrolizumab, a monoclonal antibody against PD-1, has shown long-lasting antitumor activity and manageable toxicity in several advanced cancers4. An ongoing phase 1b clinical trial showed promising clinical results with pembrolizumab treatment in advanced PD-L1-positive biliary tract cancers9. In the trial, PD-L1 positivity was defined as staining in 1% of cells in tumor nests or PD-L1-positive bands in the stroma by the 22C3 assay, and the ORR was 17.4%9.
In our study, 6 (23%) of the 26 patients with PD-L1-positive (TPS ≥ 1%) biliary tract cancers revealed partial response to pembrolizumab, and 7 (27%) showed stable disease. Notably, the responders were mostly enriched in high PD-L1 expression although they did not have MSI-H tumors. Evaluating treatment responses to pembrolizumab is clinically significant since currently, there is no effective second-line treatment agent for patients with biliary tract cancer refractory to standard chemotherapy. High PD-L1 expression was significantly associated with response to pembrolizumab in patients with biliary tract cancer, a finding in line with previous studies on other tumors such as lung cancer11. To the best of our knowledge, this is the first study reporting correlation between PD-L1 expression level and response to pembrolizumab in biliary tract cancers. Given the dramatic therapeutic response of the patients with high PD-L1 expression in our study, and the fact PD-L1 IHC is considered a cost-effective screening tool for routine usage in the clinic, PD-L1 could serve as an alternative predictive biomarker for pembrolizumab immunotherapy in advanced biliary tract cancers refractory to standard chemotherapy.
Regarding treatment-related adverse effects, pembrolizumab was generally well-tolerated as reported in a previous study9. The most-common grade III-IV adverse effects were elevated hepatic enzyme (ALT) level and fatigue. However, most of the adverse effects were manageable and reversible; thus, employing pembrolizumab in biliary tract cancer patients seems feasible.
Recently, pembrolizumab has been approved for MSI-H/MMR-deficient solid tumors regardless of tumor type15. In a recent trial of pembrolizumab in patients with previously treated, advanced noncolorectal MSI-H/MMR-deficient tumors encompassing 27 different solid tumors (KEYNOTE-158), 22 patients with biliary tract cancer were included, and the ORR of those biliary tract cancer patients was 40.9%17. However, the frequency of MSI-H in biliary tract cancer, which is generally very low1, varied from 1 to 10% in different reports18,19,20. In the present study, MSI-H was only observed in 2 (1.4%) of the 142 patients. Given the rarity of MSI-H in biliary tract cancer, we recommend PD-L1 expression to be evaluated along with MSI status when considering immunotherapy. We also correlated other biomarkers including CD8+ cell density and TILs with treatment response and found no significant associations. Among various biomarkers, only PD-L1 expression level showed reliable predictability with statistical significance. This supports the argument that PD-L1 IHC can be a useful biomarker for pembrolizumab treatment.
This study has several limitations. First, the sample size was relatively small to draw a definitive conclusion on the usefulness of the biomarkers. In addition, patients with PD-L1 negative biliary tract cancers were not included in this study. Thus, large-scale studies are warranted to determine the role of PD-L1 as a predictive marker of response to pembrolizumab in biliary tract cancers. Second, the enrollment criteria were not strict. Patients with advanced biliary tract cancer are relatively common in South Korea compared to western countries, and the Korean Ministry of Food and Drug Safety approved the usage of pembrolizumab based on the interim result of the ongoing phase 1b clinical trial9. Third, the follow-up period was insufficient to evaluate durable treatment effects of pembrolizumab, long-term disease-free survival, and overall survival. However, the responders in high PD-L1 group showed comparable progression-free survival compared to previous studies17. Fourth, MSI status was not evaluated in all patients. MSI status was evaluated in 142 out of total 175 patients. In addition, the number of MSI-H tumors was too small to determine the association between MSI status and response to pembrolizumab in biliary tract cancers. However, the very low frequency of MSI-H patients suggests less usefulness of MSI in the clinical setting, which is precisely the reason that novel biomarkers are needed. Lastly, tumor mutation burden, which is a promising biomarker for predicting response to immunotherapy21, was not evaluated in this study.
In conclusion, 8.6% of advanced biliary tract cancer patients revealed high PD-L1 expression, and MSI-H tumors were rare (1.4%) in our screening cohort. High PD-L1 expression was associated with treatment response to pembrolizumab. Although further prospective studies using a large cohort is required, PD-L1 expression can potentially serve as a valuable biomarker in identifying those patients with advanced biliary tract cancer who could benefit from pembrolizumab treatment.
Methods
Patients
Advanced biliary tract cancer patients who experienced disease progression after 1st-line chemotherapy (n = 260) were retrospectively reviewed. Among them, a total of 175 patients were screened for PD-L1 expression and MSI status at Seoul National University Bundang Hospital (Seongnam, Korea) from Feb 2018 to June 2019. All patients had a histologically confirmed diagnosis of either the gallbladder, bile duct, or ampulla of Vater cancer. Pembrolizumab was then administered solely to patients with PD-L1 positive (≥ 1%) tumors that were refractory to standard chemotherapy (gemcitabine with cisplatin), and those who could afford the high cost of treatment (The Ministry of Food and Drug Safety in South Korea approved the usage of pembrolizumab as a second-line chemotherapy in biliary tract cancers patients with positivity of PD-L1 without reimbursement since December 2017).
Study design
We divided patients treated with pembrolizumab into two groups (high PD-L1 group: PD-L1 expression ≧ 50% vs. low PD-L1 group: PD-L1 expression < 50%) and compared clinical outcomes in these two groups. The primary outcome of this study encompassed ORR and DCR with respect to PD-L1 expression level.
Pembrolizumab was administered intravenously at a fixed dose of 200 mg every 3 weeks up to the point of disease progression which was evaluated following the RECIST criteria version 1.122. Response evaluation was made by investigator assessment. To evaluate the primary outcome (ORR and DCR), the patients who could be evaluated for tumor response (via CT or MRI) were included the ‘efficacy analysis’. Patients who dropped out before evaluating tumor response were excluded in the ‘efficacy analysis’. However, among this excluded group, those who dropped out due to medical reasons such as toxicity or intolerance were included in the ‘toxicity analysis’. Patients who withdrew consent for the reasons other than medical reasons, such as financial problems or access to hospitals, were excluded in both ‘efficacy analysis’ and ‘toxicity analysis’.
The study was approved by the institutional review board of Seoul National University Bundang Hospital (Protocol No.B-1901/519-104), and informed consent was waived. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
PD-L1 immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections of biopsy or surgical specimens were used for IHC. The PharmDx assay (Dako, Carpinteria, CA, USA) was utilized, using an anti-PD-L1 22C3 mouse monoclonal primary antibody and EnVision FLEX visualization system (Agilent, Santa Clara, CA, USA) on an Autostainer Link 48 system (Dako, Carpinteria, CA, USA) along with negative control reagents and cell line run controls, as per the manufacturers’ instructions23,24. PD-L1 staining was defined as complete or partial circumferential linear cellular membrane staining at any intensity that could be differentiated from the background as well as diffuse cytoplasmic staining23,25. Tonsillar tissue was used as a positive internal control23. Positivity of PD-L1 status was determined based on a 1% threshold in tumor cells. A TPS was recorded as the overall percentage of PD-L1 stained tumor cells relative to the entire tumor area26. A CPS was recorded based on the number of PD-L1 positive tumor and immune cells in relation to total tumor cells26.
Microsatellite instability status
The patients’ MSI status was assessed by comparing the allele profiles of 5 tumor cell markers (BAT-26, BAT-25, D5S346, D17S250, and S2S123) to those in the available corresponding normal samples. Hematoxylin and eosin-stained slides were reviewed to select appropriate areas with sufficient tumor cellularity and adequate non-neoplastic tissue for macro-dissection (where applicable), and subsequently, DNA extraction was performed. PCR amplification of the extracted DNA was carried out and the PCR products were analyzed using a DNA autosequencer (ABI 3731 Genetic Analyzer; Applied Biosystems, Foster City, CA, USA). According to the Revised Bethesda Guidelines, tumors with additional alleles in ≥ 2 markers were classified as MSI-H, those with novel bands in 1 marker were defined as MSI-low, and those with identical bands in all 5 markers were classified as microsatellite stable27.
Quantification of tumor infiltrating lymphocytes (TILs) and CD8+ lymphocytes
TILs and CD8+ lymphocyte analysis was performed by one experienced pathologist (S.A). TILs were scored as a percentage of immune cells in tumor-associated stromal areas28. All mononuclear cells (including lymphocytes and plasma cells) were scored; polymorphonuclear leukocytes were excluded28. CD8+ stained slides were scanned using a high-resolution digital slide scanner up to 400 × magnification (3DHISTECH Pannoramic 250; 3DHISTECH Ltd., Budapest, Hungary), and an area of 1 mm2 was marked per tumor. For tumors with heterogeneous distribution of CD8+ lymphocytes, one hotspot area was selected per sample. The absolute number of CD8+ cells was manually counted.
CD8 and mismatch repair protein immunohistochemistry
Immunohistochemical staining was performed using the BenchMark XT autostainer (Ventana Medical Systems, Tucson, AZ, USA) with an UltraView detection kit (Ventana Medical Systems, Basel, Switzerland). Commercially-available antibodies were used for immunohistochemical staining; CD8 (Clone C8/144B, ready to use; Dako, Carpinteria, CA, USA), hMLH1 (Clone M1, ready to use; Ventana, Basel, Switzerland), hMLH2 (Clone G219-1,129, 1:100 dilution; Abnova, Taipei City, Taiwan), hMLH6 (Clone 44, 1:100 dilution; Cell Marque, Rocklin, CA, USA), and PMS2 (Clone A16-4, ready to use; Ventana Medical Systems, Basel, Switzerland). The normal lymph node was used as positive control of CD8 stain. For MLH1, MLH2, MLH6, and PMS2, inflammatory cells and stromal tissue were used as internal positive control.
Statistical analyses
All continuous variables were analyzed using t-test or Mann–Whitney U test depending on normal distribution of the dataset. The range of continuous variables was expressed using whole ranges (minimal to maximal value) with several exceptions. Dichotomous or categorical variables were compared using Chi-square test or Fisher’s exact test. Statistical analyses were performed using STATA version 15.0 (StataCorp, College Station, TX, USA) and SPSS version 25.0 (SPSS, Inc., Chicago, IL, USA). P-values < 0.05 were considered significant.
Change history
03 December 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Razumilava, N. & Gores, G. J. Cholangiocarcinoma. Lancet 383, 2168–2179. https://doi.org/10.1016/S0140-6736(13)61903-0 (2014).
Thongprasert, S. The role of chemotherapy in cholangiocarcinoma. Ann. Oncol. 16(Suppl 2), ii93–ii96. https://doi.org/10.1093/annonc/mdi712 (2005).
Valle, J. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362, 1273–1281. https://doi.org/10.1056/NEJMoa0908721 (2010).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028. https://doi.org/10.1056/NEJMoa1501824 (2015).
Sul, J. et al. FDA Approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist 21, 643–650. https://doi.org/10.1634/theoncologist.2015-0498 (2016).
Fontugne, J. et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget 8, 24644–24651. https://doi.org/10.18632/oncotarget.15602 (2017).
Walter, D. et al. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology 71, 383–392. https://doi.org/10.1111/his.13238 (2017).
Ma, K. et al. PD-L1 and PD-1 expression correlate with prognosis in extrahepatic cholangiocarcinoma. Oncol. Lett. 14, 250–256. https://doi.org/10.3892/ol.2017.6105 (2017).
Bang, Y.-J. et al. Safety and efficacy of pembrolizumab (MK-3475) in patients with advanced biliary tract cancer: interim results of KEYNOTE-028. Eur. J. Cancer 51, S112 (2015).
Mou, H. et al. Successful response to the combination of immunotherapy and chemotherapy in cholangiocarcinoma with high tumour mutational burden and PD-L1 expression: a case report. BMC Cancer 18, 1105. https://doi.org/10.1186/s12885-018-5021-2 (2018).
Aguiar, P. N. Jr. et al. The role of PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: a network meta-analysis. Immunotherapy 8, 479–488. https://doi.org/10.2217/imt-2015-0002 (2016).
Fuchs, C. S. et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4, e180013. https://doi.org/10.1001/jamaoncol.2018.0013 (2018).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567. https://doi.org/10.1038/nature14011 (2014).
Spencer, K. R. et al. Biomarkers for immunotherapy: current developments and challenges. Am. Soc. Clin. Oncol. Educ. Book 35, e493–e503. https://doi.org/10.14694/EDBK_16076610.1200/EDBK_160766 (2016).
Marcus, L., Lemery, S. J., Keegan, P. & Pazdur, R. FDA Approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-18-4070 (2019).
Rizvi, S., Khan, S. A., Hallemeier, C. L., Kelley, R. K. & Gores, G. J. Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 15, 95–111. https://doi.org/10.1038/nrclinonc.2017.157 (2018).
Marabelle, A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10. https://doi.org/10.1200/JCO.19.02105 (2020).
Silva, V. W. et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin. Clin. Oncol. 5, 62. https://doi.org/10.21037/cco.2016.10.04 (2016).
Bonneville, R. et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis. Oncol. https://doi.org/10.1200/PO.17.00073 (2017).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413. https://doi.org/10.1126/science.aan6733 (2017).
Chan, T. A. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol. 30, 44–56. https://doi.org/10.1093/annonc/mdy495 (2019).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
Ahn, S. et al. Programmed cell death ligand-1 (PD-L1) expression in extrahepatic biliary tract cancers: a comparative study using 22C3, SP263 and E1L3N anti-PD-L1 antibodies. Histopathology 75, 526–536. https://doi.org/10.1111/his.13901 (2019).
Kim, H., Kwon, H. J., Park, S. Y., Park, E. & Chung, J. H. PD-L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non-small cell lung cancer: a comparative study. Oncotarget 8, 98524–98532. https://doi.org/10.18632/oncotarget.21567 (2017).
Phillips, T. et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl. Immunohistochem. Mol. Morphol. 23, 541–549. https://doi.org/10.1097/PAI.0000000000000256 (2015).
Kulangara, K. et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch. Pathol. Lab. Med. 143, 330–337. https://doi.org/10.5858/arpa.2018-0043-OA (2019).
Umar, A. et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl. Cancer Inst. 96, 261–268. https://doi.org/10.1093/jnci/djh034 (2004).
Dieci, M. V. et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 52, 16–25. https://doi.org/10.1016/j.semcancer.2017.10.003 (2018).
Acknowledgements
We are indebted to Dr. Yul Ri Chung for editing the manuscript. This study was supported by Grant No. 02–2018-011 from SNUBH research funds.
Author information
Authors and Affiliations
Contributions
S.A. and J.H.H. conceived the idea and designed the study. J.C.L., J.K. and D.W.S. contributed to the collection of the samples and clinical data. S.A., J.C.L. and J.H.H. analyzed the data and wrote the main manuscript text. All of the authors reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahn, S., Lee, Jc., Shin, D.W. et al. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep 10, 12348 (2020). https://doi.org/10.1038/s41598-020-69366-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69366-4
This article is cited by
-
Triple therapy in biliary tract cancers: GemOX plus immune checkpoint inhibitor in combination with lenvatinib or NGS-guided targeted therapy
Journal of Cancer Research and Clinical Oncology (2023)
-
Common DNA methylation changes in biliary tract cancers identify subtypes with different immune characteristics and clinical outcomes
BMC Medicine (2022)
-
Treating Biliary Tract Cancers: New Targets and Therapies
Drugs (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.