Abstract
In order to elucidate the active polyoxotungstate (POT) species that inhibit fungal polyphenol oxidase (AbPPO4) in sodium citrate buffer at pH 6.8, four Wells–Dawson phosphotungstates [α/β-PV2WVI18O62]6− (intact form), [α2-PV2WVI17O61]10− (monolacunary), [PV2WVI15O56]12− (trilacunary) and [H2PV2WVI12O48]12− (hexalacunary) were investigated. The speciation of the POT solutions under the dopachrome assay (50 mM Na-citrate buffer, pH 6.8; L-3,4−dihydroxyphenylalanine as a substrate) conditions were determined by 183W-NMR, 31P-NMR spectroscopy and mass spectrometry. The intact Wells–Dawson POT [α/β-PV2WVI18O62]6− shows partial (~ 69%) disintegration into the monolacunary [α2-PV2WVI17O61]10− anion with moderate activity (Ki = 9.7 mM). The monolacunary [α2-PV2WVI17O61]10− retains its structural integrity and exhibits the strongest inhibition of AbPPO4 (Ki = 6.5 mM). The trilacunary POT [PV2WVI15O56]12− rearranges to the more stable monolacunary [α2-PV2WVI17O61]10− (~ 62%) accompanied by release of free phosphates and shows the weakest inhibition (Ki = 13.6 mM). The hexalacunary anion [H2PV2WVI12O48]12− undergoes time-dependent hydrolysis resulting in a mixture of [H2PV2WVI12O48]12−, [PV8WVI48O184]40−, [PV2WVI19O69(H2O)]14− and [α2-PV2WVI17O61]10− which together leads to comparable inhibitory activity (Ki = 7.5 mM) after 48 h. For the solutions of [α/β-PV2WVI18O62]6−, [α2-PV2WVI17O61]10− and [PV2WVI15O56]12− the inhibitory activity is correlated to the degree of their rearrangement to [α2-PV2WVI17O61]10−. The rearrangement of hexalacunary [H2PV2WVI12O48]12− into at least four POTs with a negligible amount of monolacunary anion interferes with the correlation of activity to the degree of their rearrangement to [α2-PV2WVI17O61]10−. The good inhibitory effect of the Wells–Dawson [α2-PV2WVI17O61]10− anion is explained by the low charge density of its protonated forms Hx[α2-PV2WVI17O61](10−x)− (x = 3 or 4) at pH 6.8.
Similar content being viewed by others
Introduction
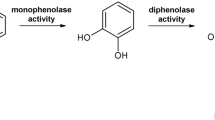
Polyphenol oxidases (PPOs) are copper-containing proteins omnipresent in animals, fungi, plants and bacteria1,2,3,4,5, with tyrosinases and catechol oxidases being prominent members of this enzyme family. Tyrosinases exhibit cresolase activity (EC 1.14.18.1; ortho-hydroxylation of monophenols to ortho-diphenols, monophenolase activity) and catecholase activity (EC 1.10.3.1; oxidation of ortho-diphenols to ortho-quinones; diphenolase activity), give rise to the rate determining step in melanogenesis and are involved in pigment coating and browning6. For its negligible lag phase and generally higher reaction velocity, the diphenolase activity of tyrosinases is usually assayed using L-DOPA (L-3,4−dihydroxyphenylalanine, Figure S1A), as the substrate for the dopachrome assay7. The polyoxotungstate (POT) inhibition parameters were determined by fitting the kinetic data to a generalized Michaelis–Menten8 model and Lineweaver–Burk plots9, accompanied by POT speciation studies applying 31P-NMR and 183W-NMR spectroscopy and mass spectrometry under physiological conditions (50 mM Na-citrate buffer, pH 6.8) with or without the substrate L-DOPA. A good mushroom PPO inhibitor is the structurally related kojic acid (Fig. S1B), which holds inhibition constant (Ki)-values in the µM range10 and acts as a competitive inhibitor.10 Mushroom (Agaricus bisporus; abbr: Ab) PPO is present in great quantity in fruiting bodies11. The enzyme has been thoroughly characterized in its structure12,13 and activity14. Following the established protocol by Pretzler et al.15, AbPPO4 was recombinantly expressed in E. coli and purified in its active form for the here presented inhibition studies.
Polyoxometalates (POMs) are metal–oxygen clusters commonly built up by W, Mo or V addenda ions, which are usually in their highest oxidation states exhibiting the electronic configuration d0 or d116,17. POMs show a widespread biological applicability such as antibacterial18 and anti-tumor activity19, and stable POTs have successfully been applied as additives in co-crystallization experiments with proteins20,21,22,23,24. Apart from the Anderson-Evans archetype25 (e.g. [TeVIWVI6O24]6− is stable in aqueous solution between pH 4.5 and 7.5) most POTs show low stability at physiological conditions, requiring solution NMR-measurements to reveal the composition under the applied conditions. As POTs possess a higher chemical stability than polyoxomolybdates (POMos), tungsten is usually selected as the addenda ion, when applying this compound class in biological investigations26.
Recently, a systematic approach varying the charge density in a series of Keggin POTs was reported by Breibeck et al.27 to characterize their inhibitory effects against recombinant AbPPO415. A detailed assignment of the active Keggin POT species was undertaken applying NMR spectroscopy27. A charge density dependence of the inhibitory capacities was derived, where the most active Keggin POT was [SiIVWVI12O40]4− (Ki = 4.7 mM), and the more stable Keggin anions with higher charge density did not inhibit diphenolase activity.
Another prominent POM scaffold investigated for biological activity is the Wells–Dawson archetype with the general formula [(Xn+)2M18O62](16−2n)−28, where M is either WVI or MoVI and Xn+ is commonly phosphorus(V) or arsenic(V). For the intact form of this cluster, 18 {MO6} octahedral units enclose both inner heteroion groups {XO4} by corner- and edge-sharing (Fig. 1). Wells–Dawson POMs exhibited antibacterial29 activity, and both the intact anion [α/β-PV2WVI18O62]6− and the hexalacunary form [H2PV2WVI12O48]12− inhibited Ca2+-ATPase, P-type ATPase30, and aquaporin-331. Transition metal-substituted Wells–Dawson POMos were used as inhibitors against α-glucosidase32 with Ki-values covering a wide range down to the µM scale.
In this study, the inhibitory effect of four structurally related Wells–Dawson POTs, intact Cat6[α/β-PV2WVI18O62]·14H2O (Cat = K+, NH4+)33,34, mono-lacunary K10[α2-PV2WVI17O61]·20H2O34,35, tri-lacunary K12[PV2WVI15O56]·24H2O35,36 and hexalacunary (NH4)12[H2PV2WVI12O48]·24H2O37,38, with net charges from 6− to 12− (Fig. 1) on the catecholase activity of AbPPO4 is explored. The focus is on the identification of the active species present under the diphenolase activity assay conditions by NMR spectroscopy and mass spectrometry. For the inhibition studies, the POTs were buffered at pH 6.8 in 50 mM Na-citrate and the obtained solutions are termed [P2W18]6− for K6[α/β-PV2WVI18O62]·14H2O and (NH4)6[α/β-PV2WVI18O62]·14H2O, [P2W17]10− for K10[α2-PV2WVI17O61]·20H2O, [P2W15]12− for K12[PV2WVI15O56]·24H2O and [P2W12]12− for (NH4)12[H2PV2WVI12O48]·24H2O to distinguish the buffered solution sample from the solid compound (see Abbreviation section in SI).
Results and discussion
Activity plots of AbPPO4 inhibited by Wells–Dawson POTs
AbPPO4 was purified and activated according to Pretzler et al.15. The ESI–MS of active AbPPO4 is presented in Fig. S2 and Table S1, the protein sequence of AbPPO4 in Fig. S3 and the SDS-PAGE analysis of AbPPO4 is shown in Figs. S4 and S5. The protocols for the synthesis of the Wells–Dawson POTs were taken from published procedures (cf. Table S2) and their identity was confirmed by IR (Fig. S6, Table S3) in the solid state and by 31P- and 183W-NMR in solutions (Figs. S7–S13). To investigate the Wells–Dawson POT-mediated inhibition of AbPPO4, the corresponding POT ([P2W18]6−, [P2W17]10−, [P2W15]12−, [P2W12]12−) was dissolved in 50 mM Na-citrate buffer (pH 6.8), where the enzyme shows its maximal activity4,15. The diphenolase activity of tyrosinase was monitored using 1 mM L-DOPA (Fig. S1A) as the substrate. Considering that the Wells–Dawson POT stability is pH and possibly time dependent, a speciation study was performed which is detailed in the paragraph "Speciation of Wells–Dawson POT by 183W-NMR and 31P-NMR analyses at pH 6.8". The concentration of the investigated Wells–Dawson phosphotungstates was in the range between 0 and 5 mM. If enzymatic inhibition was observed, data were taken in triplicates and fitted with a hyperbolic function (cf. SI Eq. (5)) from a mixed inhibition model to evaluate the Ki and α-parameters. All four solutions [P2W18]6−, [P2W17]10−, [P2W15]12− and [P2W12]12− showed a mixed-type inhibition with Ki values in the mM range, binding both to the free enzyme and to the enzyme–substrate complex39 (Table 1, Fig. 2). As a positive control for the inhibition of AbPPO4 diphenolase activity and for validation of the kinetic methodology, the well-characterized natural PPO-inhibitor kojic acid10 (Fig. S1B) was additionally tested and evaluated applying exactly the same mathematical model. The organic inhibitor kojic acid features a Ki in the µM range corresponding to a much higher affinity to the enzyme as revealed for the Wells–Dawson POTs. The Ki values previously reported for Keggin POTs [XWVI12O40]n− (X = PV, SiIV, BIII, AlII, H22+, BeII), which were obtained under the same conditions, are also in the mM range and vary from 4.7 mM (X = SiIV) to 25.6 mM (X = PV)27. The Ki values obtained using the Michaelis–Menten model, which is the generally accepted method for calculating the enzyme kinetic parameters, are used as primary values to compare the POTs’ activities.
Activity plots of AbPPO4 with four Wells–Dawson phosphotungstates [P2W18]6−, [P2W17]10−, [P2W15]12− and [P2W12]12−. The dopachrome assay was performed using 1 mM L-DOPA as the substrate in 50 mM Na-citrate buffer at pH 6.8. The measurements were taken in triplicates and a hyperbolic curve fit (Eq. (5), SI) was performed. The kinetic inhibition parameters are summarized in Table 1.
To evaluate the inhibitory effect, the activity curves for four Wells–Dawson phosphotungstates were plotted in the concentration range 0–5 mM (Fig. 2). Among the Wells–Dawson clusters, [P2W17]10− showed the greatest inhibitory effect (Ki = 6.5 mM) and the fitted α-parameter suggests a mixed mode of inhibition (Table 1). According to their Ki values [P2W17]10− (Ki = 6.5 mM) and [P2W12]12− (Ki = 7.5 mM) exhibited nearly identical inhibitory activity and [P2W18]6− (Ki = 9.7 mM) and [P2W15]12− (Ki = 13.6 mM) showed a lower inhibition capacity.
Lineweaver–Burk evaluation of inhibition types
The type of enzymatic inhibition is usually investigated by linear plots according to Lineweaver–Burk, also allowing for further validation of the inhibitory constant Ki.9 For [P2W18]6−, [P2W17]10−, [P2W15]12− and [P2W12]12− as well as for the kojic acid control measured in our previous study27, the dopachrome assay was repeated at five different substrate concentrations (varying from 0.4 to 1.5 mM) and three different inhibitor concentrations, respectively (SI section 7, Figs. S14–S17, Tables S4, S5). Each POT analysis yielded a set of three lines intersecting in a common point. The slopes (SI Eq. (13), insets in Figs. S14–S17) and ordinate intercepts of these regression lines were further evaluated to validate the inhibitory constant Ki and the α-parameter from the non-linear regression procedure. Therefore, the slopes were plotted against the used inhibitor concentrations to give lines intersecting the abscissa at -Ki. Similarly, the Lineweaver–Burk ordinate intercepts were evaluated for the α-parameter (cf. Table S5 and Table 1). In good accordance with their structural similarity, the respective intersection point of the three Lineweaver–Burk lines is the third quadrant for all four Wells–Dawson POT solutions [P2W18]6−, [P2W17]10−, [P2W15]12− and [P2W12]12−, which indicated mixed-type inhibition39,40 previously found for POT representatives of the Keggin archetype27.
Speciation of Wells–Dawson POT by 183W-NMR and 31P-NMR analyses at pH 6.8
The detailed structural information about species under the respective experimental conditions is essential for understanding of POT activity17. All Wells–Dawson POTs were dissolved in reaction buffer (50 mM Na-citrate, pH 6.8) and were adjusted with 1 M HCl or 1 M NaOH to pH 6.8 for measurements with AbPPO4, where the enzyme shows its maximal activity4,15. Upon dissolution of [P2W18]6− and [P2W17]10−, the pH of the buffer slightly decreased, whereas [P2W15]12− and [P2W12]12− behaved as bases. 31P-NMR in combination with 183W-NMR is established as a useful technique to determine the structural composition of the Wells–Dawson POTs16 and was previously successfully applied to elucidate the speciation of Keggin—type POTs in an analogous study27. The concentration of all species was calculated based on 31P-NMR peaks integration. Since the stock solutions of each Wells–Dawson POT was used up to three days after preparation and 183W-NMR acquisition takes 60 h, 31P-NMR spectra for all four solutions have been recorded 1 h and 48 h after preparation to check if speciation changes occur over time. Under the experimental conditions (50 mM Na-citrate, pH 6.8) [P2W18]6− showed partial hydrolysis of intact [α/β-PV2WVI18O62]6− (Fig. 1A) to monolacunary (Fig. 1B) [α2-PV2WVI17O61]10− (Figs. 3A, S8B). In the solution of intact POT one hour after preparation 12% of [α2-PV2WVI17O61]10− was detected based on 31P-NMR peaks integration (Fig. 3A), whereas 48 h after dissolution the concentration of monolacunary anion increased to 69%. The 183W-NMR spectrum of [P2W18]6− (Fig. S8B) is in agreement with 31P-NMR data and demonstrates 9 signals for the mono-lacunary anion and 6 additional signals related to a mixture of intact [α/β-PV2WVI18O62]6− isomers. 31P-NMR studies showed that the solution of monolacunary POT [P2W17]10− exhibited [α2-PV2WVI17O61]10− as the only species present in the freshly prepared solution (Fig. 3B), and as the dominant anion (92%) after 48 h of solution aging. The 31P-NMR spectrum of tri-lacunary POT [P2W15]12− (Fig. 3C) demonstrates the fast rearrangement of the tri-lacunary anion to 62% of monolacunary [α2-PV2WVI17O61]10− in both fresh and in 48 h aged solutions with 35% of free phosphate anions HxPO4x−3 and 3% remaining unidentified phosphotungstates. The 183 W-NMR spectra of monolacunary [P2W17]10− (Fig. S8A) and trilacunary [P2W15]12− (Fig. S8C) are in agreement with 31P-NMR data confirming the presence of only the monolacunary anion by 9 signals. The 31P-NMR spectrum of fresh hexalacunary POT [P2W12]12− (Fig. 3D) points to the presence of unhydrolyzed hexalacunary [H2PV2WVI12O48]12− (72%) together with a low amount of monolacunary [α2-PV2WVI17O61]10− (20%). After 48 h, the signals corresponding to hexalacunary [H2PV2WVI12O48]12− and monolacunary [α2-PV2WVI17O61]10− decreased to 9% and 3%, respectively, while two intense signals at − 7.5 and − 9.5 ppm appeared. These signals are of comparable intensities, but do not correspond to the monolacunary [α1-PV2WVI17O61]10− isomer.34 An anion which can give rise to a signal at − 9.5 ppm is [PV2WVI19O69(H2O)]14−, which contains two A-α-[PVWVI9O34]9- halves linked via one WVI ion in the equatorial plane and was previously reported as an intermediate species in phosphotungstate solutions as well as isolated in the solid state41,42. The signal at − 7.5 ppm corresponds to [PV8WVI48O184]40−, which was previously detected in solutions with a high amount of NH 4+37. The presence of four different POTs in [P2W12]12− 48 h after preparation renders the detection of all species’ signals in the 183W-NMR spectrum impossible due to their low concentrations and the low abundancy (14%) of the 183W isotope (Fig. S8D). The most intense signals at -190.3, -191.6 and -211.3 ppm correspond to three types of W ions in cyclic [PV8WVI48O184]40−43. 31P-NMR spectra recorded 96 h after preparation are identical to those recorded after 48 h for [P2W18]6−, [P2W17]10− and [P2W15]12−. In the 31P-NMR spectrum of [P2W12]12−, the signal at − 8.3 ppm corresponding to hexalacunary [H2PV2WVI12O48]12− disappeared completely after 96 h. In every 31P-NMR analysis, peaks for free phosphate were observed, indicating the release of HxPO4x−3 from the POT clusters as a consequence of partial decomposition or rearrangement (Fig. 3). The weak signals between − 12 and − 7 ppm in the 31P-NMR spectra (Fig. 3) with a total amount of no more than 19% of all POT signals, correspond to solution intermediates of unknown identity that have been reported previously44. The inhibitory activity of Wells–Dawson solutions does not correlate with the presence of any of those intermediates.
31P-NMR spectra of [P2W18]6−, [P2W17]10−, [P2W15]12− and [P2W12]12− in 50 mM Na-citrate buffer at pH 6.8 (Table S2) recorded 1 h and 48 h after preparation. A) educts: [α-PV2WVI18O62]6− and [β-PV2WVI18O62]6−; products: fresh—[α/β -PV2WVI18O62]6− (88%) and [α2-PV2WVI17O61]10− (12%), after 48 h – [α/β -PV2WVI18O62]6− (15%) and [α2-PV2WVI17O61]10− (69%); B) educt [α2-PV2WVI17O61]10−; products: fresh—[α2-PV2WVI17O61]10− (100%), after 48 h—[α2-PV2WVI17O61]10− (92%); C) educt: [PV2WVI15O56]12−; products: fresh—[α2-PV2WVI17O61]10− (62%), after 48 h—[α2-PV2WVI17O61]10− (61%); D) educt: [H2PV2WVI12O48]12−; products: fresh—[H2PV2WVI12O48]12− (72%), [α2-PV2WVI17O61]10− (20%), after 48 h—[PV8WVI48O184]40− (23%), [PV2WVI19O69(H2O)]14− (22%), [H2PV2WVI12O48]12− (9%), [α2-PV2WVI17O61]10− (3%). Weak signals in the range between − 10 and − 7 ppm correspond to unstable solution intermediates with a total of 9% for [P2W18]6− (A); 8% for [P2W17]10− (B); 5% for [P2W15]12− (C) and 19% for [P2W12]12− (D) in aged solutions based on integrated POT signals34,41,44, Signal assignment is based on references34,37,41. Color code: {WO6} octahedra, white; {PO4} tetrahedra, green, red, blue, pink, orange, burgundy; O atoms, red.
Influence of the substrate L-DOPA at pH 6.8 on POT's speciation
During the kinetic measurement of intact [P2W18]6−, the solution immediately turned blue due to the intervalence charge transfer of WV–O–WVI ↔ WVI–O–WV after addition of L-DOPA, hinting towards the reduction of POTs in the presence of L-DOPA (Fig. S18). Many POTs are redox active and can easily accept electrons45. The cyclic voltammogram of the intact Wells–Dawson anion [α-PV2WVI18O62]6− displays notable proton-coupled electron redox activity, which allows this molecule to reversibly accept up to 18 electrons in aqueous solution at pH 446. In contrast, lacunary anions (Fig. 1B–D) accept electrons less easily, for example, the reduction energy of monolacunary [α2-P2WVI17O61]10− is less favorable by 1.6 eV than for intact [PV2WVI18O62]6−47. Since a reduction occurs only in solution of intact [P2W18]6− in the presence of L-DOPA and no color change and no change in speciation (Figs. S9, S11, Table S2) were observed for the other three POT samples [P2W17]10−, [P2W15]12− and [P2W12]12−, it can be concluded that only the intact [PV2WVI18O62]6− accepts electrons in the presence of L-DOPA. This reduction and protonation from [PV2WVI18O62]6− to H2[PV2WVI12WV6O62]10− has been previously reported at pH 6.848. The reduction might affect inhibitory activity of [P2W18]6− based on the lower value of R2 for [P2W18]6− as compared to the ones observed for the other Wells–Dawson POTs [P2W17]10−, [P2W15]12− and [P2W12]12− (Table 1).
Charge density dependence of inhibitory effects of Wells–Dawson POT species
The charge density of POMs, expressed in number of charges (q) per addenda metal ions (m), is a criterion to characterize the chaotropic behavior of POMs (Table S6)49,50,51,52,53. Recently, it was shown that the affinity of POMs towards biomolecules is attributable to their superchaotropic character, and POMs with moderate charge densities (q/m = 0.33) interact considerably strong with surfaces of different or mixed polarities, which are present in protein molecules54. The activity of [P2W18]6−, [P2W17]10− and [P2W15]12− solutions correlates with the amount of the dominant monolacunary anion [α2-PV2WVI17O61]10− in Na-citrate buffer at pH 6.8 (Fig. 3, Scheme S1). For the unprotonated form of the active monolacunary Wells–Dawson anion [α2-PV2WVI17O61]10− the q/m ratio is 0.59 (Table 2), however, the protonation of this anion in neutral solutions has previously been shown by electrochemical analysis in combination with theoretical calculations47,55. For POMs the proton affinity difference between the terminal and bridging oxygen is around 11 kcal mol−156, proving that the four oxygen atoms surrounding the lacuna are much more basic than the bridging and terminal O atoms (Fig. 1B), and thus prone to protonation. Electrospray-ionization mass spectrometry (ESI–MS) has already been successfully used for the protonation states assignment of Wells–Dawson POTs containing different heteroatoms57. Consequently, ESI–MS has been applied to investigate the protonation state of monolacunary [α2-PV2WVI17O61]10−. When recording ESI–MS for POMs in Na-citrate buffer, the relative intensity of citrate anion signals is almost 100%, suppressing POM signals. Hence, the measurements had to be carried out in water at pH 6.4 (adjusted with HCl), where [α2-PV2WVI17O61]10− is the predominant species (Fig. S19), as well as in a CH3CN/CH3OH/H2O mixture. The spectra recorded in both solvents show signals for NaH4[α2-PV2WVI17O61]5- at 838.0 and NaKH3[α2-PV2WVI17O61]5- at 845.6 m/z, while species with lower protonation states have not been detected (Fig. S19). Therefore, the monolacunary anion [α2-PV2WVI17O61]10− is present as the three-fold protonated anion H3[α2-PV2WVI17O61]7- with a charge density of 0.41 and the four-fold protonated anion H4[α2-PV2WVI17O61]6− with a charge density of 0.35. The predominant species in [P2W12]12− is the wheel-shaped [PV8WVI48O184]40− (23%), which can be protonated to H16[PV8WVI48O184]24−58 q/m = 0.5. Due to the compositional complexity of the hexalacunary anion it is impossible to accurately correlate its activity with the POTs’ charge density.
Conclusions
The inhibitory effects of four Wells–Dawson phosphotungstates starting from intact, mono-, three- and hexalacunary forms against AbPPO4 were investigated with a focus on speciation under the dopachrome assay conditions. During the investigation of [P2W18]6−, [P2W17]10− and [P2W15]12−, the inhibition effect was assigned to the stable POT species Hx[α2-PV2WVI17O61](10−x)− (x = 3 or 4), to which intact [α/β-PV2WVI18O62]6− and trilacunary [PV2WVI15O56]12− rearrange in significant quantities (Fig. 4). Interestingly, hexalacunary [P2W12]12− demonstrates a more complex and time-dependent scenario with re-arrangement to the mixture of [PV8WVI48O184]40− (23%), [PV2WVI19O69(H2O)]14− (22%), [H2PV2WVI12O48]12− (9%), [α2-PV2WVI17O61]10− (3%) and free phosphate (24%), which does not allow us to unambiguously assign activity to one or several POT anions. The magnitude of the inhibitory activities correlated with the amount of Hx[α2-PV2WVI17O61](10−x)- (x = 3 or 4) in the case of [P2W18]6− (Ki = 9.7 mM), [P2W17]10− (Ki = 6.5 mM) and [P2W15]12− (Ki = 7.5 mM), which was quantified by 31P-NMR peaks integration.
The POTs’ inhibitory activities are compared with the main species at pH 6.8 and at assay conditions. The intensity of the blue arrows correlates with the relative rearrangement to Hx[α2-PV2WVI17O61](10−x)− (x = 3 or 4) for the different inhibitors at pH 6.8. The more Hx[α2-PV2WVI17O61](10−x)− (x = 3 or 4) is in the sample, the higher the observed inhibitory activity of the POT. For [P2W12]12− two predominant species are shown. n = 7–16 in Hn[PV8WVI48O184](40−n)- according to58. Color code: {WO6} octahedra, green; {PO4} tetrahedra, turquoise.
Methods
Protocols for synthesis, spectroscopic assignments and kinetic curves are discussed in the supplementary information. All chemicals have been purchased from Sigma-Aldrich (Vienna, Austria) and Carl-Roth (Karlsruhe, Germany) and were at least of analytical grade. They were used without further purification.
Preparation of AbPPO4 in its active form
For the preparation of active AbPPO4, the procedure published by Pretzler et al.15 was used. Briefly, latent (inactive) AbPPO4 was expressed with an N-terminal glutathione-S-transferase tag in E. coli BL21(DE3). For the protein expression, ZYM-5052 medium59 without addition of trace elements was used for 20 h at 20 °C, before 0.5 mM CuSO4 was added to the medium and incubation was continued for another 20 h. The cells were resuspended in Tris/HCl buffer.
The cell lysis was done via French press. After centrifugation, the supernatant was loaded onto an affinity chromatography GSTrap Fast Flow column and GST-AbPPO4 was obtained after elution with glutathione. The GST tag was cut by digestion with HRV-3C protease. After another affinity chromatography step the latent AbPPO4 was obtained in the flow-through. For activation of latent AbPPO4 for inhibition studies the C-terminal cap was removed from the PPO with Proteinase K and the activated form was purified via size-exclusion chromatography in 50 mM Na-citrate buffer at pH 6.8.
Synthesis of α-Wells Dawson POTs
The Wells–Dawson POTs were synthesized according to the procedures given in Table S2 in the Supplementary Information. The starting K6[α/β-PV2WVI18O62]·14 H2O and (NH4)6[α/β-PV2WVI18O62]·14 H2O were synthesized as mixtures of alpha and beta isomers precisely following the procedure from ref.34. K6[α/β-PV2WVI18O62]·14 H2O and (NH4)6[α/β-PV2WVI18O62]·14 H2O are both suitable for synthesis of lacunary derivatives34. Slight modifications during the preparations are given below:
-
(1)
(NH4)6[α/β-PV2WVI18O68]·14H2O: During the synthesis no bromine water was added.
-
(2)
(NH4)12[α-H2PV2WVI12O48]·24H2O: The precipitate was collected after reaction overnight.
IR spectroscopy
A Bruker Vertex 70 IR Spectrometer equipped with a single-reflection diamond-ATR unit was used to verify the structure of the applied POTs. The distortion vibrations of W–O–W arise at 400 to 900 cm−1, the W=O stretching vibration occurs in the range of 930–960 cm−1 and the P=O vibrations appear between 960 and 1200 cm−1 (Fig. S6, Table S3).
Electrospray-ionization mass spectrometry
Analysis was performed with an ESI–Qq–oaRTOF supplied by Bruker Daltonics Ltd. Bruker Daltonics Data Analysis software was used to analyze the results. The measurements were carried out in H2O and in a mixture of CH3CN/MeOH/H2O, collected in negative ion mode and with the spectrometer calibrated with the standard tune—mix to give an accuracy of better than 5 ppm in the region of m/z 100–1900.
Nuclear magnetic resonance spectroscopy
183W-NMR and 31P-NMR were recorded with a Bruker FT-NMR spectrometer Avance Neo 500 MHz (Bruker, Rheinstetten, Germany) at 25 °C. Chemical shifts were measured relative to 1 M Na2WO4 and 85% H3PO4. 183W-NMR samples were prepared in 2.7 mL Na-citrate buffer (50 mM, pH 6.8) with a POT concentration of 2 mM with or without 1 mM L-DOPA and measured in 10 mm tubes. The experimental time was ca. 60 h, with a standard pulse program at 20.836 MHz and a 63° flip angle with 1 s relaxation delay. Subsequently, 31P-NMR was measured at 202.53 MHz.
183W-NMR
183W-NMR was performed in 50 mM Na-citrate buffer at pH 6.8 to obtain insight into the chemical speciation and hydrolytic stability of POTs. The peaks of the 183W-NMR for [P2W18]6− are shifted by approx. 4–5 ppm downfield relative to the assignment in the literature (Table S2). The slight shifting can be explained by different buffer conditions and ionic strength of the solution with regard to the reference data.
The 183W-NMR speciation indicates the presence of [α2-PV2WVI17O61]10− anions in [P2W18]6−, [P2W17]10−, [P2W15]12− at pH 6.8 (Figs. S8, S12). For the 183W-NMR of [P2W18]6− at pH 4 two lines (1:2) were assigned (Fig. S7), whereas for the same compound at neutral pH a mixture of [α-PV2WVI18O62]6− (1:2) and [α2-PV2WVI17O61]10− (2:2:2:2:1:2:2:2:2) was observed (Fig. S8). Also, the β-isomer was detected at pH 4 and pH 6.8, respectively (Figs. S7, S8). Interestingly, the spectra for [P2W18]6− at pH 6.8 with L-DOPA exhibits only signals for [α2-PV2WVI17O61]10− (Fig. S8). Upon storage of [P2W18]6− for 28 d, low amounts of the α-isomer were found, but no β-isomer was detected (Fig. S12).
31P-NMR
The presence of [α2-PV2WVI17O61]10− in the 183W-NMR at pH 6.8 was confirmed by two respective signals in the 31P-NMR (Figs. 3, S10). The signals at 0 or higher ppm values were assigned to HxPO4x-3 (x = 1, 2), indicating the decomposition of POTs. The signals at − 7.3 ppm and − 14.5 ppm were assigned to [α2-PV2WVI17O61]10−.
Electrospray ionization mass spectrometry of AbPPO4
The mass spectrum (Fig. S2) was recorded with a LTQ Orbitrap Velos Mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) fitted with a nanospray ion source, coupled to a nano HPLC-system (UltiMate 3000, Dionex), followed by deconvolution with Bruker Compass Software.
The sample was loaded on a trap column (µ-Precolumn 5 mm × 300 µ i.d. C4 PepMapp300, 5 µm, 300 Å, Thermo Scientific) with 0.1% trifluoroacetic acid (TFA). The separation of the sample was implemented on a C4 analytical column 50 cm × 75 µm Accucore C4, 2.6 µm, 150 Å (Thermo Fisher Scientific) at a flow rate of 300 nL/min. Mobile Phase A consisted of 2% ACN, 98% H2O and 0.1% formic acid (FA). Mobile Phase B comprised 80% ACN, 20% H2O and 0.1% FA.
Dopachrome assay
UV–VIS measurements were carried out on a Shimadzu UV 2401PC following the specific absorption of dopachrome at 475 nm (ε = 3700 M−1 cm−17). For all measurements, 50 mM Na-citrate at pH 6.8 was used as the buffer agent to ensure physiological conditions. The stock solutions of POTs were prepared on the day of measurements and used within 3 days.
For a general 1 mL reaction setup in a polystyrene cuvette, 1 µg/mL AbPPO4 (~ 23 nM) was used to catalyze the reaction. The relative inhibition was investigated using 1 mM L-DOPA as the substrate and applying an inhibitor concentration range from 0 to 5 mM to obtain activity curves. For the Lineweaver–Burk plots, a L-DOPA concentration range of 0.4–1.5 mM was assayed for three different POT concentrations, respectively.
References
Mayer, A. M. & Harel, E. Polyphenol oxidase in plants. Phytochemistry 18, 193–215. https://doi.org/10.1016/0031-9422(79)80057-6 (1979).
Mayer, A. M. Polyphenol oxidase in plants and fungi: Going places? A review. Phytochemistry 67, 2318–2331. https://doi.org/10.1016/j.phytochem.2006.08.006 (2006).
Fernandez, E., Sanchez-Amat, A. & Solano, F. Location and catalytic characteristics of a multipotent bacterial polyphenol oxidase. Pigment Cell Res. 12, 331–339. https://doi.org/10.1111/j.1600-0749.1999.tb00767.x (1999).
Pretzler, M. & Rompel, A. What causes the different functionality in type-III-copper enzymes? A state of the art perspective. Inorg. Chim. Acta 481, 25–31. https://doi.org/10.1016/j.ica.2017.04.041 (2018).
Kampatsikas, I. & Rompel, A. Similar but still different: Which amino acid residues are responsible for varying activities in type-III copper enzymes?. ChemBioChem 22, 1161–1175. https://doi.org/10.1002/cbic.202000647 (2021).
Rodríguez-López, J. N., Tudela, J., Varón, R., García-Carmona, F. & García-Cánovas, F. Analysis of a kinetic model for melanin biosynthesis pathway. J. Biol. Chem. 267, 3801–3810 (1992).
Winder, A. J. & Harris, H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur. J. Biochem. 198, 317–326. https://doi.org/10.1111/j.1432-1033.1991.tb16018.x (1991).
Johnson, K. A. A century of enzyme kinetic analysis, 1913 to 2013. FEBS Lett. 587, 2753–2766. https://doi.org/10.1016/j.febslet.2013.07.012 (2013).
Waldrop, G. L. A qualitative approach to enzyme inhibition. Biochem. Mol. Biol. Educ. 37, 11–15. https://doi.org/10.1002/bmb.20243 (2009).
Lima, C. R. et al. Combined kinetic studies and computational analysis on kojic acid analogs as tyrosinase inhibitors. Molecules 19, 9591–9605. https://doi.org/10.3390/molecules19079591 (2014).
Pretzler, M., Bijelic, A. & Rompel, A. Fungal tyrosinases: why mushrooms turn brown. In Elsevier Reference Module in Chemistry, Molecular Sciences and Chemical Engineering (ed. Reedijk, J.) (Elsevier, 2015). https://doi.org/10.1016/B978-0-12−409547-2.11521-5.
Mauracher, S. G., Molitor, C., Al-Oweini, R., Kortz, U. & Rompel, A. Latent and active abPPO4 mushroom tyrosinases cocrystallized with hexatungstotellurate(VI) in a single crystal. Acta Cryst. D70, 2301–2315. https://doi.org/10.1107/S1399004714013777 (2014).
Mauracher, S. G., Molitor, C., Al-Oweini, R., Kortz, U. & Rompel, A. Crystallization and preliminary X-ray crystallographic analysis of latent isoform PPO4 mushroom (Agaricus bisporus) tyrosinase. Acta Cryst. F70, 263–266. https://doi.org/10.1107/S2053230X14000582 (2014).
Mauracher, S. G. et al. High level protein-purification allows the unambiguous polypeptide determination of latent isoform PPO4 of mushroom tyrosinase. Phytochemistry 99, 14–25. https://doi.org/10.1016/j.phytochem.2013.12.016 (2014).
Pretzler, M., Bijelic, A. & Rompel, A. Heterologous expression and characterization of functional mushroom tyrosinase (AbPPO4). Sci. Rep. 7, 1810. https://doi.org/10.1038/s41598-017-01813-1 (2017).
Pope, M. T. Heteropoly and Isopoly Oxometalates (Springer, 1983).
Gumerova, N. I. & Rompel, A. Polyoxometalates in solution: speciation under spotlight. Chem. Soc. Rev. 49, 7568–7601. https://doi.org/10.1039/D0CS00392A (2020).
Bijelic, A., Aureliano, M. & Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 54, 1153–1169. https://doi.org/10.1039/C7CC07549A (2018).
Bijelic, A., Aureliano, M. & Rompel, A. Polyoxometalates as potential next-generation metallodrugs in the combat against cancer. Angew. Chem. Int. Ed. 58, 2980–2999. https://doi.org/10.1002/ange.201803868 (2019).
Bijelic, A. & Rompel, A. The use of polyoxometalates in protein crystallography: An attempt to widen a well-known bottleneck. Coord. Chem. Rev. 299, 22–38. https://doi.org/10.1002/ange.201803868 (2015).
Bijelic, A. & Rompel, A. Ten good reasons for the use of the Tellurium-centered Anderson-Evans polyoxotungstate in protein crystallography. Acc. Chem. Res. 50, 1441–1448. https://doi.org/10.1021/acs.accounts.7b00109 (2017).
Bijelic, A. & Rompel, A. Polyoxometalates: More than a phasing tool in protein crystallography. ChemTexts 4, 10. https://doi.org/10.1007/s40828-018-0064-1 (2018).
Breibeck, J., Bijelic, A. & Rompel, A. Transition metal-substituted Keggin polyoxotungstates enabling covalent attachment to proteinase K upon cocrystallization. Chem. Commun. 55, 11519–11522. https://doi.org/10.1039/c9cc05818d (2019).
Mac Sweeney, A. et al. The crystallization additive hexatungstotellurate promotes the crystallization of the HSP70 nucleotide binding domain into two different crystal forms. PLoS ONE 13(6), e0199639. https://doi.org/10.1371/journal.pone.0199639 (2018).
Blazevic, A. & Rompel, A. The Anderson-Evans polyoxometalate: From inorganic building blocks via hybrid organic-inorganic structures to tomorrows “Bio-POM”. Coord. Chem. Rev. 307, 42–64. https://doi.org/10.1016/j.ccr.2015.07.001 (2016).
Gumerova, N. I. & Rompel, A. Interweaving disciplines to advance chemistry: Applying polyoxometalates in biology. Inorg. Chem. 60, 6109–6114. https://doi.org/10.1021/acs.inorgchem.1c00125 (2021).
Breibeck, J., Gumerova, N. I., Boesen, B. B., Galanski, M. & Rompel, A. Keggin-type polyoxotungstates as mushroom tyrosinase inhibitors: A speciation study. Sci. Rep. 9, 5183. https://doi.org/10.1038/s41598-019-41261-7 (2019).
Briand, L. & Baronetti, G. The state of the art on Wells–Dawson heteropoly-compounds: A review of their properties and applications. Appl. Catal. A 256, 37–50. https://doi.org/10.1016/S0926-860X(03)00387-9 (2003).
Gumerova, N. I. et al. Anitbacterial activity of polyoxometalates against Moraxella catarrhalis. Front. Chem. 6, 336. https://doi.org/10.3389/fchem.2018.00036 (2018).
Gumerova, N. I. et al. The P-type ATPase inhibiting potential of polyoxotungstates. Metallomics 10, 287–295. https://doi.org/10.1039/C7MT00279C (2018).
Pimpão, C. et al. The aquaporin-3 inhibiting potential of polyoxotungstates. Int. J. Mol. Sci. 21, 2467. https://doi.org/10.3390/ijms21072467 (2020).
Hu, J.-J. et al. Transition metal substituted polyoxometalates as α-glucosidase inhibitors. Eur. J. Inorg. Chem. 3270–3276, 2019. https://doi.org/10.1002/ejic.201900306 (2019).
Dawson, B. The structure of the 9(18)-heteropoly anion in potassium 9(18)-tungstophosphate, K6[P2W18O62]14H2O. Acta Cryst. 6, 113–126. https://doi.org/10.1107/S0365110X53000466/ (1952).
Ginsberg, A. P. Inorganic synthesis. Wiley Online Library 27, 104–111 (1990).
Müller, A. et al. „Adding“ stable functional complementary, nucleophilic and electrophilic clusters: a synthetic route to [{(SiW11O39)}]Mo3S4(H2O)3(µ-OH)}2]10− and [{(P2W17O61)Mo3S4(H2O)3(µ-OH)}2]14− as examples. Chem. Commun. 13, 1189–1190. https://doi.org/10.1039/a903170g (1999).
Finke, R. G., Lyon, D. K., Nomiya, K. & Weakley, T. J. R. Structure of nonasodium – triniobatopentadecawolframatodiphosphate-acetonitrile-water (1/2/23), Na9[P2W15Nb3O62] 2CH3CN 23H2O. Acta Cryst. C46, 1592–1596. https://doi.org/10.1107/S0108270190000038 (1990).
Boyd, T., Mitchell, S. G., Gabb, D., Long, D.-L. & Cronin, L. Investigating cation binding in the polyoxometalate-super-crown [P8W48O184]40−. Chem. Eur. J. 17, 12010−12014. https://doi.org/10.1002/chem.201101666 (2011).
Mal, S. S. & Kortz, U. The wheel-shaped Cu20 tungstophosphate [Cu20Cl(OH)24(H2O)12(P8W48O184)]25- ion. Angew. Chem. Int. Ed. 24, 3777–3780. https://doi.org/10.1002/anie.200500682 (2005).
Copeland, R. A. Evaluation of enzyme inhibitors in drug discovery: A guide for medicinal chemists and pharmacologists. Methods Biochem. Anal. 46, 1–265. https://doi.org/10.1002/9781118540398 (2005).
Wang, Z.-M. et al. Rational modification of donepezil as multifunctional acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 123, 282–297. https://doi.org/10.1016/j.ejmech.2016.07.052 (2016).
Maksimovskaya, R. I. & Maksimov, G. M. 31P NMR studies of hydrolytic conversions of 12−tungstophosphoric heteropolyacid. Coord. Chem. Rev. 385, 81–99. https://doi.org/10.1016/j.ccr.2019.01.014 (2019).
Tourné, C. M. & Tourné, G. F. Aquanonadecatungstodiphosphate(14–) polyanion, [P2W19O69(OH2)]14–: X-ray crystallographic structure of its potassium salt, chemical relationships in the tungstophosphate system, and conversion into the diaquaicosatungstodiphosphate [P2W20O70(OH2)2]10−. J. Chem. Soc. Dalton Trans. https://doi.org/10.1039/DT9880002411 (1988).
Haouas, M. et al. Investigation of the protonation state of the macrocyclic {HnP8W48O184} anion by modeling 183W NMR chemical shifts. New J. Chem. 41, 6112–6119. https://doi.org/10.1039/c7nj00915a (2017).
Massart, R., Contant, R., Fruchart, J. M., Ciabrini, J. P. & Fournier, M. Phosphorus-31 NMR studies on molybdic and tungstic heteropolyanions. Correlation between structure and chemical shift. Inorg. Chem. 16, 2916−2921. https://doi.org/10.1021/ic50177a049 (1977).
Gumerova, N. I. & Rompel, A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2, 0112. https://doi.org/10.1038/s41570-018-0112 (2018).
Chen, J.-J., Symes, M. D. & Cronin, L. Highly reduced and protonated aqueous solutions of [P2W18O62]6− for on-demand hydrogen generation and energy storage. Nat. Chem. 10, 1042–1047. https://doi.org/10.1038/s41557-018-0109-5 (2018).
López, X. Effect of protonation, composition and isomerism on the redox properties and electron (de)localization of classical polyoxometalates. Phys. Sci. Rev. 2, 20170137. https://doi.org/10.1515/psr-2017-0137 (2017).
Sadakane, M. & Steckhan, E. Electrochemical properties of polyoxometalates as electrocatalysts. Chem. Rev. 98, 219–237. https://doi.org/10.1021/cr96043a (1997).
Assaf, K. et al. Water structure recovery in chaotropic anion recognition: high-affinity binding of dodecaborate clusters to γ-cyclodextrin. Angew. Chem. Int. Ed. 54, 6852–6856. https://doi.org/10.1002/anie.201412485 (2015).
Assaf, K. & Nau, W. The chaotropic effect as an assembly motif in chemistry. Angew. Chem. Int. Ed. 57, 13968–13981. https://doi.org/10.1002/anie.201804597 (2018).
Buchecker, T. et al. Polyoxometalates in the Hofmeister series. Chem. Commun. 54, 1833–1836. https://doi.org/10.1039/C7CC09113C (2018).
Naskar, B., Diat, O., Nardello-Rataj, V. & Bauduin, P. Nanometer-size polyoxometalate anions adsorb strongly on neutral soft surfaces. J. Phys. Chem. C 119, 20985–20992. https://doi.org/10.1021/acs.jpcc.5b06273 (2015).
Buchecker, T. et al. Self-assembly of short chain poly-N-isopropylacrylamid induced by superchaotropic keggin polyoxometalates: From globules to sheets. J. Am. Chem. Soc. 141, 6890–6899. https://doi.org/10.1021/jacs.8b12181 (2019).
Solé-Daura, A., Poblet, J. M. & Carbó, J. J. Structure-activity relationships for the affinity of chaotropic polyoxometalate anions towards proteins. Chem. Eur. J. 26, 5799–5809. https://doi.org/10.1002/chem.201905533 (2020).
Asakura, T., Donnet, L., Picart, S. & Adnet, J.-M. Extraction of hetero polyanions, P2W17O6110−, P2W18O626−, SiW11O398- by TBP. J. Radioanal. Nuclear Chem. 246, 651–656. https://doi.org/10.1023/A:1006795929703 (2000).
López, X., Bo, C. & Poblet, J. M. Electronic properties of polyoxometalates: Electron and proton affinity of mixed-addenda Keggin and Wells−Dawson anions. J. Am. Chem. Soc. 124, 12574–12582. https://doi.org/10.1021/ja020407z (2002).
Long, D.-L., Streb, C., Song, Y.-F., Mitchell, S. & Cronin, L. Unravelling the complexities of polyoxometalates in solution using mass spectrometry: Protonation versus heteroatom inclusion. J. Am. Chem. Soc. 130, 1830–1832. https://doi.org/10.1021/ja075940z (2008).
Baroudi, I. et al. Supramolecular assembly of gelatin and inorganic polyanions: Fine-tuning the mechanical properties of nanocomposites by varying their composition and microstructure. Chem. Mater. 27, 1452–1464. https://doi.org/10.1021/cm502605q (2015).
Studier, F. W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 41, 207–234. https://doi.org/10.1016/j.pep.2005.01.016 (2015).
Acknowledgements
This research was funded by the Austrian Science Fund (FWF): P27534, P32326 and P33089 (to A.R.) and P33927 (to N.I.G.) and by the University of Vienna, which is gratefully acknowledged. The authors wish to thank Anna Fabisikova, MSc for ESI-MS measurements, Ricarda Ofenschüssl and Ing. Susanne Felsinger for 31P NMR measurements, Dipl.-Ing. Matthias Pretzler for valuable discussions concerning this work and Elias Tanuhadi, MSc for proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
Design of the study (R.L., J.B., A.R.); conduction of the study (R.L., J.B., N.I.G.), data collection (M.S.G.), data collection, analysis, and interpretation (R.L., J.B., N.I.G.); Manuscript preparation and review (R.L., J.B., N.I.G., M.S.G., A.R.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lampl, R., Breibeck, J., Gumerova, N.I. et al. Wells–Dawson phosphotungstates as mushroom tyrosinase inhibitors: a speciation study. Sci Rep 11, 19354 (2021). https://doi.org/10.1038/s41598-021-96491-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96491-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.