Abstract
The prognosis of out of cardiac arrest is poor and most cardiac arrest patients suffered from the non-shockable rhythm especially in patients without pre-existing cardiovascular diseases and medication prescription. Beta-blocker (ß-blocker) therapy has been shown to improve outcomes in cardiovascular diseases such as heart failure, ischemia related cardiac, and brain injuries. Therefore, we investigated whether prior ß-blockers use was associated with reduced mortality in patients with cardiac arrest and non-shockable rhythm. We conducted a population-based retrospective cohort study using multivariate propensity score–based regression to control for differences among patients with cardiac arrest. A total of 104,568 adult patients suffering a non-traumatic and non-shockable rhythm cardiac arrest between 2005 and 2011 were identified. ß-blocker prescription at least 30 days prior to the cardiac arrest event was defines as the ß-blockers group. We chose 12.5 mg carvedilol as the cut-off value and defined greater or equal to carvedilol 12.5 mg per day and its equivalent dose as high-dose group. After multivariate propensity score–based logistic regression analysis, patients with prior ß-blockers use were associated with better 1-year survival [adjusted odds ratio (OR), 1.15, 95% confidence interval (CI) 1.01–1.30; P = 0.031]. Compared to non-ß-blocker use group and prior low-dose ß-blockers use group, prior high-dose ß-blockers use group was associated with higher mechanical ventilator wean success rate (adjusted OR 1.19, 95% CI 1.01–1.41, P = 0.042). In conclusion, prior high dose ß-blockers use was associated with a better 1-year survival and higher weaning rate in patients with non-shockable cardiac arrest.
Similar content being viewed by others
Introduction
More than 356,000 people experience out-of-hospital cardiac arrest (OHCA) annually in the U.S., and almost 90% cases are considered fatal1. Pharmacotherapy can prevent disease progression; and effective resuscitation and rapid care for OHCA can lead to better outcomes; however, the long-term survival remains poor. Cardiac arrest with non-shockable rhythm is more prevalent at present and is associated with higher risk of mortality than shockable rhythm2. Also, people suffering cardiac arrest with non-shockable rhythm have less predisposing cardiac disease3.
Patients might experience complex pathophysiological processes such as brain injury, cardiac dysfunction, global ischemia–reperfusion injuries and a sepsis-like syndrome with cytokine storms through the duration of cardiac arrest4. Beta blockers (β-blocker) are drugs widely used to treat patients with cardiac arrhythmia, heart failure, and coronary artery disease5. β-blockers use is associated with better prognosis in patients with heart failure, myocardial infarction, cardiac arrhythmia, and acute stroke6,7,8. Whether prior β-blockers use improves survival in patients with cardiac arrest and non-shockable rhythm remains unclear. Therefore, this study aimed to determine if the prior use of β-blocker is associated with better survival and improved clinical and functional outcomes in OHCA patients with non-traumatic and non-shockable rhythms.
Materials and methods
Data sources
The mandatory health insurance in Taiwan, which now covers more than 99% of the Taiwanese population (23.78 million people), began in 1995 through the National Health Insurance (NHI) program9. All medical institutions contracted with the NHI program are required to submit standard computerized claims documents for medical expenses. Thus, all the details of claims, patients’ demographics, and medical care procedures are stored in the National Health Insurance Research Database (NHIRD). This retrospective and observational study was based on a nationwide population cohort. The study used electronic health records data as well as unstructured text found in clinical notes and reports accumulated between 2005 and 2011. The codes of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) were used for disease diagnoses. To conform to the rules of data privacy, personal information was encrypted, and was transformed to a de-identified manner. The study protocol was approved by the National Taiwan University Hospital Research Ethics Committee (study no. 201811086RINA) and waived the requirement of informed consent. All study methods were carried out in accordance with relevant guidelines and regulations.
Patient selection and follow-up
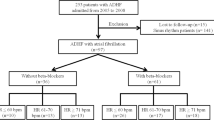
The study population included all patients receiving cardiopulmonary resuscitation (CPR) at the emergency room. These patients would be coded with a procedure code “47029c”. To appropriately select post-cardiac arrest patients, patients with (1) non-level 1 triage, (2) trauma-related event experience, (3) more than 6 h stay in emergency room, (4) shockable rhythm, and (5) < 18 years old were excluded from the study (Fig. 1). Patients presenting to emergency room in Taiwan are allocated a triage category based on the time in which they need medical attention. People who need to have treatment immediately or within two minutes are categorized as Level 1(resuscitation) and they are in an immediately life-threatening condition. Reportedly, only 3.9% of patients were assigned into levels 110. Previous study revealed that the hospital mortality was associated with the ER boarding time. Patients stayed longer than 6 h in emergency room (coded in our National Health Insurance Research Database (NHIRD) were excluded to minimize the confounding effects of inadequate post-cardiac arrest care11.
Patients were followed-up from the index date of cardiac arrest until 1-year survival or death. The “β-blocker group” is composed of cardiac arrest patients using β-blockers continuously for at least 30 days before the index date. Patients without β-blocker use in the 30 days before the cardiac arrest event were included in the control group or “non-β-blocker group.” To investigate the dose effect of β-blockers on the 1-year survival in these patients, they were categorized to high-dose if the mean daily dose was greater or equal to carvedilol 12.5 mg or its equivalent dose. The Taiwan National Health Research Institute definitions of urbanization, hospital classification, and qualifications of medical center criteria have been previously described12,13 (Fig. 1).
Outcome measures
The primary end point was 1-year survival. Additional clinical outcomes included survival to ICU admission and survival to discharge. The functional outcomes measured were ventilator wean success rate at 1 month.
Statistical analysis
Descriptive statistics were computed for the categorical and continuous variables. We compared baseline characteristics of the 2 groups using the χ2 test for categorical variables and the t test for continuous variables. In addition to sex and age at index date, comorbidities at baseline and 1-year period were extracted from both outpatient and inpatient records and classified according to the Deyo-Charlson Comorbidity Index (Deyo-CCI)14.
Patients in the β-blocker and non-β-blocker groups were then matched by Propensity score (PS) at a 1:1 ratio sampling without replacement to reduce selection bias between the groups. Table 1 showed the results of logistic regression for PS estimation. Kaplan–Meier survival curves were analyzed using the stratified log-rank test to evaluate the β-blockers beneficial effect on 1-year survival. If the imbalance existed after PS matching, multivariate logistic regression analysis after PS matching (double-adjustment) could be performed to remove confounding15,16. The independent effects of prior β-blocker use on 1-year survival were analyzed by multivariate PS-based logistic regression analysis. The model was adjusted by potential confounders such as age, sex, drug use, CCI scores, underlying comorbidities like HTN, diabetes, coronary artery disease, congestive heart failure, atrial fibrillation, CKD, malignancy, COPD and asthma, urbanization level, the event year, resuscitation in medical center, hypothermia therapy, and achieved of coronary angiography. Subgroup analyses of 1-year survival were used for β-blockers and non-β-blockers groups based on clinical characteristics such as age, gender, CCI scores, diabetes mellitus, hypertension, dyslipidemia, CAD, CHF, atrial fibrillation, CKD, COPD and asthma. Differing criteria in sensitivity analysis were used to validate regression model findings. All analyses were performed with SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina). All p-values reported were 2-tailed, and the significant level was set at < 0.05.
Results
Overall, 104,568 patients were included in the analysis (Fig. 1), of which, 13,880 patients (13.3%) were β-blocker users. Patients in the β-blocker group had more comorbidities such as hypertension, diabetes malleus, coronary artery disease, congestive heart failure, and chronic kidney disease. Conversely, several patients in the control group had a history of malignancy, COPD, and asthma. The CCI score was higher and procedures for coronary angiography were performed more often in the β-blocker group. After PS matching, no significant differences in most comorbidities, gender, or year of resuscitation were noted between these groups except that patients in the non-β-blocker group were older, more hypertensive, had history of COPD, and less coronary artery disease. Our results showed that the survival to ICU admission (25.7% vs. 23.1%; P < 0.0001), survival to hospital discharge (15.5% vs. 13.7%; P < 0.0001), and 1-year survival (4.2% vs. 3.7%; P = 0.02) were better in the prior β-blocker use group after PS matching (Table 2). The Kaplan–Meier survival curve demonstrated better 1-year survival in the prior β-blockers use group (Fig. 2A, stratified log-rank test; P < 0.0001).
One-year survival rate for cardiac arrest people with or without prior ß-blockers use. (a) Kaplan–Meier one year survival curve in patients with/without prior ß-blockers use. (b) Multiple logistic regression analysis of (1) survival to hospital admission (2) survival to discharge (3) one year survival rate (4) weaning off ventilator rate among patients receiving high dose, low dose and no ß-blocker (*P < 0.05 by stratified log-rank test).
Under the multivariate PS-based logistic regression analysis, patients with prior ß-blockers use were associated with better 1-year survival under the (OR, 1.15, 95% CI 1.01–1.30; P = 0.031) after adjusting age, gender, medications use such as antiplatelet agents and ACEI/ARB, CCI scores, clinical covariates such as diabetes mellitus, hypertension, dyslipidemia, CAD, CHF, atrial fibrillation, CKD, COPD, urbanization level, the event year and the management of cardiac arrest(Table 3). A beneficial outcome of 1-year survival was observed for patients who were younger, were females, had a history of COPD, and had higher CCI scores (Table 3). Cardiac resuscitation that was performed at the medical center and required a lower amount of adrenaline (epinephrine) was associated with better chances of 1-year survival. In comparison to those who did not undergo coronary angiography, patients who underwent coronary angiography during hospitalization also had better chances of 1-year survival.
The mean daily dose in both groups is listed in Table 4. After using Carvedilol 12.5 mg and its equivalent dose as cutoff value, the mean daily dose greater or equal to Carvedilol 12.5 mg (or its equivalent dose) was defined as high-dose group. After multivariate PS-based logistic regression analysis, both patients in the high-dose (n = 7021) and low-dose (n = 6859) prior β-blockers use groups had greater odds of survival to hospital admission (adjusted OR: 1.16, 95% CI 1.09–1.25, P < 0.0001 and adjusted OR: 1.08, 95% CI 1.01–1.16, P = 0.0216, respectively ) and survival to discharge (adjusted OR: 1.18, 95% CI 1.09–1.28, P < 0.0001, and adjusted OR: 1.04, 95% CI 0.95–1.13, P = 0.41, respectively) than those in the control group. In addition, the high-dose prior β-blockers use group had better chance of 1-year survival (adjusted OR: 1.27, 95% CI 1.09–1.47, P = 0.0021) and higher ventilator wean rate (adjusted OR: 1.19, 95% CI 1.01–1.411, P = 0.0423) than the control group after multivariate PS-based logistic regression analysis (Fig. 2B).
Also, subgroup analysis was performed in patients with prior β-blockers use, we found that compared to patents with prior β-blockers use and younger than 70 years old, patients with prior β-blocker use and more than 70 years old are associated with a better 1-year survival. (adjusted OR: 1.12 vs. 1.62, P-value of interaction = 0.013). Similar finding was also found in patients with the history of diabetes (adjusted OR: 1.16 vs. 1.68, P-value of interaction = 0.003), hypertension (adjusted OR: 1.08 vs. 1.43, P-value of interaction = 0.005), and COPD (adjusted OR: 1.16 vs. 1.86, P-value of interaction = 0.008), and with a CCI score > 3 (OR: 1.18 vs. 1.74 P-value of interaction = 0.002). The remaining subgroups were not significantly different on the outcome of 1-year survival. (Fig. 3). Further large multicenter prospective studies are required to verify the better 1-year survival outcome of prior β-blocker use in patients > 70 years old, with history of HTN, diabetes, COPD or CCI score > 3.
As shown in Table 5, sensitivity analysis excluded patients with comorbidities such as COPD, asthma or malignancy, prior to cardiac arrest. Patients receiving no epinephrine or only 1 dose (due to rhythm conversion, without resorting to anti-arrhythmic agents) were also excluded in the sensitivity analysis. The relationships between high- or low-dose β-blocker treatment and 1-year survival, as well as survival to ICU admission and survival to hospital discharge, remained consistent.
Discussion
Our nationwide cohort study showed that a greater odd of better 1-year survival and higher weaning rate of ventilator were observed in the prior high-dose β-blocker use group under the multivariate PS-matched regression analysis method. Reportedly, the chance of 1-year survival after cardiac arrest was higher in patients with favorable neurologic outcomes at hospital discharge17. From our analysis of ventilator wean rates, pre-β-blocker use not only improved the long-term clinical outcome but also improved the immediate functional outcomes after cardiac arrest. Better outcomes of previous ß-blockers use in patients with non-shockable cardiac arrest were unchanged even after sensitivity analysis.
The Charlson Comorbidity Index (CCI) score is a measure of the burden of disease arising from multiple comorbidities and a higher CCI score was associated with greater mortality risk18. The existence of some comorbidity may trigger the initiation of appropriate therapy to reduce that mortality risk. High CCI scores might be associated with more intensive treatment, and this could lead to inadequate prediction of 1-year survival due to the presence of a treatment paradox19.
Cardiac arrest patients experience post cardiac arrest syndrome, which comprises anoxic brain injury, post cardiac arrest myocardial dysfunction, the global ischemic-reperfusion injuries, and responsible for mortality and morbidity in these patients20. Cessation of blood circulation leads to ischemia reperfusion injuries in major organs, including the brain and the heart. The ischemic event initiates a cascade of circulating inflammatory cytokines, resulting in excessive sympathoadrenal activation and endothelial damage. The pathological sympathetic activation after acute ischemic stroke has been repeatedly observed in acute stroke and is related to poorer outcomes21.β-blockers use before ischemia-related brain injuries showed beneficial effects including a milder stroke severity and lower mortality22. In animal models, β-blockers administered before the induction of experimental ischemia lead to a reduction in infarct volume by 40%23. The high susceptibility to pneumonia caused by the sympathetic activation in post-stroke immunosuppression has been extensively discussed. Stroke treatment with β-blockers has been associated with reduced mortality24.
Several studies have shown the protective effect of β-blockers on ischemia-related cardiac injury23,24. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II) trial, the first efficacy trial investigating all-cause mortality in patients with chronic heart failure on β-blocker therapy, showed a 32% risk reduction in total mortality25. The use of pre-procedural β-blockers was associated with a lower risk of in-hospital cardiac death in patients with acute coronary syndrome undergoing percutaneous coronary intervention26. The perioperative cardioprotective effect made by β-blockers may be multifactorial. First, the negative inotropic and chronotropic effects made byβ-blockers decrease myocardial oxygen demand. Also, β-blockers could modulate myocardial inflammatory responses such as cytokine release and leukocyte recruitment27. Moreover, the myocardial metabolism was shift from fatty acid oxidation toward glucose uptake28. Therefore, it is reasonable to suggest that prior β-blockers use might mitigate the magnitude of post cardiac arrest syndrome, which results in improved clinical and functional outcomes and overall performance survival.
Prior systematic review of clinical trials about ß-blockers use in patients with heart failure has established higher dose ß-blocker does not ameliorate better outcomes29. Take CIBIS II study for example, the reduced mortality with bisoprolol was little different regardless of the dose level (high dose (10 mg/day), moderate dose (5 mg or 7.5 mg/day) and lowest dose (1.25, 2.5 or 3.75 mg/day)30. The incidence and severity of adverse drug effects are often dose related. ß-blockers can be effective in lower dose. In our high dose prior β-blockers use group, the mean daily dose was 4.4 mg (bisoprolol) or 25.6 mg (carvedilol). But the mean daily dose in low dose prior β-blockers use group was only 1.8 mg (bisoprolol) or 1.8 mg (carvedilol). The insufficient dose of ß-blocker use might explain the insignificant survival benefit in the low dose ß-blocker group.
The study has some limitations. Detailed neurologic outcomes were not available from the NHIRD. Instead, we evaluated the overall performance outcomes by ventilator wean success rate at 1 month. Also, resuscitation information at the area of occurrence was not accessible. However, the quality of resuscitation performance was not related to the prior use of β-blockers.
Conclusion
In conclusion, patients with prior high-dose ß-blockers use before cardiac arrest are associated with better chance of 1-year survival and higher weaning rate in a propensity score-matched nationwide cohort study. Further large multicenter prospective studies are required to confirm whether prior ß-blockers use before cardiac arrest leads to improved clinical outcomes in cardiac arrest patients with non-shockable rhythm.
Data availability
The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2018 update: A report From the American Heart Association. Circulation 137, e467–e492 (2018).
Daya, M. R. et al. Out-of-hospital cardiac arrest survival improving over time: Results from the Resuscitation Outcomes Consortium (ROC). Resuscitation 91, 108–115 (2015).
Granfeldt, A. et al. Clinical predictors of shockable versus non-shockable rhythms in patients with out-of-hospital cardiac arrest. Resuscitation 108, 40–47 (2016).
Adrie, C. et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 106, 562–568 (2002).
Foody, J. M., Farrell, M. H. & Krumholz, H. M. beta-Blocker therapy in heart failure: Scientific review. JAMA 287, 883–889 (2002).
Thygesen, K. et al. Fourth universal definition of myocardial infarction. Eur. Heart J. 40, 237–269 (2018).
Farshi, R., Kistner, D., Sarma, J. S. M., Longmate, J. A. & Singh, B. N. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: A crossover open-label study of five drug regimens. J. Am. Coll. Cardiol. 33, 304–310 (1999).
Sykora, M. et al. β-blockers, pneumonia, and outcome after ischemic stroke. Stroke 46, 1269–1274 (2015).
Cheng, S. H. & Chiang, T. L. The effect of universal health insurance on health care utilization in Taiwan: Results from a natural experiment. J. Am. Med. Assoc. 278, 89–93 (1997).
Ng, C. J. et al. Validation of the Taiwan triage and acuity scale: A new computerised five-level triage system. Emerg. Med. J. 28, 1026–1031 (2011).
Jo, S. et al. ED crowding is associated with inpatient mortality among critically ill patients admitted via the ED: Post hoc analysis from a retrospective study. Am. J. Emerg. Med. 33, 1725–1731 (2015).
Huang, C. H. et al. Relationship between statin use and outcomes in patients having cardiac arrest (from a Nationwide Cohort Study in Taiwan). Am. J. Cardiol. 123, 1572–1579 (2019).
Lin, Y. J., Tian, W. H. & Chen, C. C. Urbanization and the utilization of outpatient services under National Health Insurance in Taiwan. Health Policy (Amsterdam, Netherlands). 103, 236–243 (2011).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron Dis. 40, 373–383 (1987).
Hsu, W. T. et al. Effect of renin-angiotensin-aldosterone system inhibitors on short-term mortality after sepsis: A population-based cohort study. Hypertension 75(2), 483–491 (2020).
Nguyen, T. L. et al. Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Med. Res. Methodol. 17, 78 (2017).
Sander, D., Winbeck, K., Klingelhöfer, J., Etgen, T. & Conrad, B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology 57, 833–838 (2001).
Poupin, P. et al. Prognostic value of Charlson Comorbidity Index in the elderly with a cardioverter defibrillator implantation. Int. J. Cardiol. 314, 64–69 (2020).
Uffen, J. W. et al. Retrospective study on the possible existence of a treatment paradox in sepsis scores in the emergency department. BMJ Open 11, e046518 (2021).
Maynard, C. et al. Effect of prehospital induction of mild hypothermia on 3-month neurological status and 1-year survival among adults with cardiac arrest: Long-term follow-up of a randomized, clinical trial. J Am Heart Assoc. 4, e001693 (2015).
Bro-Jeppesen, J. et al. Endothelial activation/injury and associations with severity of post-cardiac arrest syndrome and mortality after out-of-hospital cardiac arrest. Resuscitation 107, 71–79 (2016).
Savitz, S. I. et al. The novel β-blocker, carvedilol, provides neuroprotection in transient focal stroke. J. Cerebral Blood Flow Metab. 20, 1197–1204 (2000).
Gheorghiade, M., Colucci, W. S. & Swedberg, K. β-blockers in chronic heart failure. Circulation 107, 1570–1575 (2003).
Yang, J. H. et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardio Intervent. 7, 592–601 (2014).
CIBIS-II Investigators and Committees. Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet (London, England). 353, 9–13 (1999).
Kim, B. S. et al. Effect of pre-procedural beta-blocker on clinical outcome after percutaneous coronary intervention in acute coronary syndrome. Int. Heart J. 60, 1284–1292 (2019).
Prabhu, S. D., Chandrasekar, B., Murray, D. R. & Freeman, G. L. beta-adrenergic blockade in developing heart failure: Effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation 101, 2103–2109 (2000).
Bangalor, S., Messerli, F. H., Kostis, J. B. & Pepine, C. J. Cardiovascular protection using beta-blockers: A critical review of the evidence. J. Am. Coll. Cardiol. 50, 563–572 (2007).
McAlister, F. A., Wiebe, N., Ezekowitz, J. A., Leung, A. A. & Armstrong, P. W. Meta-analysis: Beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann. Intern. Med. 150, 784–794 (2009).
Simon, T., Mary-Krause, M., Funck-Brentano, C., Lecgat, O. & Jaillon, P. Bisoprolol dose-response relationship in patients with congestive heart failure: A subgroup anlaysis in the cardiac insufficiency Bisoprolol study (CIBIS II). Eur. Heart J. 24, 552–559 (2003).
Acknowledgements
The authors also thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
H.C.H., C.W.J., and H.H.C. contributed to study conception and design. H.C.H. and C.W.J. contributed to the acquisition of the data. H.H.C., C.K.L., Y.P.H. and T.M.S. contributed to the analysis and/or interpretation of data. All authors contributed to the drafting or critical revision of the article for important intellectual content. All authors gave final approval of the version of the article to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, HC., Yu, PH., Tsai, MS. et al. Prior beta-blocker treatment improves outcomes in out-of-hospital cardiac arrest patients with non-shockable rhythms. Sci Rep 11, 16804 (2021). https://doi.org/10.1038/s41598-021-96070-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96070-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.