Abstract
The constrained total energy expenditure (TEE) model posits that progressive increases in physical activity (PA) lead to increases in TEE; but after certain PA threshold, TEE plateaus. Then, a compensatory reduction in the expenditure of non-essential activities constrains the TEE. We hypothesized that high PA levels as locomotion associate with a compensatory attenuation in arm movements. We included 209 adults (64% females, mean [SD] age 32.1 [15.0] years) and 105 children (40% females, age 10.0 [1.1] years). Subjects wore, simultaneously, one accelerometer in the non-dominant wrist and another in the hip for ≥ 4 days. We analyzed the association between wrist-measured (arm movements plus locomotion) and hip-measured PA (locomotion). We also analyzed how the capacity to dissociate arm movements from locomotion influences total PA. In adults, the association between wrist-measured and hip-measured PA was better described by a quadratic than a linear model (Quadratic-R2 = 0.54 vs. Linear-R2 = 0.52; P = 0.003). Above the 80th percentile of hip-measured PA, wrist-measured PA plateaued. In children, there was no evidence that a quadratic model fitted the association between wrist-measured and hip-measured PA better than a linear model (R2 = 0.58 in both models, P = 0.25). In adults and children, those with the highest capacity to dissociate arm movements from locomotion—i.e. higher arm movements for a given locomotion—reached the highest total PA. We conclude that, in adults, elevated locomotion associates with a compensatory reduction in arm movements (probably non-essential fidgeting) that partially explains the constrained TEE model. Subjects with the lowest arm compensation reach the highest total PA.
Similar content being viewed by others
Introduction
The constrained total energy expenditure (TEE) model posits that, in humans and other endothermic species, TEE is homeostatically constrained within a narrow range1. The model predicts that, within low-to-moderate physical activity (PA) levels, increases in PA lead to increases in TEE. Yet after reaching higher PA levels, further increases in PA do not increase TEE1. Instead, high PA levels would entail reallocation of the constrained TEE budget from non-essential physiological activities (e.g. excessive innate immunity) to PA. This energy trade-off may explain some health benefits of regular PA2. For example, regular PA would decrease the energy available for inflammation, thus preventing diseases associated with chronic systemic inflammation3.
In adults, the model was initially supported by data showing similar TEE among populations with markedly different PA levels4,5. But more compelling evidence was obtained from a cross-sectional analysis of 332 adults with a wide range of PA patterns6. Therein, PA was measured by hip-worn accelerometers, and TEE by doubly labeled water. Among subjects with PA levels below the ~ 70th percentile of their measured PA span, PA was directly associated to TEE. Nevertheless, among subjects with PA levels above such threshold, PA and TEE were unrelated; higher PA levels were thus not associated with higher TEE6. This observation supports that TEE was constrained, and suggests that the TEE budget should have been reallocated to sustain such a high PA level. The authors hypothesized that the energy expended in non-muscular activities (e.g. immunity, reproduction) was partially reallocated to PA6.
Data comparing children from industrialized populations (US/UK; n = 44) and a forager-horticulturalist population (Shuar; n = 44) suggest that the model also operates during childhood7. In that study, PA level was measured by hip-worn accelerometers, and TEE by doubly labeled water. Although the Shuar children had higher PA levels than the US/UK children, TEE was not different between groups. Immune activity was also higher among Shuar children. Thus, to sustain PA levels and immune activity in Shuar children, a TEE reallocation comprising reductions in the energy expended in other physiological activities should have occurred.
Taken together, studies using hip-worn accelerometers to measure PA support the constrained TEE model in adults and children. But how this TEE constraint is achieved in face of high hip-measured PA levels is unknown. The reallocation of energy from non-essential, non-muscular physiological activities has been proposed2,6,7. It may also involve a compensatory decrease in muscular activities that hip-worn accelerometers cannot detect, such as arm movements8. Perhaps, above certain hip-measured PA (mostly determined by locomotion), a compensatory decrease in non-essential arm movements (e.g. fidgeting) helps to maintain TEE within its limits. Evidence in adults suggests that when hip-measured PA is zero (no locomotion), there are still ~ 600 kcal/d theoretically attributed to PA. This energy expenditure may partially correspond to non-essential arm movements that can be avoided in case of elevated locomotion. This compensation, along with the reallocation of energy expended in other non-essential activities, may have evolved to allow humans to maintain the long walking distances required for survival in the past5.
To test such hypothesis, we analyzed cross-sectional data from adults and children that worn—simultaneously—accelerometers in the hip and the non-dominant wrist. We hypothesized that, at low hip-measured PA levels, wrist-measured PA and hip-measured PA are directly associated; nevertheless, beyond a certain hip-measured PA threshold, the association plateaus. This would suggest that high levels of locomotion lead to reductions in arm movements, thus partially explaining the constrained TEE model. The aim of the study was to analyze the association between wrist-measured PA and hip-measured PA in adults and children.
Results
Association patterns between wrist-measured PA and hip-measured PA
We conducted a secondary analysis of the data of 209 adults from two clinical trials (NCT023651299 and NCT03334357)10, and of 105 children from another clinical trial (NCT02295072)11 (see Methods, "Study design and subjects" subsection, for details). All subjects wore—simultaneously—one accelerometer in the non-dominant wrist and another in the right hip for ≥ 4 days. Table 1 shows the participant's main characteristics. The raw data from the accelerometers were processed to remove the gravity and noise components; Euclidean norm minus one G were then calculated in 5-s epochs, and PA was expressed in milli-gravitational units (mg; see Methods, "Physical activity" subsection, for details)12,13. To determine the shape of the association between wrist-measured PA and hip-measured PA, we first calculated the R2 for linear and quadratic regression models. Then, we iteratively removed subjects at low deciles of hip-measured PA to assess the association with wrist-measured PA above increasing hip-measured PA thresholds.
For adults, the association between wrist-measured PA and hip-measured PA showed a higher R2 using a quadratic model than a linear model (Fig. 1A). The extra sum-of-squares F test confirmed that the quadratic model fitted better the data than the linear model (F [DFn, DFd] = 9.104 [1, 206]; P = 0.003, n = 209). When all adults were considered, wrist-measured PA and hip-measured PA correlated directly (Pearson r = 0.72, P < 0.001, n = 209). Nevertheless, the correlation became progressively weaker by iteratively excluding the lowest hip-measured PA deciles, becoming non-significant after the deciles 1st-8th had been excluded (Fig. 1B). Similarly, the unstandardized ß-coefficient [95% CI] of the linear regression between wrist-measured PA and hip-measured PA was not different from zero after excluding the deciles 1st-8th (0.52 [− 0.38–1.43], n = 41; Fig. 1C). We thus identified the 80th percentile of hip-measured PA (i.e. 14.33 mg) as a threshold beyond which there is no evidence of an association between wrist-measured PA and hip-measured PA (Fig. 1D). The association between wrist-measured PA and hip-measured PA below such a threshold was statistically significant, with a y-intercept [95% CI] different from zero (6.59 mg [4.05–9.13]; Fig. 1D). We then tested whether the lack of evidence for an association observed beyond the 80th percentile threshold was explained by the small sample size (n = 41). To that end, we analyzed the association between wrist-measured PA and hip-measured PA including the 41 adults with the lowest hip-measured PA. The association was significant (Pearson r = 0.51, P = 0.001, n = 41), suggesting that the sample size did not explain the effect observed beyond the 80th percentile threshold.
Association between wrist-measured and hip-measured physical activity [PA] in adults. (A) Quadratic [dashed line] and linear [solid line] regression models. (B) Pearson r [circles] and P values [squares; blue for statistically significant, and red for non-significant] for the associations between wrist-measured PA and hip-measured PA that include subjects from different deciles of hip-measured PA until the 10th decile; e.g. values for decile 9th indicate a correlation that includes subjects from the 9th to the 10th deciles. (C) Unstandardized ß-coefficients and 95% confidence intervals for the linear regression between wrist-measured PA vs. hip-measured PA that include subjects spanning different deciles of hip-measured PA, as explained in B. Blue symbols indicate significantly different from zero; red symbols indicate non-significantly different from zero (D) Threshold of hip-measured PA [dotted line; 14.33 mg] beyond which the association with wrist-measured PA becomes non-significant. n = 209.
For children, the association between wrist-measured PA and hip-measured PA showed a similar R2 using a quadratic model or a linear model (Fig. 2A). The extra sum-of-squares F test confirmed that there was no evidence that the quadratic model fitted better the data than the linear model (F [DFn, DFd] = 1.349 [1, 102]; P = 0.248, n = 105); since the linear model was simpler, this was deemed the preferred model. When all children were considered, wrist-measured PA and hip-measured PA correlated directly (Pearson r = 0.76, P < 0.001, n = 105). Notably, the correlation remained significant when the lowest hip-measured PA deciles were iteratively excluded; indeed, when 1st-9th deciles had been excluded, wrist-measured PA and hip-measured PA were still associated (Pearson r = 0.68, P = 0.029, n = 10; Fig. 2B). Similarly, the unstandardized ß-coefficient of the linear regression between wrist-measured PA and hip-measured PA was always different from zero (Fig. 2C). Therefore, we did not observe any hip-measured PA threshold beyond which wrist-measured PA plateaued. The association between wrist-measured PA and hip-measured PA had a y-intercept [95% CI] different from zero (11.59 mg [6.81–16.37], n = 105; Fig. 2A).
Association between wrist-measured and hip-measured physical activity [PA] in children. (A) Quadratic [dashed line] and linear [solid line] regression models. (B) Pearson r [circles] and P values [squares] for the associations between wrist-measured PA and hip-measured PA that include subjects from different deciles of hip-measured PA until the 10th decile; e.g. values for decile 9th indicate a correlation that includes subjects from the 9th to the 10th deciles. (C) Unstandardized ß-coefficients and 95% confidence intervals for the linear regression between wrist-measured PA vs. hip-measured PA that include subjects spanning different deciles of hip-measured PA, as explained in B. n = 105.
Dissociation of locomotion and arm movements at high total PA
We conducted complementary analyses to study how the capacity to dissociate arm movements from locomotion influences total PA. When accelerometers are worn on the hip, they mostly detect accelerations from activities that include locomotion (e.g. walking), but cannot detect accelerations from arm movements. In contrast, when accelerometers are worn on the wrist, they detect accelerations from activities that include locomotion as well as arm movements (e.g. arms swing during locomotion, occupational activities, fidgeting). As an approach to isolate the accelerations produced by arm movements, we adjusted wrist-measured PA for hip-measured PA (see Methods, "Statistical analyses" subsection, for details). This new index—wrist-measured PAADJ—is thus a surrogate of the accelerations produced by arm movements. Total PA was then calculated by adding hip-measured PA (i.e. locomotion) to wrist-measured PAADJ (i.e. arm movements).
In adults, as expected, hip-measured PA and wrist-measured PAADJ associated directly to total PA (Pearson r = 0.63 and 0.77, respectively; P < 0.001, n = 209). Notably, the slope [95% CI] of the linear regression for wrist-measured PAADJ (vs. total PA) was steeper than the slope of the linear regression for hip-measured PA (0.59 [0.52–0.66] vs. 0.40 [0.33–0.47], respectively; F [DFn, DFd] = 15.74 [1, 414]; P < 0.001, n = 209; Fig. 3A). In children, also as expected, hip-measured PA and wrist-measured PAADJ associated directly to total PA (Pearson r = 0.57 and 0.81, respectively; P < 0.001, n = 105). The slope [95% CI] of the linear regression for wrist-measured PAADJ (vs. total PA) was also steeper than the slope of the linear regression for hip-measured PA (0.67 [0.57–0.76] vs. 0.32 [0.23–0.42], respectively; F [DFn, DFd] = 27.26 [1, 206]; P < 0.001, n = 105; Fig. 3B).
Resting metabolic rate is unrelated to PA
We analyzed the association between resting metabolic rate (RMR) and the indexes of PA in adults. For analyses, RMR was first adjusted for lean mass (RMRADJ). There was no evidence for an association of RMRADJ vs. hip-measured PA, wrist-measured PA, wrist-measured PAADJ, or total PA (Fig. 4).
Association between resting metabolic rate [RMR] and physical activity [PA] in adults. RMR adjusted for lean mass [RMRADJ] was associated with (A) hip-measured PA, (B) wrist-measured PA, (C) wrist-measured PA adjusted for hip-measured PA [wrist-measured PAADJ], or (D) total PA [hip-measured PA plus wrist-measured PAADJ]. n = 169.
Effects of sex and trial
Our databases included males and females. To ascertain how sex influenced our results, we repeated our main analyses stratified by sex. Table S1 shows the main characteristics of the adults and children stratified by sex. In adults, wrist-measured PA and hip-measured PA were similar in males and females (Table S1). In male adults, a quadratic model fitted better the association between wrist-measured PA and hip-measured PA than a linear model (F [DFn, DFd] = 13.69 [1, 72]; P < 0.001, n = 75; Fig. S1A). Nevertheless, in female adults, there was no evidence for such an effect (F [DFn, DFd] = 0.002 [1, 131]; P = 0.966, n = 134; Fig. S1B). These data indicate that our main findings in adults are mostly explained by males. In children, males had higher wrist-measured PA and hip-measured PA than females (Table S1). In male children, there was no evidence that a quadratic model fitted better the data than a linear model (F [DFn, DFd] = 1.602 [1, 60]; P = 0.210, n = 63; Fig. S1C). This was also the case for female children (F [DFn, DFd] = 1.162 [1, 39]; P = 0.287, n = 42; Fig. S1D). Our main results in children thus appear independent of sex.
Finally, our adult sample included a clinical trial in young adults (18–25 years; NCT02365129)9 and another in middle-age adults (45–65 years; NCT03334357)10. To ascertain how the characteristics of these trials influenced our results, we repeated our main analyses stratified by trial (young adults, middle-age adults). Table S2 shows the main characteristics of the adults stratified by trial. Middle-age adults had higher wrist-measured PA and hip-measured PA than young adults (Table S2). In young adults, a quadratic model fitted better the association between wrist-measured PA and hip-measured PA than a linear model (F [DFn, DFd] = 7.780 [1, 140]; P = 0.006, n = 143; Fig. S2A). In middle-age adults, a quadratic model also trended to fit better the association between wrist-measured PA and hip-measured PA than a linear model (F [DFn, DFd] = 2.709 [1, 63]; P = 0.104, n = 66; Fig. S2B). These findings suggest the trial had no major impact in our main results.
Discussion
In humans, TEE is proposed to be maintained within a narrow physiological range, thus constraining excessive elevations induced by PA5,6,7. This constraint may have evolved as a mechanism to allow humans and other species to increase foraging effort without requiring greater dietary intake1. Thus, the energy to sustain foraging or other PA would be obtained by reallocating expended energy from other non-essential physiological activities1,2. What those “other” activities are is unknown. Herein, we tested the hypothesis that arm movements may be involved. We found that, in adults, the higher the PA measured by hip-worn accelerometers the higher the PA measured by wrist-worn accelerometers; however, beyond a certain hip-measured PA level, wrist-measured PA plateaued. This suggests that at high levels of locomotion (i.e. hip-measured PA), a compensation that reduces arm movements ensues. We speculate that this compensation limits the energy expended by non-essential PA (e.g. fidgeting) to reallocate such energy to locomotion, and may partly explain the resistance to weight loss of PA interventions14. In children, however, the direct association between wrist-measured PA and hip-measured PA did not plateau. Thus, our data do not support a compensation involving arm movements during childhood.
By simultaneously wearing accelerometers in the hip and the wrist, we intended to discriminate different components of the total PA. In adults, we observed that wrist-measured PA and hip-measured PA were directly associated. A quadratic model fitted the data better than a linear model. This indicates that as hip-measured PA increases, the increases in wrist-measured PA are progressively attenuated. Previous studies have reported linear associations between wrist-measured PA and hip-measured PA15,16. Nevertheless, whether a quadratic regression fitted better the data than a linear regression does not seem to have been tested. We thus replicated and then re-analyzed the scatterplots by Kamada et al. (Fig. 1A in ref.15) and Dieu et al. (Fig. 2 in ref.16). In the data by Kamada et al., which included 94 elderly women15, a quadratic model trended to fit better the data than a linear model (R2 = 0.60 and 0.58, respectively; F [DFn, DFd] = 3.752 [1, 91]; P = 0.055). This agrees with our current findings. In the data by Dieu et al., which included 40 young adults16, there was no evidence that a quadratic model fitted the data better than a linear model (R2 = 0.78 in both models; F [DFn, DFd] = 0.849 [1, 37]; P = 0.362).
At least two factors may explain the discrepancy of the findings from the data by Dieu et al.16 vs. our findings and those from the data by Kamada et al.15. First, the sample size by Dieu et al.16 was the smallest. Second, PA data by Dieu et al. was obtained during 1 day of measurement16, whereas we obtained data from at least 4 days (minimum 4 days, maximum 8 days); Kamada et al. measured subjects for 7 days15. Therefore, it is possible that the compensation in arm movement is not evident within a single day, but only over longer periods (≥ 4 days).
In adults, Pontzer et al.6 identified a hip-measured PA threshold beyond which TEE plateaus. Herein we used a similar analytical approach to identify a hip-measured PA threshold beyond which wrist-measured PA plateaus. We found this threshold at the 80th percentile of the hip-measured PA of our sample distribution (14.33 mg). We speculate that beyond this threshold a compensation that reduces non-essential arm movement—e.g. fidgeting—ensues to constrain TEE (note that arms swinging for locomotion would continue increasing). This supports the previous hypothesis for a compensatory reduction in non-exercise activity thermogenesis17. In contrast, we found that, below the threshold, wrist-measured PA and hip-measured PA were directly associated. Of note, the y-intercept of the association was different from zero. This indicates that when hip-measured PA is zero (no locomotion), a remaining wrist-measured PA is expected (e.g. fidgeting, occupational activities). In agreement, Pontzer et al.6 observed that when hip-measured PA was zero, the energy expenditure theoretically attributed to PA was ~ 600 kcal/day. They thus proposed that other non-muscular physiological activities were responsible for those 600 kcal/day. Our current data suggest that arm movement may be at least partially responsible. Arm movements—along with the thermic effect of food—may also explain why the ratio TEE/RMR (so named PAL: Physical Activity Level) reaches 1.2 in non-ambulant subjects18. But the proportion of those 600 kcal/day explained by arm movements cannot be ascertained with our data. Previous evidence demonstrates that fidgeting increases TEE by 0.6 kcal/min (46%) while sitting19. Also, analyses using calorimetric chambers have shown that spontaneous PA—with fidgeting as a major component—accounts for 100–800 kcal/day20. The energy expended in fidgeting, which is a non-essential PA, might thus represent an important proportion of the energy available for reallocation.
Previous evidence obtained during a 20-week running competition also suggests an energy reallocation from arm movements to locomotion21. In that study, TEE was measured by doubly labeled water, and then parsed into its components: RMR (measured by indirect calorimetry or estimated by equations), thermic effect of food (assumed as 10% of TEE), and running cost (estimated by equations). The first week of the race, there were ~ 600 kcal/day from the measured TEE budget that could not be attributed to RMR, thermic effect of food, or running cost. This energy thus represented “other” unidentified physiological activities. By the last week of the race, measured TEE was almost fully explained by RMR, the thermic effect of food, and running cost. Thus, the energy expended in “other” physiological activities had decreased21. Based on our current data, we speculate that non-essential arm movements were part of the “other” activities measured the first week of the race. And as the distance ran accumulated, a compensation to reduce this non-essential activity (and probably others too) may have ensued. Notably, for the reallocation of energy from non-essential, non-muscular physiological activities to take place, a period of several weeks has been proposed2. In contrast, the compensation in the arm movements could be an earlier mechanism to constrain TEE.
In children, however, our data do not support a similar compensation as in adults. The reason for the difference in children vs. adults is unknown. A recent report showed similar TEE among children from industrialized (US/UK) and from forager-horticulturalist (Shuar) populations, thus supporting the constrained TEE model7. Therein, TEE budget was parsed into its classic components: RMR (measured by indirect calorimetry), thermic effect of food (assumed as 10% of TEE), and PA (the remainder, commonly named as physical activity energy expenditure). Intriguingly, even though hip-measured PA was higher in Shuar than US/UK children, the physical activity energy expenditure was higher in US/UK children7. Although factors such as movement efficiency may be involved, the result suggested that physical activity energy expenditure included energy from activities not attributed to hip-measured PA. We speculate that arm movements are among those activities. The fact that we have not observed a compensation in our current study may result from the children not being active enough. Regardless, strictly considering our current data, the high levels of locomotion (i.e. hip-measured PA) among Shuar children would not lead to a reduction of arm movements. Instead, locomotion may have led to decreases in other non-muscular, non-essential activities to maintain TEE within its physiological range. An attenuated circadian variation in RMR among Shuar children was proposed as an alternative7.
We conducted complementary analyses to gain insight into the association between locomotion and arm movements. As an attempt to isolate the arm movements, we adjusted wrist-measured PA for hip-measured PA. We then considered as total PA the sum of this wrist-measured PAADJ and hip-measured PA. As expected, total PA was directly associated with its components. But notably, the slope of the association was higher for wrist-measured PAADJ than for hip-measured PA, in both adults and children. These findings suggest that the individuals with the highest total PA were those with the largest dissociation between locomotion and arm movements. In other words, those individuals manifested the lowest compensation in arm movements. This capacity for dissociation may benefit health. Previous studies have shown that the energy expended in spontaneous PA (e.g. fidgeting) associates inversely with fat and weight gain22,23. Additionally, fidgeting has been associated with a better post-prandial glycemic response24, and also with a constitutional leanness phenotype25. Thus, individuals who can increase their locomotion without having a compensatory decrease in arm movements may manifest resistance to obesity and its associated complications. Future studies should test such hypothesis.
The constrained TEE model posits that, to sustain high PA levels, energy trade-offs should occur among the components of the TEE budget2. Evidence in adults suggests that the energy allocated to RMR does not participate in these trade-offs. Pontzer et al.6 found no evidence for an association between hip-measured PA and RMR (adjusted for confounding variables). Herein we confirmed those findings. We further showed no evidence for an association between RMR (adjusted for lean mass) vs. wrist-measured PA, wrist-measured PAADJ, or total PA. Together, data suggest that physiological activities not accounted for by RMR are involved in the energy trade-offs. What proportion of this energy is explained by arm movements and by non-muscular activities is unknown. Future studies are required to fill this gap in knowledge.
The data analyzed herein are from studies designed for different purposes than our current analyses. Indeed, low levels of self-reported PA were among the inclusion criteria in both adult studies9,10. This limits the possibility of identifying a compensation in arm movements, which supposedly occurs among highly active individuals. Despite this limitation, we observed such a compensation above the 80th percentile of hip-measured PA. Notably, our sensitivity analyses in adults suggest that the compensation may occur only in males, but future studies should confirm this finding. In the case of the children cohort, PA levels were not among the inclusion/exclusion criteria11. Nevertheless, having excess body weight was an inclusion criterion, which may indicate that the children were not highly active. Also, previous data show that children from industrialized populations have lower hip-measured PA than children from forager-horticulturalist populations7. It is thus possible that we did not observe a compensation in arm movements because our children were not active enough. Finally, we speculate that high levels of locomotion result in decreases mostly of fidgeting, instead of other activities such as occupational activities. If there is a compensatory reduction in arm movements—as our adult data suggest–, those arm movements should be "non-essential". Certain occupational activities could be considered as "essential" for adults, e.g. typing on the computer for work; whereas moving the arms because of nervousness (an example of fidgeting) is not "essential". Nevertheless, note that fidgeting cannot be distinguished from occupational or household activities in our data.
In conclusion, we have shown that a compensatory reduction in arm movements may be involved in the constrained TEE model in adults. We speculate that at high levels of locomotion, there is a reduction in non-essential arm movements that, along with reallocation from yet unidentified physiological activities, allow locomotion to be sustained. The inclusion of both hip-worn and wrist-worn accelerometers will be valuable to better understand the constrained TEE model in future studies. This information will help to improve the effectiveness of interventions aimed at preventing and treating obesity.
Methods
Study design and subjects
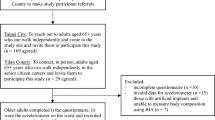
The study was a cross-sectional analysis of the baseline measurements of previous clinical trials in adults and children. For the adult sample, we included two clinical trials involving similar experimental procedures (NCT023651299 and NCT03334357)10. Adults were 18–65 years old, performed < 20 min of regular PA on 3 days/week (self-reported), had a stable body weight (< 3 kg change over the last 3 months), were not involved in weight loss programs, were non-smokers, took no medication, and suffered no chronic disease. No female subject was pregnant. Written informed consent was obtained from all subjects. The study protocols were approved by the Committee for Research Involving Human Subjects at the University of Granada (Spain; no. 924), the Servicio Andaluz de Salud (Centro de Granada, CEI-Granada, Spain), and the Human Research Ethics Committee of the Junta de Andalucía (Spain; no. 0838-N-2017). All the methods were performed in accordance with the declaration of Helsinki. The complete adult sample included 232 subjects, but only 211 met the inclusion criteria for accelerometer data (see "Physical activity" subsection). Moreover, two adults were excluded because they classified as extreme outliers for hip-measured PA (i.e. values higher than: Q3 + 3 × [Q3 − Q1]).
For the child sample, we included a single clinical trial (NCT02295072)11. Children were 8–12 years old, had overweight or obesity according to the World Obesity Federation criteria26,27, and had no physical disabilities or neurological disorders that limited exercise. No girl had started menstruating. Written informed consent was obtained from the parents of the children. The study protocols were approved by the Human Research Ethics Committee of the University of Granada (Spain; no. 848). All the methods were performed in accordance with the declaration of Helsinki. The complete children sample included 115 subjects, but only 105 met the inclusion criteria for accelerometer data (see "Physical activity" subsection).
Body composition
Body weight and height were measured with an electronic scale and a stadiometer (SECA, Hamburg, Germany), with subjects wearing light underclothes. Whole-body fat mass and lean mass were measured by dual-energy X-ray absorptiometry following the manufacturer’s instructions (Discovery Wi DXA, Hologic, Inc., Bedford, MA, USA).
Physical activity
Accelerometers (ActiGraph GT3X + , Pensacola, FL, USA) were worn on the non-dominant wrist and the right hip (on the iliac crest) for seven consecutive days. Subjects were required to remove the accelerometers only during water-based activities, and to register these non-wear time periods in a log. Sleeping times were also registered in the log. Accelerometers were initialized to record accelerations at 100 Hz with a dynamic range of ± 6 G. Raw accelerations were downloaded with the ActiLife software (ActiGraph, Pensacola, FL, USA) and then processed in the R package GGIR v.1.5.1228.
The processing steps in the GGIR were: (i) auto-calibration of the data according to local gravity acceleration13; (ii) detection of the non-wear time based on the raw acceleration recorded in the three axes12; (iii) detection of abnormally high accelerations related to malfunctioning of devices; (iv) calculation of the Euclidean norm minus one G, with negative values rounded to zero, aggregated in 5-s epochs12; (v) imputation of non-wear time and abnormal accelerations identified by using data from the rest of the days in the same time interval; (vi) identification of waking and sleeping time using an automated algorithm guided by the self-reported sleep logs29,30; and (vii) calculation of the average acceleration of all valid 5-s epochs on each day.
A day was considered as valid if it included ≥ 10 h of register during waking time, and ≥ 4 h of register during sleeping time. For the current analyses, we included only subjects with ≥ 4 valid days in both the wrist-worn and hip-worn accelerometers. Data from weekdays and weekends were weighed as: [(mean of weekdays × 5) + (mean of weekend days × 2) / 7]. Hip-measured PA and wrist-measured PA were expressed as milli-gravitational units (i.e. mg).
Resting metabolic rate (RMR)
RMR was measured only in adults, and following recent recommendations31. Subjects were instructed to avoid moderate/vigorous activities during the 24–48 h before the test day. The evening before the measurement, the subjects consumed a standardized meal ad libitum. After a ~ 12-h overnight fast, subjects came to the lab in a motorized vehicle. RMR measurements were performed between 7:00 AM and 11:00 AM in a quiet room at 22–24 °C and 35–45% humidity. Subjects laid on a reclined bed in supine position for 20 min before beginning the measurement. During measurement, subjects were covered with a bed sheet and instructed to stay awake, breathe normally, avoid fidgeting and remain silent. Gas exchange (O2 consumption, CO2 production) was measured by indirect calorimetry over 30 min using a CPX Ultima CardiO2 or a CCM Express metabolic cart (Medical Graphics Corp, St. Paul, MN, USA). Data were processed using the MGCDiagnostic Breeze Suite 8.1.0.54 SP7 software (Medical Graphics Corp., St. Paul, MN, USA). Only the final 25 min of measurement were considered for the analysis, using the steady-state method for gas exchange data selection32,33. RMR (in kcal/day) was estimated using the Weir’s abbreviated Eq. 34.
Statistical analyses
Data are presented as mean [standard deviation] (minimum − maximum). Data for adults and children were analyzed separately. IBM SPSS Statistics version 26 or GraphPad Prism version 8.4.2 were used for analyses. A P < 0.05 was considered significant.
The extra sum-of-squares F test was used to determine the regression model that fitted the data better; the null hypothesis was that a linear regression fitted the data better, while the alternative hypothesis was that a quadratic model did so. Pearson r values (2-tailed) and linear regression ß-coefficients [95% CI] were computed for subjects spanning variable hip-measured PA deciles: 1st to 10th, 2nd to 10th, 3rd to 10th, and so on.
To adjust wrist-measured PA for hip-measured PA, we calculated the residuals of the regression model that fitted the best the association between wrist-measured PA and hip-measured PA (i.e. quadratic model for adults, and linear model for children; Residual = measured value − predicted value). To avoid using positive and negative residual values when presenting the data, we added those residuals to the mean wrist-measured activity of the group, as previously done6. This new index—wrist-measured PAADJ—is thus a surrogate of the accelerations produced by arm movements. Total PA was then calculated by adding hip-measured PA (i.e. locomotion) to wrist-measured PAADJ (i.e. arm movements). The slopes of the regressions hip-measured PA or wrist-measured PAADJ vs. total PA were compared with an F test.
A stepwise multiple linear regression was used to identify significant predictors of RMR in adults (n = 169). Seven candidate predictors were included: fat mass, lean mass, height, weight, body mass index, age and sex. After correcting for the family-wise error rate (P-value = 0.05/7 = 0.007), only lean mass appeared as a significant predictor (P < 0.001, R2 = 0.48). We thus computed an adjusted RMR—i.e. RMRADJ—by adding the residuals from the “RMR ~ lean mass” linear regression to the mean RMR of the adults. The association of RMRADJ with the indexes of PA was assessed using the Pearson r test.
For continuous variables, differences between sexes or trials were tested with Student’s t test (2-tailed). Differences in the proportion of sexes between adult trials were tested with Chi-Square test.
References
Pontzer, H. Constrained total energy expenditure and the evolutionary biology of energy balance. Exerc. Sport Sci. Rev. 43, 110–116 (2015).
Pontzer, H. Energy constraint as a novel mechanism linking exercise and health. Physiology (Bethesda). 33, 384–393 (2018).
Gleeson, M. et al. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–610 (2011).
Pontzer, H. et al. Hunter–Gatherer energetics and human obesity. PLoS ONE 7, e40503 (2012).
Pontzer, H., Wood, B. M. & Raichlen, D. A. Hunter-gatherers as models in public health. Obes. Rev. 19, 24–35 (2018).
Pontzer, H. et al. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr. Biol. 26, 410–417 (2016).
Urlacher, S. S. et al. Constraint and trade-offs regulate energy expenditure during childhood. Sci. Adv. 5, eaax1065 (2019).
Ellis, K. et al. A random forest classifier for the prediction of energy expenditure and type of physical activity from wrist and hip accelerometers. Physiol. Meas. 35, 2191–2203 (2014).
Sanchez-Delgado, G. et al. Activating brown adipose tissue through exercise (ACTIBATE) in young adults: rationale, design and methodology. Contemp. Clin. Trials 45, 416–425 (2015).
Amaro-Gahete, F. J. et al. Exercise training as S-Klotho protein stimulator in sedentary healthy adults: rationale, design, and methodology. Contemp. Clin. Trials Commun. 11, 10–19 (2018).
Cadenas-Sánchez, C. et al. An exercise-based randomized controlled trial on brain, cognition, physical health and mental health in overweight/obese children (ActiveBrains project): rationale, design and methods. Contemp. Clin. Trials 47, 315–324 (2016).
van Hees, V. T. et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS ONE 8, e61691 (2013).
van Hees, V. T. et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J. Appl. Physiol. 117, 738–744 (2014).
Melanson, E. L., Keadle, S. K., Donnelly, J. E., Braun, B. & King, N. A. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med. Sci. Sports Exerc. 45, 1600–1609 (2013).
Kamada, M., Shiroma, E. J., Harris, T. B. & Lee, I.-M. Comparison of physical activity assessed using hip- and wrist-worn accelerometers. Gait Posture 44, 23–28 (2016).
Dieu, O. et al. Physical activity using wrist-worn accelerometers: comparison of dominant and non-dominant wrist. Clin. Physiol. Funct. Imaging 37, 525–529 (2017).
Melby, C. L., Paris, H. L., Sayer, R. D., Bell, C. & Hill, J. O. Increasing energy flux to maintain diet-induced weight loss. Nutrients 11, 2533 (2019).
Black, A. E., Coward, W. A., Cole, T. J. & Prentice, A. M. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur. J. Clin. Nutr. 50, 72–92 (1996).
Levine, J. A., Schleusner, S. J. & Jensen, M. D. Energy expenditure of nonexercise activity. Am. J. Clin. Nutr. 72, 1451–1454 (2000).
Ravussin, E., Lillioja, S., Anderson, T. E., Christin, L. & Bogardus, C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J. Clin. Invest. 78, 1568–1578 (1986).
Thurber, C. et al. Extreme events reveal an alimentary limit on sustained maximal human energy expenditure. Sci. Adv. 5, eaa0341 (2019).
Zurlo, F. et al. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am. J. Physiol. Endocrinol. Metab. 263, 296–300 (1992).
Levine, J. A., Eberhardt, N. L. & Jensen, M. D. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283, 212–214 (1999).
Kousta, E. et al. Delayed metabolic and thermogenic response to a mixed meal in normoglycemic European women with previous gestational diabetes. J. Clin. Endocrinol. Metab. 87, 3407–3412 (2002).
Marra, M. et al. BMR variability in women of different weight. Clin. Nutr. 26, 567–572 (2007).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 7, 284–294 (2012).
Bervoets, L. & Massa, G. Defining morbid obesity in children based on BMI 40 at age 18 using the extended international (IOTF) cut-offs. Pediatr. Obes. 9, e94–e98 (2014).
Migueles, J. H., Rowlands, A. V., Huber, F., Sabia, S. & van Hees, V. T. GGIR: a research community-driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J. Meas. Phys. Behav. 2, 188–196 (2019).
van Hees, V. T. et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS ONE 10, e0142533 (2015).
van Hees, V. T. et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci. Rep. 8, 12975 (2018).
Fullmer, S. et al. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically Ill individuals. J. Acad. Nutr. Diet. 115, 1417-1446.e2 (2015).
Sanchez-Delgado, G. et al. Reliability of resting metabolic rate measurements in young adults: Impact of methods for data analysis. Clin. Nutr. 37, 1618–1624 (2018).
Alcantara, J. M. A., Sanchez-Delgado, G., Amaro-Gahete, F. J., Galgani, J. E. & Ruiz, J. R. Impact of the method used to select gas exchange data for estimating the resting metabolic rate, as supplied by breath-by-breath metabolic carts. Nutrients 12, 487 (2020).
Weir, J. B. D. B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 109, 1–9 (1949).
Acknowledgements
Thanks to Dr. Herman Pontzer (Duke University) for his valuable feedback. We also thank the following agencies for their funding: Fondo Nacional de Desarrollo Científico y Tecnológico (11180361 to R.F.-V.); Spanish Ministry of Education, Culture and Sport (FPU15/04059 to J.M.A.A.; FPU15/02645 to J.H.M.; FPU14/04172 to F.J.A.-G.); University of Granada (Plan Propio de Investigación 2019 [Programa Contratos-Puente] to F.J.A.-G.; Plan Propio de Investigación 2016 [Excellence actions: Unit of Excellence on Exercise and Health UCEES]); Spanish Ministry of Economy and Competitiveness (ACTIBATE study; ACTIVEBRAINS study); Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI13/01393 to ACTIBATE study); Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades and European Regional Development Funds (FEDER: ref. SOMM17/6107/UGR to ACTIBATE study); Redes Temáticas de Investigación Cooperativa RETIC (Red SAMID RD16/0022 to ACTIBATE study); EXERNET Research Network on Exercise and Health in Special Populations (DEP2005-00046/ACTI); Fundación Iberoamericana de Nutrición (ACTIBATE study); AstraZeneca HealthCare Foundation (ACTIBATE study); PTA 12264-I to FIT-AGEING study.
Author information
Authors and Affiliations
Contributions
R.F.V. conceived the study, analyzed the data, interpreted the data, and drafted the manuscript; J.M.A.A. conceived the study, and also acquired, processed and interpreted the data; J.E.G. conceived the study and interpreted the data; F.M.A. acquired, processed and interpreted the data; J.H.M. acquired, processed and interpreted the data; F.J.A.G. acquired, processed and interpreted the data; F.B.O. conceived the study and interpreted the data; I.L. conceived the study and interpreted the data; J.R.R. conceived the study and interpreted the data. All authors revised critically the manuscript, and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Verdejo, R., Alcantara, J.M.A., Galgani, J.E. et al. Deciphering the constrained total energy expenditure model in humans by associating accelerometer-measured physical activity from wrist and hip. Sci Rep 11, 12302 (2021). https://doi.org/10.1038/s41598-021-91750-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91750-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.