Abstract
The aim of this study was to test the utility of haemodynamic and autonomic variables (e.g. peripheral chemoreflex sensitivity [PCheS], blood pressure variability [BPV]) for the prediction of individual performance (marathon time and VO2max) in older men. The post-competition vasodilation and sympathetic vasomotor tone predict the marathon performance in younger men, but their prognostic relevance in older men remains unknown. The peripheral chemoreflex restrains exercise-induced vasodilation via sympathetically-mediated mechanism, what makes it a plausible candidate for the individual performance marker. 23 men aged ≥ 50 year competing in the Wroclaw Marathon underwent an evaluation of: resting haemodynamic parameters, PCheS with two methods: transient hypoxia and breath-holding test (BHT), cardiac barosensitivity, heart rate variability (HRV) and BPV, plasma renin and aldosterone, VO2max in a cardiopulmonary exercise test (CPET). All tests were conducted twice: before and after the race, except for transient hypoxia and CPET which were performed once, before the race. Fast marathon performance and high VO2max were correlated with: low ventilatory responsiveness to hypoxia (r = − 0.53, r = 0.67, respectively) and pre-race BHT (r = − 0.47, r = 0.51, respectively), (1) greater SD of beat-to-beat SBP (all p < 0.05). Fast performance was related with an enhanced pre-race vascular response to BHT (r = − 0.59, p = 0.005). The variables found by other studies to predict the marathon performance in younger men: post-competition vasodilation, sympathetic vasomotor tone (LF-BPV) and HRV were not associated with the individual performance in our population. The results suggest that PCheS (ventilatory response) predicts individual performance (marathon time and VO2max) in men aged ≥ 50 yeat. Although cause-effect relationship including the role of peripheral chemoreceptors in restraining the post-competition vasodilation via the sympathetic vasoconstrictor outflow may be hypothesized to underline these findings, the lack of correlation between individual performance and both, the post-competition vasodilation and the sympathetic vasomotor tone argues against such explanation. Vascular responsiveness to breath-holding appears to be of certain value for predicting individual performance in this population, however.
Similar content being viewed by others
Introduction
Over the past half-century, the marathon has evolved from an Olympic discipline to a mass social phenomenon, with thousands of participants competing in very popular runs organized in nearly every city throughout the world today1,2. The tremendous growth in popularity of the marathon running can be illustrated by the number of finishers in the New York City Marathon, the world’s largest one: > 12 K in 1980, > 30 K in 1997, > 44 K in 2010 and > 53 K in 20193. As another example, nearly half a million runners have submitted their application for the 2020 London Marathon4. As a consequence of the boom, researches on physiology and training aspects of marathon running (in particular, fast performance determinants) have been attracting increasing attention of thousands of professional as well as non-professional athletes around the world1,5.
Noteworthy, middle-aged and older adults represent a substantial and growing proportion of the marathon-running population. Over the years, the mean age of the finishers of the New York City Marathon increased from ~ 35 y in the 1970s to ~ 41 y in the 2010s6 with almost 30% of all male finishers in 2019 being 50 years old or older7. This underscores the need for further in-depth studies on the physiology of marathon running with advanced age.
Given that the autonomic nervous system (ANS) is a master regulator of visceral functions at rest and during the exercise, an indirect “autonomic monitoring” (including ANS surrogate measures: haemodynamic parameters and heart and blood pressure variability analysis) has been proposed as a useful tool for predicting the performance1,5. In an elegant field study by Gratze et al.8 five haemodynamic monitors were used simultaneously to assess circulatory and ANS parameters in a group of 51 male marathon runners within 2 h after completion of the Graz marathon. The authors reported enhanced postcompetition vasodilation and low sympathetic vasomotor tone to be major predictors of fast marathon performance. However, subjects studied by Gratze et al. were relatively young (mean age: ~ 40 y, range: 26–57 y). Given that aging is characterized by an impaired endothelium-dependent vasodilation9 and sympathetic neurovascular transduction10,11 and by an increased basal sympathetic drive12,13, these results do not necessarily apply to men over 50 years of age.
Secondly, most previous studies in this field have assessed cardiac baroreflex sensitivity as a plausible marker of athletic performance8,14, but have ignored the peripheral chemoreceptor mechanism, possibly due to technical difficulties. However, the observations that the peripheral chemoreflex restrain the exercise-induced vasodilation via increased sympathetic vasoconstrictor outflow15,16,17 provide strong rationale for considering peripheral chemoreflex sensitivity (PCheS) as a possible important predictor of marathon performance. Thirdly, plasma renin activity was shown to be inversely associated with subject’s physical fitness18,19 and was proposed as a marker of sympathetic activity20, given its putative role in the autonomic regulation of cardiovascular control21. Therefore, the components of renin–angiotensin–aldosterone system were included in the study as potential candidates for the predictors of individual performance.
To address these knowledge gaps, we used an approach similar to that described by Gratze et al.8 in a group of men aged 50 years and older competing in the 37th PKO Wroclaw Marathon (Wroclaw, Poland, 19th September 2019). We aimed to test whether the parameters of individual performance (the competition time and maximal oxygen consumption, VO2max, as assessed with the treadmill cardiopulmonary test) in healthy middle-aged and older men are associated with: (1) haemodynamic parameters measured in a continuous and non-invasive fashion, (2) spontaneous cardiac baroreflex sensitivity (cBRS) estimated non-invasively with the sequence method, (3) PCheS as assessed by the ‘gold standard’ transient hypoxia method and recently proposed breath-holding method, (4) autonomic balance as assessed by heart rate variability (HRV) and blood pressure variability (BPV) analysis and (5) plasma renin and aldosterone levels. All the tests listed were conducted twice: before and immediately after the race, except for the transient hypoxia test and the cardiopulmonary exercise test which were performed once, before the race.
Methods
Ethical approval and study population
Twenty-six healthy men aged 50 years and older participating in the 37th PKO Wroclaw Marathon (Wroclaw, Poland, 19th September 2019) were recruited prospectively. All participants were free from any acute illness or chronic disease and not taking regular medication. The study protocol was approved by the local Institutional Ethics Committee (Senacka Komisja ds. Badań Naukowych przy Akademii Wychowania Fizycznego we Wrocławiu). All participants gave written informed consent. The research was conducted in accordance with the Declaration of Helsinki.
Out of 26 men enrolled, one subject failed to complete the marathon and the post-race haemodynamic recordings collected from two other subjects were of unacceptable quality due to technical issues (frequent Nexfin finger-cuff oscillations). These subjects were excluded from the analysis and the final sample included 23 participants. For two subjects, oscillations of the Nexfin finger cuff were limited to the post-race BHT. Therefore, we decided to exclude these subjects from the analysis of BHT-SVR slope only, given that none of the other parameters analysed in the study were affected. Blood sample cannot be obtained from one patient after the race.
Given that the study protocol required all the laboratory experiments to be carried out on the same day (4 days before the run) and that the transient hypoxia test is a time-consuming procedure, the test was conducted in a subgroup of the study participants (N = 18).
Study protocol
Figure 1 outlines the study protocol. All laboratory and field experiments were performed in the order listed below.
Laboratory experiments
All participants were admitted to the laboratory (Department of Physiology, Wroclaw Medical University) once, 4 days before the marathon, for: standard physical examination, measurement of haemodynamic parameters at rest (resting recording), the assessment of PCheS with the transient hypoxia method and/or the breath-holding method and the cardiopulmonary exercise testing on a treadmill.
Field experiments
On the day of the marathon, venous blood sample was collected by venepuncture twice, within 1 h before the race and within 30 min after the race. Blood samples were centrifuged immediately and the plasma separated and frozen at − 70 °C for subsequent analysis. The measurement of haemodynamic parameters at rest (resting recording) and the assessment of PCheS with the breath-holding method were performed once, within 1 h after the race.
Given that the transient hypoxia test is time-consuming (one experimental session lasts typically 40–60 min) and technically demanding (e.g. requires breathing circuit and nitrogen tank), the field assessment of PCheS after the race was relied on the BHT only.
Resting recordings before and after the marathon were used for the evaluation of: (1) cBRS with the sequence method, and (2) HRV and BPV analysis. Five-minute segment of the entire > 10-min resting recording, free of movement artefacts, was selected and used for the calculation of cBRS, HRV and BPV.
Measurement of haemodynamic parameters at rest
Beat-to-beat cardiovascular parameters heart rate (HR, beats/min.), RR interval (RRI, ms), systolic (SBP, mmHg), diastolic (DBP, mmHg) and mean arterial blood pressure (MAP, mmHg), stroke volume (SV, mL/beat), cardiac output (CO, L/min), and systemic vascular resistance (SVR, dyn × s/cm5) were derived from the Nexfin monitor (BMEYE B.V., The Netherlands). Nexfin employs the volume-clamp technique to measure finger arterial blood pressure in a beat-to-beat fashion22 and built-in algorithms to reconstruct brachial pressure from finger pressure waveform23. The height correction unit of the Nexfin device is used to compensate for a difference in height (and thereby, hydrostatic pressure) between the finger and the heart. SV, CO and SVR are estimated employing the Nexfin pulse-contour method23. Nexfin HR measurement was based on finger pressure waveform analysis.
10-min resting recording of haemodynamic parameters was performed with the subject lying supine. All haemodynamic parameters were monitored for > 3 min prior to the resting recording to ensure stabilization of haemodynamics and data from this period were not included in the analysis.
Baroreflex sensitivity assessment using the sequence method
Beat-to-beat SBP and RRI from the resting recording were used for the cBRS estimation by the sequence method24. All sequences of ≥ 3 cardiac cycles in which SBP increases or decreases by ≥ 1 mmHg were accompanied by, respectively, RRI lengthening or shortening by ≥ 5 ms, were isolated and slope of the regression line relating SBP and RRI was computed for each sequence isolated. SBP values were paired with accompanying RRI values (SBP–ECG delay of 0 beat), as this approach was reported to be the most appropriate25. Sequences with R2 < 0.85 were excluded from the analysis. cBRS (ms/mmHg) was computed as the average of all slopes.
Peripheral chemoreflex sensitivity assessment
The transient hypoxia test26,27 is a well-established method for the evaluation of PCheS, that is believed to represent a relatively “pure” response from peripheral chemoreceptors, unlike the steady-state hypoxia tests reflecting a more systemic response to hypoxia28. However, due to technical difficulties, it cannot be performed in the field. Therefore, a novel promising, yet not fully established method, the breath-holding test29,30 has been used to assess the PCheS before and after the race, in addition to the transient hypoxia testing carried out before the race only.
Peripheral chemoreflex sensitivity assessment using the transient hypoxia method
The transient hypoxia method was used for the evaluation of PCheS, as described previously26,27. The subject lies supine and breaths through the oronasal mask (7450 series V2 oro-nasal mask, Hans Rudolph Inc., USA) connected to a two-way non-rebreathing valve (Hans Rudolph Inc.). The inspiratory side of the valve is connected to a three-way stopcock-type valve (Hans Rudolph Inc.), allowing the operator to switch between room air and 60-L bag filled with pure nitrogen.
All haemodynamic parameters were measured with the Nexfin monitor as described above (see: Measurement of haemodynamic parameters at rest), except for HR, which was calculated from the ECG (BioAmp, ADInstruments, New Zealand). Respiratory flow was measured continuously with a 1000 L/min flowhead (MLT3000L, ADInstruments) placed on the inspiratory side of the breathing circuit and a differential pressure transducer (FE141 Spirometer, ADInstruments), and used to calculate instantaneous values of tidal volume (VT, L), breathing rate (BR, breaths/min) and minute ventilation (VIns, L/min). Blood oxygen saturation (SpO2, %) was measured with the Nexfin monitor and an ear probe (Masimo Corporation, USA). All data were recorded at a sampling frequency of 1 kHz using data acquisition hardware (PowerLab 16/30, ADInstruments) and software (LabChart 8, ADInstruments).
During the test, the subject is repeatedly switched from breathing room air to pure N2 from the bag and then back to room air, in order to evoke a transient decrease in SpO2. The test includes four to seven N2-breathing episodes, each lasting three to eight breaths. The length of the consecutive N2-breathing episodes is changed randomly in order to collect a range of SpO2 decreases (SpO2 nadirs) from 70 to 90%.
Quantifying respiratory responses to transient hypoxia
The arithmetic average of three largest consecutive VIns values (referring to three consecutive breaths) within a time-window starting 5 s before the SpO2 nadir and ending 30 s after the SpO2 nadir was calculated for each N2-breathing episode and plotted against corresponding SpO2 nadir (post-hypoxic VIns-to-SpO2). Pre-hypoxic VIns-to-SpO2 was calculated from 90-s period immediately preceding N2-breathing episode, by plotting 90-s average of VIns against 90-s average of SpO2. Finally, a regression line relating all pre- and post-hypoxic VIns-to-SpO2 was drawn and the slope of the line was defined as the minute ventilation response to transient hypoxia (VIns-Hypo, L/min/SpO2%). The tidal volume response to transient hypoxia (VT-Hypo, L/SpO2%) was calculated analogously, but VT was used instead of VIns. Given the inverse relation between SpO2 and breathing (VIns and VT), lower (more negative) values of the slope (VIns-Hypo and VT-Hypo) indicate greater ventilatory responsiveness to hypoxia, whereas higher (less negative) values indicate lower ventilatory responsiveness to hypoxia.
Quantifying haemodynamic responses to transient hypoxia
To reduce the possible confounding effect of spontaneous (e.g. respiratory-induced) fluctuations in haemodynamic parameters, a moving average (10-s window) was used to smooth the data28. The following haemodynamic responses to transient hypoxia: heart rate response (HR-Hypo, beats/min/SpO2%), systolic blood pressure response (SBP-Hypo, mmHg/SpO2%), mean arterial blood pressure response (MAP-Hypo, mmHg/SpO2%), and systemic vascular resistance response (SVR-Hypo, dyn × s/cm5/SpO2%) were calculated analogously to the minute ventilation to transient hypoxia, using HR, SBP, MAP or SVR, respectively, instead of VIns. The only difference was that the single highest value (and not the average of three consecutive largest values) of HR, SBP or MAP within the time-window was used for calculation of HR-Hypo, SBP-Hypo and MAP-Hypo, respectively, and the single lowest value of SVR within the time-window was used for calculation of SVR-Hypo.
Peripheral chemoreflex sensitivity assessment using the breath-holding method
The breath-holding test (BHT) for PCheS evaluation, as proposed by Trembach and Zabolotskikh29, was performed either immediately after the resting recording (in subjects not undergoing PCheS assessment with the transient hypoxia test) or immediately after PCheS assessment with the transient hypoxia. In both scenarios, the measuring instruments used in the preceding stage were utilized, except for the oronasal mask which was removed after completion of the transient hypoxia test.
The test was performed with the subject lying supine. The subject was asked to hold his/her breath at about 2/3 of the vital capacity for as long as possible, and the duration of the voluntary apnoea was measured from the end of inspiration to the onset of reflex contractions of the diaphragm as assessed by palpation. The breath-hold manoeuvre was performed two to three times with ≥ 5 min pause between consecutive runs and the average duration of the voluntary apnoea was taken as a measure of PCheS (BHT apnoea duration, s). Several papers reported high agreement between BHT and single-breath CO2 method for PCheS evaluation in healthy subjects29,31 and heart failure patients30, however to the authors’ knowledge, the agreement between BHT and the transient hypoxia test has not yet been investigated.
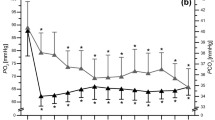
Although not considered in the original version of the BHT, SVR response to the breath-holding was calculated from beat-to-beat recordings, covering the interval between 20th heart beat before the onset of the apnoea and 51st heart beat following the onset of the apnoea (72 beats in total), and expressed as the slope of the regression line. The procedure is shown on Fig. 2A. Responsiveness to BHT in terms of SVR (BHT-SVR slope, dyn × s/cm5/ beat) was defined as a mean of all slopes calculated from all episodes of voluntary apnoea.
Vascular (SVR) responsiveness to the breath-holding test (BHT). (A) Calculation of the SVR response to a single voluntary apnoea episode. (B) Pre- and post-race values of the BHT-SVR slope. (C) Beat-to-beat SVR response to the BHT in fast finishers versus slow finishers (classified according to the median of the marathon time; whiskers indicate standard error). (D) The competition time plotted against the pre-race BHT-SVR slope.
Heart rate variability and blood pressure variability analysis
Ectopic beats, if present, were corrected by interpolation. Standard autoregressive methods were used for the spectral analysis of HRV32. The following HRV and BPV indices were computed: standard deviation of RRIs (SDNN, ms), the percentage of adjacent RRIs with a difference of duration > 50 ms (pNN50, %), the power of HRV spectrum within low-frequency range (0.04–0.15 Hz) and high-frequency range (0.15–0.40 Hz) expressed in normalized units (LF-HRV and HF-HRV, respectively, nu), standard deviation of beat-to-beat SBP (BPV-SD, mmHg), the power of DBP variability spectrum within low-frequency range (0.05–0.17 Hz, LF-BPV)14,32. HRV parameters were calculated based on Nexfin-derived blood pressure signal. SDNN is believed to reflect overall autonomic regulation of the heart function; pNN50 and HF-HRV are markers of the vagal tone, while the physiological background of LF-HRV includes sympathetic or mixed sympathetic and parasympathetic activity33.
Maximal oxygen consumption measurement
All subjects underwent a maximal cardiopulmonary exercise test on a motorised treadmill (Trackmaster TMX425, Full Vision, Inc., KS, USA). After a 5-min running at 8 km/h (warm-up), the protocol started and a treadmill speed was increased by 1 km/h every 2 min, in a stepwise fashion. The treadmill inclination was kept constant at 1°. The protocol was continued until exhaustion.
Oxygen uptake (VO2, mL/min/kg), and instantaneous minute ventilation (VE, L/min) were measured breath-by-breath (Cosmed Quark CPET, Rome, Italy) and averaged every 30 s. The highest values of VO2 and VE were taken as VO2max (mL/kg/min) and maximal minute ventilation (VEmax, mL/min/kg) respectively.
Blood chemistry
Plasma renin (µIU/mL) and aldosterone (ng/dL) levels were measured using chemiluminescence immunoassay (CLIA) technique on an automated analyser (Liaison, Diasorin, Dartford, UK).
Data and statistical analyses
Statistica (Statsoft, USA), Matlab 2016a (Mathworks, USA), LabChart (ADInstruments) and CardioSeries Software (http://www.danielpenteado.com) were used for data analysis. Kolmogorov–Smirnov test was used to assess the normality of distribution of continuous variables and the results were ensured by visual inspection of frequency histograms. For the parameters measured twice, before and after the competition, pre-race values were tested only. Data are presented as mean ± standard deviation (for continuous normally distributed variables), median with lower and upper quartile (for continuous variables that did not follow a normal distribution) or number and percentage (for categorical data). For the parameters measured twice (before and after the race), mean percentage difference ± standard deviation was presented.
Paired Student’s t test was used for within-subject comparisons. Pearson’s linear correlation coefficient was used to assess the relations between variables, in particular between the parameters of individual performance (the marathon time and VO2max) and the haemodynamic and autonomic parameters (predictors). In order to reduce the number of correlations carried out and avoid redundancy a priori, we decided to analyse the correlations for: (1) pre-race values of all haemodynamic and autonomic parameters, (2) pre- versus post-race changes for these parameters only which had changed significantly after the race. Therefore, neither post-race values of all parameters nor the pre- versus post-race changes of the parameters not affected by the competition were included in the correlation analysis.
For all haemodynamic and autonomic parameters, a general linear model was used to explore the effect of interaction between pre-race value of a given parameter and pre- versus post-race change in the given parameter ([pre-race × change] interaction) on the dependent variable: the marathon time or VO2max. To further investigate the nature of the observed significant pre-race × change interactions for a given parameter, the subjects were dichotomized according to the median of the pre-race value of the parameter, and linear correlations between the pre- versus post-race change versus the marathon time or VO2max were assessed in both groups.
Due to the exploratory nature of the study, we decided not to adjust the significance level to account for multiple testing. A value of p < 0.05 was considered statistically significant.
In addition to the analysis of correlation, we attempted to compare the marathon time between the subjects with PCheS within the normal range (normal PCheS) and the subjects with peripheral chemoreceptor hypersensitivity (high PCheS), according to the cut-off value of VIns-Hypo commonly used in clinical studies on PCheS in heart failure patients34,35,36. Based on these studies, the value of VIns-Hypo of − 0.7 L/min/SpO2% was used to allocate the subjects into the normal PCheS group (VIns-Hypo between zero and − 0.7 L/min/SpO2%) or high PCheS group (VIns-Hypo more negative than − 0.7 L/min/SpO2%).
Last, in order to better visualize the SVR response to breath-holding in slow versus fast finishers, the subjects were dichotomized according to the median of the marathon time in our population (266 min.).
Ethics approval
The study protocol was approved by the local Institutional Ethics Committee (Senacka Komisja ds. Badań Naukowych przy Akademii Wychowania Fizycznego we Wrocławiu). The research was conducted in accordance with the Declaration of Helsinki.
Consent to participate
All participants gave written informed consent.
Consent for publication
All participants gave written informed consent.
Results
Baseline characteristics of the study participants are summarized in Table 1.
Haemodynamic parameters
As expected, the post-race CO was higher, and the post-race values of RRI, SBP, MAP, SV and SVR were lower as compared with the pre-race values (all p < 0.01, Table 2).
Pre- versus post-race change in RRI was correlated with both, the competition time (r = 0.46, p = 0.02) and VO2max (r = − 0.46, p = 0.03), while the correlation between the change in CO and the competition time was not statistically significant (r = − 0.40, p = 0.06). No other relations between the haemodynamic parameters and the parameters of individual performance (the competition time or VO2max) were found (all p ≥ 0.15, Table 3). Furthermore, no significant pre-race × change interactions were identified (Table 3).
Given that the post-exercise alterations in total peripheral resistance have been shown previously to be associated with marathon performance8, scatter plot of pre- versus post-race change in SVR against competition time was presented (Fig. 3).
Spontaneous cardiac baroreflex sensitivity
cBRS decreased after the competition (Table 4). Neither pre-race cBRS nor the change in cBRS were correlated to the competition time (r = − 0.18, p = 0.42 and r = 0.06, p = 0.79, respectively) or VO2max (r = 0.01, p = 0.96 and r = − 0.30, p = 0.17, respectively). Furthermore, no significant pre-race × change interactions were found (p = 0.23 and 0.84 for the competition time and VO2max, respectively).
Peripheral chemoreflex sensitivity
The pre-race values for PCheS as assessed with the transient hypoxia test and the pre- and post-race values for PCheS as assessed with the BHT were presented in Table 4. BHT apnoea time did not change after the race, while the BHT-SVR slope was lowered (Table 4, Fig. 2B).
Marathon time was inversely correlated with a ventilatory response from the peripheral chemoreceptors, as assessed by the transient hypoxia (VIns-Hypo vs. competition time: r = − 0.53, p = 0.02, Fig. 4A, VT-Hypo vs. competition time, r = − 0.50, p = 0.03) and the BHT (pre-race BHT apnoea time vs. competition time, r = − 0.47, p = 0.02, Fig. 4B). In line with this, VO2max was correlated with VIns-Hypo (r = 0.67, p = 0.002, Fig. 4C), VT-Hypo (r = 0.55, p = 0.02) and pre-race BHT apnoea time (r = 0.51, p = 0.01, Fig. 4D), thereby providing further support for the postulated link between peripheral chemosensitivity and individual performance.
Correlations between the ventilatory response to the chemoreceptor-stimulating manoeuvres (transient hypoxia and breath-holding) and the parameters of individual performance: the marathon time and VO2max. (A) The competition time plotted against VIns-Hypo (minute ventilation-response to the transient hypoxia). (B) The competition time plotted against the pre-race BHT apnoea duration. (C) VO2max plotted against VIns-Hypo (minute ventilation-response to the transient hypoxia). (D) VO2max plotted against the pre-race BHT apnoea duration.
High VIns-Hypo group was characterized by significantly worse competition time as compared with normal VIns-Hypo group (282 ± 26 vs. 242 ± 35 min., respectively, p = 0.03, Fig. 5).
Regarding the haemodynamic responses to the chemoreceptor-stimulating manoeuvres, pre-race BHT-SVR slope was correlated with the marathon outcome (r = − 0.59, p = 0.005, Fig. 2D). Figure 2C presents mean beat-to-beat SVR response to the breath-holding test in fast vs. slow finishers (classified according to the median marathon time). We did not observe any significant associations between a change in BHT-SVR slope versus the competition time (r = 0.05, p = 0.84) and pre-race BHT-SVR slope or pre- versus post-race change in BHT-SVR slope and VO2max (r = 0.36, p = 0.11 and r = − 0.33, p = 0.15, respectively). Similarly, no statistically significant linear correlations were found between the competition time or VO2max and the transient hypoxia test-derived responses: HR-Hypo (r = 0.25, p = 0.32 and r = 0.23, p = 0.35, respectively), SBP-Hypo (r = − 0.03, p = 0.90 and r = 0.05, p = 0.83, respectively), MAP-Hypo (r = − 0.04, p = 0.87 and r = 0.13, p = 0.60, respectively), SVR-Hypo (r = − 0.17, p = 0.49 and r = − 0.30, p = 0.23, respectively). Furthermore, we did not find any significant pre-race × change interaction for the BHT-apnoea time (p = 0.16 and 0.76, for the marathon time and VO2max, respectively) or the BHT-SVR slope (p = 0.27 and 0.49, for the marathon time and VO2max, respectively).
Heart rate variability and blood pressure variability
The post-race pNN50, HF-HRV and SDNN were diminished as compared with the pre-race values, while the LF-HRV increased after the race. BPV-SD did not differ between and after the race, whereas LF-BPV was increased after the race (Table 4).
No significant correlations between the parameters of individual performance and pre-race HRV parameters or pre- versus post-race changes in HRV parameters were found, although an inverse correlation between pNN50 and the marathon time approached significance (r = − 0.37, p = 0.08, Table 5). Similarly, we did not identify any significant pre-race × change interactions for HRV indices (Table 5).
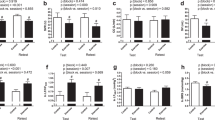
Pre-race BPV-SD was related with both, the competition time (r = − 0.42, p = 0.04) and the VO2max (r = 0.64, p = 0.001, Table 5, Fig. 6). We found no correlation between the competition time or VO2max and LF-BPV (pre-race values or pre- versus post-run changes, Table 5), however statistically significant pre-race × change interaction was identified (p = 0.05, Table 5). To explore this interaction further, the subjects were divided into low- and high pre-race LF-BPV group according to the median of the pre-race LF-BPV. Interestingly, in the low pre-race LF-BPV group, the competition time correlated strongly with the change in BPV-LF (r = 0.75, p = 0.005), indicating that the fast marathon performance is accompanied by little or no increase in LF-BPV in subjects with initially low LF-BPV, whereas no such relation was found in subjects with high LF-BPV before the run (Fig. 7).
Plasma renin and aldosterone levels
Plasma renin and aldosterone values were elevated after the run (Table 4).
We found no relations between the parameters of individual performance (competition time and VO2max) and the pre-race plasma renin (r = 0.11, p = 0.61 and r = − 0.12, p = 0.57, respectively) and aldosterone (r = − 0.20, p = 0.37 and r = 0.25, p = 0.25, respectively) levels or pre- versus post-race changes in plasma renin (r = − 0.19, p = 0.40 and r = − 0.03, p = 0.90, respectively) or aldosterone levels (r = 0.10, p = 0.65 and r = − 0.19, p = 0.39, respectively). There were no significant pre-race × change interactions for renin (p = 0.55 and 0.61 for the marathon time and VO2max, respectively) or aldosterone (p = 0.78 and 0.59, for the marathon time and VO2max, respectively).
Discussion
To our knowledge, this is the first study addressing the utility of the autonomic variables as predictors of the marathon performance in healthy men aged 50 years and older. The pattern of relations we observed is in stark contrast to that reported for younger competitors8. We found that: (1) low ventilatory responsiveness to the manoeuvres targeting peripheral chemoreflex (the transient hypoxia test and the breath-holding test), and (2) enhanced vasoconstrictor responsiveness to the breath-holding are the predictors of fast marathon performance in healthy men over 50 years of age; (3) neither the post-exercise vasodilation nor the markers of sympathetic outflow to the vessels (LF-BPV) or sympathovagal control of the heart (HRV analysis) predicts the marathon outcome in this population. The major strength of our study is that we used two different methods for the peripheral chemoreflex sensitivity assessment (the transient hypoxia test and the breath-holding test), as well as two parameters of individual performance, namely the marathon completion time and the VO2max as assessed with the treadmill cardiopulmonary exercise test. Of importance, our major results displayed similar pattern regardless the method for the peripheral chemoreflex sensitivity evaluation considered.
We are not aware of any previous studies demonstrating the relation between peripheral chemoreceptor sensitivity and marathon performance in the middle-aged and older men. Fast performance was associated with low ventilatory response to transient hypoxia and breath-holding in our study. Given that the link between the marathon performance and autonomic regulation of the cardiovascular system (as assessed by HRV and BPV5,8,14 and beat-to-beat cBRS5,8,14) has been repeatedly reported in younger subjects1, the sparseness of data on peripheral chemosensitivity in this context may appear surprising. Of note, however, role of peripheral chemoreflex in regulation of circulation during the exercise has been largely underestimated37 until the series of elegant studies by Stickland et al.15,16,17. They have shown, in chronically instrumented dogs, that transient inhibition of carotid chemoreceptors during mild exercise leads to an abrupt vasodilation in the hindlimb, and this effect is abolished by α-adrenergic receptor blockade or carotid body denervation15. Subsequently, they translated these findings to humans and found that transient suppression of carotid chemosensory activity during exercise decreases sympathetic vasoconstrictor outflow16 and increases muscle blood flow and conductance17.
Therefore, given that the peripheral chemoreflex was shown to restrain the exercise-induced vasodilation via increased sympathetic vasoconstrictor outflow15,16,17 and both aforementioned factors—namely, exercise vasodilation and sympathetic regulation of vasomotor tone—were reported to be major predictors of the marathon performance8, it is tempting to speculate that the peripheral chemosensitivity influences the marathon outcome by the effect on exercise vasodilation. Alternatively, peripheral chemoreflex may impact the marathon performance by the effect on exercise hyperpnoea. Our results are discussed in relation to both scenarios below, although it needs to be emphasized that the study design does not permit any conclusions regarding causality.
Does the peripheral chemoreflex impact marathon performance via its effect on exercise-induced vasodilation?
Surprisingly, fast performance was associated with greater SVR increase in response to breath-holding test. Given that an inverse relationship between sympathetic responsiveness and sympathetic tone was reported previously in young volunteers38, one might conclude that greater SVR responsiveness to stressors reflects merely a decreased tonic sympathetic outflow to the vessels, resulting in lower SVR at rest. However, resting SVR was similar in fast vs. slow finishers, both before and after the run (Fig. 2C). Clearly, the observation that fast finishers were characterized by low ventilatory and high vascular responsiveness to peripheral chemoreflex stimulation does not support our hypothesis that low peripheral chemosensitivity results in low vasoconstrictor drive to vessels, greater exercise-induced vasodilation and eventually, better marathon performance. Moreover, vascular response to hypoxia was not associated with individual performance in our study.
The following two points should be considered, however. First, chemoreceptors’ ventilatory responsiveness does not necessarily mirror the vascular27 or sympathetic39 responsiveness and the existence of distinct connections from the carotid body into the nervous system, targeting different reflexes (e.g. ventilatory and sympathetic) was postulated40. Second, chemoreceptors’ acute sensitivity to stressors does not necessarily parallel chemoreceptors’ tonicity40. In other words, enhanced vascular response to stressor (e.g. breath-holding) is not necessarily a marker of high tonic vasomotor tone (eg. due to increased chemoreceptor tonicity). Sympathetic tone and sympathetic responsiveness should not be equated with one another38.
Does the peripheral chemoreflex impact marathon performance via its effect on exercise hyperpnoea?
Hypoxic ventilatory response at rest was reported to be lower in athletes, including marathon runners, in some41,42,43,44, but not all studies45,46. Mean hypoxic ventilatory response value (defined as change in minute ventilation) in the current study (VIns-Hypo: − 0.42 ± 0.32 L/min/SpO2%) was close to the values reported by our27,47,48 and other groups26,28,49.
Although circulating levels of the major carotid chemoreceptor stimuli (PO2, PCO2, lactate, K+) do not change significantly during the exercise50, several lines of evidence support a role of carotid chemoreceptors as a source of tonic excitatory drive for breathing which is equally important at rest as well as during the exercise50,51. Firstly, in animal models, carotid body denervation leads to hypoventilation at rest and during exercise52,53,54. Secondly, suppression of peripheral chemoreceptor activity with hyperoxia decreases ventilation at rest and during exercise in humans55,56,57. Thirdly, asthmatic patients who had undergone carotid-body resection for the relief of dyspnoea were characterized by lower rate of increase in ventilation to its steady-state, higher PCO2 level and less marked hyperpnoea above the anaerobic threshold as compared with age-matched controls58. It should be underlined, however, that there is still huge controversy over whether peripheral chemoreceptor mechanism contributes significantly to the control of breathing during exercise50,59.
We did not collect respiratory data during or immediately after the run and therefore the existence of a relation between chemosensitivity and exercise hyperpnoea cannot be verified directly in the current study. However, lack of linear relation between hypoxic ventilatory response and maximal minute ventilation during the cardiopulmonary exercise test (r = 0.03, p = 0.89) argues against this explanation.
Post-competition vasodilation does not predict the marathon performance in the older men
Gratze et al.8 found robust correlations between the marathon time and both: (1) the pre- versus post-race change in SVR (r = 0.29) and (2) the pre-race LF-BPV (r = 0.48) in the group of relatively young males (mean age: ~ 40 y, range: 26–57 y) and concluded that sympathetic vasomotor control and post-competition vasodilation are critical determinants of the marathon outcome. Employing a similar approach, we failed to replicate this finding in healthy men aged 50 years or older. The change in SVR did not correlate with the marathon outcome or VO2max in our study. Regarding LF-BPV, no significant correlations between performance and the pre-race LF-BPV or the change in LF-BPV were found in the whole group. However, analysis of the effect of interaction between the pre-race LF-BPV and the change in LF-BPV revealed that fast marathon time is accompanied by little or no increase in LF-BPV after the run in the subjects with initially low LF-BPV only. Therefore, it is plausible that that the pre- versuss post-race change in the sympathetic vasomotor outflow has a certain prognostic value for athletic performance in middle-aged and older man with low tonic sympathetic drive to the vessels. Nevertheless, predictive value limited to subjects with low resting sympathetic tone precludes the widespread usage of LF-BPV for predicting the marathon outcome. Interestingly, however, the two other physiological measures predicted the competition time in the whole group in our study. We found that fast finishers displayed higher vascular responsiveness to the breath-holding test (r = − 0.59) and greater overall BPV (expressed as BPV-SD, r = − 0.42).
Peripheral vasodilator capacity declines with aging in healthy humans60 and this is particularly manifested during the exercise61,62. Multiple factors are involved, however, and, for instance, regular aerobic exercise was shown to restore vasomotor responsiveness in older healthy subjects to levels similar to those in young adults63. In line with this, an average change in SVR found in the current study (decrease by ~ 20%) is slightly lower as compared with the value reported by Gratze et al. (decrease by ~ 30–35%).
Factors attributed to age-dependent impairment of vasodilator capacity include endothelium-dependent (e.g. decreased NO bioavailability60) and non-endothelium-dependent mechanisms (e.g. increased sympathetic vasoconstrictor outflow62). As sympathetic vasomotor tone was shown previously to predict the marathon outcome8, age-dependent alterations in sympathetic control will be outlined herein. Aging is associated with an increased overall sympathetic activity at rest64,65,66,67,68,69. However, it does not necessarily translate into higher blood pressure and α1-desensitization70 and impaired functional sympatholysis62 have been proposed as underlying mechanisms. In line with this, Hart et al.71 reported an inverse relationship between sympathetic vasoconstrictor activity (as assessed directly using microneurography) and vascular conductance in young men (mean age ~ 27 y), while no such relation was found in a group of older men (mean age ~ 61 y). In other words, it seems that neural control of the vessels is impaired in older men. In young adults, cardiac output is a key compensatory mechanism that maintains blood pressure at an appropriate level when tonic sympathetic outflow to the vessels is low (and therefore, SVR is decreased as well)71. Literature data regarding the sympathetic vascular responsiveness to stressors in the older are less consistent. The older subjects displayed blunted MSNA response to head-up tilting68 or nitroprusside injection72, enhanced MSNA response to phenylephrine injection72 or whole-body cooling73, and unchanged MSNA responsiveness to mental stress74.
Keeping in mind that the sympathetic control of the vessels is impaired in the older men, we suggest the following interpretation of our results, revolving around a trade-off between the peripheral vasodilation (aimed to enhance oxygen supply to the working muscles) and a need to maintain blood pressure within a normal range during the exercise. In younger subjects, high maximal cardiac output compensates for an enhanced peripheral vasodilation so effectively that maximal (or close to maximal) level of the peripheral vasodilation may be triggered. As a result inter-individual differences in maximal peripheral vasodilation translate to better or worse perfusion of the muscles, and eventually, better or worse performance. In older subjects however, maximal cardiac output is decreased75, and thereby not capable to effectively offset a fall in SVR. Consequently, maximal peripheral vasodilation is rarely achieved and the body’s ability to quickly reroute the blood from one vascular bed to another is by far more important for the individual performance than the maximal achievable level of vasodilation. Given the age-related deterioration of the vascular control, mentioned above, the vascular responsiveness becomes a crucial predictor of the individual performance in the older subjects.
Importantly, our observation that in subjects with low tonic vasomotor drive, better marathon outcome is accompanied by little increase in the vasomotor drive after the run fits into this explanation given that an inverse relationship between the sympathetic responsiveness and the sympathetic tone has been reported38. In these subjects, low sympathetic tone during the run could be compensated by the remarkable increase in cardiac output.
Our results corroborate the findings by Ng et al.76 who reported higher sympathetic vasomotor control at rest and during the exposure to laboratory stressors (cold pressor test and isometric handgrip) in healthy older (over 60 y) endurance athletes as compared to age-matched untrained controls. Furthermore, this effect may be limited to older athletes, given that in younger subjects, 4–6 weeks of dynamic training was reported to decrease MSNA response to exercise77,78.
In the study by Gratze et al.79, a subgroup of marathon runners experiencing pre-syncopal symptoms during active standing test (performed 2 h after the run) was identified. The authors claimed, based on LF-BPV changes, that those runners have exhausted their sympathetic reserve – the ability to further increase sympathetic vasomotor drive. These results underscore the importance of the fully functional sympathetic regulation of blood vessels for the marathon outcome. Given that maximal cardiac output decreases with age75, preserved vascular responsiveness allowing for redistributing the blood from non-active areas to the working muscles would be of particular importance in the older athletes.
Of note, however, Gratze et al. reported no differences in pre-race values of autonomic and haemodynamic parameters (at rest or during active standing) between the males experiencing- vs. non-experiencing pre-syncopal symptoms. Moreover, the competition time was virtually identical between both groups. In stark contrast, in the current report, fast marathon performance was accompanied by an elevated pre-race SVR responsiveness to a stressor (breath-holding) in healthy middle-aged and older men.
LF-BPV was not related with the parameters of individual performance in the whole group in our study and it corroborates the aforementioned hypothesis based on the impaired neural control of the vessels in the older males. However, another index of the BPV—standard deviation of the beat-to-beat SBP (BPV-SD) correlated with both, the competition time and VO2max. We believe it provides further support for our explanation, given that SD-based index is vulnerable to random, non-rhythmic changes in the variable and does not necessarily reflect rhythmical oscillations that may result from tonic neural influences. In this context, BPV-SD is rather a marker of vascular responsiveness to random environmental changes than a marker of a neutrally-driven tonic control of the vasculature, thereby being closer to BHT-SVR than to LF-BPV.
Does the heart rate variability analysis identify fast finishers among older male marathon runners?
Multiple studies demonstrated that HRV declines with advancing age80,81 and training improves HRV in the older individuals82,83. Furthermore, training-induced changes in HRV were shown to predict individual performance5,83,84,85. The effect of ageing on HRV appears to be more complex, however. In a large sample of > 1.700 subjects aged from 40 to 100 years, a linear decrease with age in overall autonomic regulation of the heart function was found to be accompanied by the U-shaped pattern of changes of the vagally-mediated indices (e.g. pNN50) with the nadir at the seventh decade86. Lack of any significant change in HRV with healthy ageing has been also reported87.
In our study, fast finishers tended to display higher values of a vagally-mediated pNN50 parameter before the race (p = 0.08). Although taking into consideration a relatively small sample size, we cannot refute the possibility that HRV parameters are linked to the marathon performance in the older athletes, the observation that other ANS-dependent parameters outperformed the most popular HRV indices seems still worth emphasizing.
Study limitations
We acknowledge that the design of our study precludes conclusions on causal associations. The other limitation of our study is the small sample size which probably constrained the capacity to detect predictors of the individual performance. Nevertheless, we believe utilizing two different methods for the PCheS evaluation and two different indices of the individual performance substantially decreased the risk of false positive results, thereby providing a reasonable compensation for the small sample size. Furthermore, sample size of < 30 subjects is common in the studies of similar-design in this field (e.g. 27 athletes studied before and after Ironman competition in Gratze et al.14, 9 athletes studied before and after a mountain marathon in Murrell et al.88). The results should be interpreted with caution given the high number of tests performed which increases the probability of false-positive results.
Most previous studies utilized linear regression analysis to identify associations between autonomic variables and athletic performance, despite the fact that these relations do not necessarily follow a linear relationship, as it was shown for cognitive performance89. In order to identify possible non-linear relationships, we have used the second-order (quadratic) regression (see: supplementary figure 1). However, the major results of the study did not change.
Furthermore, we did not measure muscle sympathetic nerve activity (MSNA) with microneurography77,78. MSNA data would shed more light on the alterations in sympathetic outflow in fast and slow finishers.
Given that the autonomic tonicity cannot be equated to the autonomic responsiveness and both are likely to carry different predictive value, the other standard and well-established stress tests (e.g. active standing test, tilt-table test or the cold pressor test90) should be considered in future studies on physiological predictors of athletic performance.
Conclusions
Low ventilatory response from the peripheral receptors (as assessed by the transient hypoxia or the breath-holding test) is a marker of fast marathon performance and high VO2max in healthy men aged ≥ 50 y. The underlying mechanism remains unknown, given that neither the post-competition vasodilation nor the sympathetic vasomotor tone (as assessed with the LF-BPV) predicted the marathon outcome or VO2max in this population. Interestingly, high vascular responsiveness (as assessed by the SVR change to breath-holding) appears to be beneficial in this population.
Data availability
All data relevant to the study are presented in this article. The data are not available due to the potential for a breach of confidentiality.
Code availability
Commercially available software was used: STATISTICA v.12.1 (Statsoft, Tulsa, OK, USA), LabChart v.8 (Dunedin, New Zealand). Baroreflex sensitivity was calculated using CardioSeries 2.4 available upon request from Dr Daniel Penteado (www.danielpenteado.com).
References
Pagani, M. & Lucini, D. Can autonomic monitoring predict results in distance runners?. Am. J. Physiol. Heart Circ. Physiol. 296, H1721–H1722 (2009).
Zinner, C. & Sperlich, B. Marathon Running: Physiology, Psychology, Nutrition and Training Aspects. (Springer, 2016). https://doi.org/10.1007/978-3-319-29728-6.
New York City Marathon Race Results 2019. http://www.marathonguide.com/results/browse.cfm?MIDD=472191103.
London Marathon 2020 Entries By the Numbers. https://www.runnersworld.com/news/a27557006/london-marathon-entries/.
Manzi, V. et al. Dose-response relationship of autonomic nervous system responses to individualized training impulse in marathon runners. Am. J. Physiol. Hear. Circ. Physiol. 296, H1733 (2009).
Vitti, A., Nikolaidis, P. T., Villiger, E., Onywera, V. & Knechtle, B. The, “New York City Marathon”: participation and performance trends of 1.2M runners during half-century. Res. Sport. Med. 28, 121–137 (2020).
Overview - TCS New York City Marathon 2019. https://results.nyrr.org/event/M2019/overview.
Gratze, G., Mayer, H., Luft, F. C. & Skrabal, F. Determinants of fast marathon performance: low basal sympathetic drive, enhanced postcompetition vasodilatation and preserved cardiac performance after competition. Br. J. Sports Med. 42, 882–888 (2008).
Gerhard, M., Roddy, M. A., Creager, S. J. & Creager, M. A. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27, 849–853 (1996).
Hart, E. C. et al. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension 54, 127–133 (2009).
Briant, L. J. B. et al. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J. Physiol. 594, 4753–4768 (2016).
Sundlöf, G. & Wallin, B. G. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J. Physiol. 274, 621–637 (1978).
Ziegler, M. G., Lake, C. R. & Kopin, I. J. Plasma noradrenaline increases with age. Nature 261, 333–335 (1976).
Gratze, G. et al. Hemodynamic and autonomic changes induced by Ironman: prediction of competition time by blood pressure variability. J. Appl. Physiol. 99, 1728–1735 (2005).
Stickland, M. K., Miller, J. D., Smith, C. A. & Dempsey, J. A. Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ. Res. 100, 1371–1378 (2007).
Stickland, M. K., Morgan, B. J. & Dempsey, J. A. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J. Physiol. 586, 1743–1754 (2008).
Stickland, M. K. et al. Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J. Physiol. 589, 6219–6230 (2011).
M’Buyamba-Kabangu, J. R., Fagard, R., Lijnen, P. & Amery, A. Relationship between plasma renin activity and physical fitness in normal subjects. Eur. J. Appl. Physiol. Occup. Physiol. 53, 304–307 (1985).
Goessler, K., Polito, M. & Cornelissen, V. A. Effect of exercise training on the renin-angiotensin-aldosterone system in healthy individuals: a systematic review and meta-analysis. Hypertens. Res. 39, 119–126 (2016).
Hamer, M. et al. Plasma renin responses to mental stress and carotid intima-media thickness in black Africans: the SABPA study. J. Hum. Hypertens. 25, 437–443 (2011).
Volpe, M., Battistoni, A., Chin, D., Rubattu, S. & Tocci, G. Renin as a biomarker of cardiovascular disease in clinical practice. Nutr. Metab. Cardiovasc. Dis. 22, 312–317 (2012).
Penáz, J. Criteria for set point estimation in the volume clamp method of blood pressure measurement. Physiol. Res. 41, 5–10 (1992).
Bogert, L. W. J. et al. Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia 65, 1119–1125 (2010).
Parati, G. et al. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension (Dallas, Tex. 1979) 12, 214–222 (1988).
Davies, L. C. et al. Effect of altering conditions of the sequence method on baroreflex sensitivity. J. Hypertens. 19, 1279–1287 (2001).
Edelman, N. H., Epstein, P. E., Lahiri, S. & Cherniack, N. S. Ventilatory responses to transient hypoxia and hypercapnia in man. Respir. Physiol. 17, 302–314 (1973).
Paleczny, B. et al. Hypoxic tachycardia is not a result of increased respiratory activity in healthy subjects. Exp. Physiol. https://doi.org/10.1113/EP087233 (2019).
Pfoh, J. R. et al. Comparing and characterizing transient and steady-state tests of the peripheral chemoreflex in humans. Exp. Physiol. 101, 432–447 (2016).
Trembach, N. & Zabolotskikh, I. Breath-holding test in evaluation of peripheral chemoreflex sensitivity in healthy subjects. Respir. Physiol. Neurobiol. 235, 79–82 (2017).
Trembach, N. & Zabolotskikh, I. Evaluation of breath-holding test in assessment of peripheral chemoreflex sensitivity in patients with chronic heart failure. Open Respir. Med. J. 11, 67–74 (2017).
Trembach, N. & Zabolotskikh, I. The influence of age on interaction between breath-holding test and single-breath carbon dioxide test. Biomed. Res. Int. 2017, 1010289 (2017).
Parati, G., Saul, J. P., Di Rienzo, M. & Mancia, G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension (Dallas, Tex., 1979) 25, 1276–1286 (1995).
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–65 (1996).
Giannoni, A. et al. Clinical significance of chemosensitivity in chronic heart failure: influence on neurohormonal derangement Cheyne-Stokes respiration and arrhythmias. Clin. Sci. 114, 489–497 (2008).
Ponikowski, P. et al. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation 104, 544–549 (2001).
Niewinski, P. et al. Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. J. Card. Fail. 19, 408–415 (2013).
Silva, B. M., Fernandes, I. A. & Vianna, L. C. Welcome the carotid chemoreflex to the ‘neural control of the circulation during exercise’ club. J. Physiol. 590, 2835–2836 (2012).
Charkoudian, N. et al. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J. Physiol. 572, 821–827 (2006).
Keir, D. A., Duffin, J., Millar, P. J. & Floras, J. S. Simultaneous assessment of central and peripheral chemoreflex regulation of muscle sympathetic nerve activity and ventilation in healthy young men. J. Physiol. 597, 3281–3296 (2019).
Paton, J. F. R. et al. Revelations about carotid body function through its pathological role in resistant hypertension. Curr. Hypertens. Rep. 15, 273–280 (2013).
Byrne-Quinn, E., Weil, J. V., Sodal, I. E., Filley, G. F. & Grover, R. F. Ventilatory control in the athlete. J. Appl. Physiol. 30, 91–98 (1971).
Scoggin, C. H., Doekel, R. D., Kryger, M. H., Zwillich, C. W. & Weil, J. V. Familial aspects of decreased hypoxic drive in endurance athletes. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 44, 464–468 (1978).
Ohyabu, Y. et al. Ventilatory and heart rate chemosensitivity in track-and-field athletes. Eur. J. Appl. Physiol. Occup. Physiol. 59, 460–464 (1990).
Schoene, R. B., Robertson, H. T., Pierson, D. J. & Peterson, A. P. Respiratory drives and exercise in menstrual cycles of athletic and nonathletic women. J. Appl. Physiol. 50, 1300–1305 (1981).
Godfrey, S., Edwards, R. H. T., Copland, G. M. & Gross, P. L. Chemosensitivity in normal subjects, athletes, and patients with chronic airways obstruction. J. Appl. Physiol. 30, 193–199 (1971).
Mahler, D. A., Moritz, E. D. & Loke, J. Ventilatory responses at rest and during exercise in marathon runners. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 52, 388–392 (1982).
Tubek, S. et al. Effects of selective carotid body stimulation with adenosine in conscious humans. J. Physiol. 594, 6225–6240 (2016).
Paleczny, B. et al. Age-related reflex responses from peripheral and central chemoreceptors in healthy men. Clin. Auton. Res. 24, 285–296 (2014).
Chua, T. P. & Coats, A. J. The reproducibility and comparability of tests of the peripheral chemoreflex: comparing the transient hypoxic ventilatory drive test and the single-breath carbon dioxide response test in healthy subjects. Eur. J. Clin. Invest. 25, 887–892 (1995).
Forster, H. V., Haouzi, P. & Dempsey, J. A. Control of breathing during exercise. Compr. Physiol. 2, 743–777 (2012).
Smith, C. A., Forster, H. V., Blain, G. M. & Dempsey, J. A. An interdependent model of central/peripheral chemoreception: Evidence and implications for ventilatory control. Respir. Physiol. Neurobiol. 173, 288–297 (2010).
Bisgard, G. E., Forster, H. V., Mesina, J. & Sarazin, R. G. Role of the carotid body in hyperpnea of moderate exercise in goats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 52, 1216–1222 (1982).
Bouverot, P., Collin, R., Favier, R., Flandrois, R. & Sébert, P. Carotid chemoreceptor function in ventilatory and circulatory O2 convection of exercising dogs at low and high altitude. Respir. Physiol. 43, 147–167 (1981).
Pan, L. G. et al. Important role of carotid afferents in control of breathing. J. Appl. Physiol. 85, 1299–1306 (1998).
Jeyaranjan, R., Goode, R., Beamish, S. & Duffin, J. The contribution of peripheral chemoreceptors to ventilation during heavy exercise. Respir. Physiol. 68, 203–213 (1987).
Masuda, A. et al. Estimation of peripheral chemoreceptor contribution to exercise hyperpnea in man. Jpn. J. Physiol. 38, 607–618 (1988).
St. Croix, C. M., Cunningham, D. A., Paterson, D. H. & Kowalchuk, J. M. Peripheral Chemoreflex drive in moderate-intensity exercise. Can. J. Appl. Physiol. 21, 285–299 (1996).
Wasserman, K., Whipp, B. J., Koyal, S. N. & Cleary, M. G. Effect of carotid body resection on ventilatory and acid base control during exercise. J. Appl. Physiol. 39, 354–358 (1975).
Parkes, M. J. Evaluating the importance of the carotid chemoreceptors in controlling breathing during exercise in man. Biomed Res. Int. 2013, 893506 (2013).
Seals, D. R., Jablonski, K. L. & Donato, A. J. Aging and vascular endothelial function in humans. Clin. Sci. 120, 357–375 (2011).
Proctor, D. N. & Parker, B. A. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13, 315–327 (2006).
Dinenno, F. A. & Joyner, M. J. α-Adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13, 329–341 (2006).
DeSouza, C. A. et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102, 1351–1357 (2000).
Rowe, J. W. & Troen, B. R. Sympathetic nervous system and aging in man. Endocr. Rev. 1, 167–179 (1980).
Christensen, N. J. Catecholamines and sympathetic nervous activity in the elderly. Acta Med. Scand. 214, 52–63 (2009).
Mazzeo, R. S., Rajkumar, C., Jennings, G. & Esler, M. Norepinephrine spillover at rest and during submaximal exercise in young and old subjects. J. Appl. Physiol. 82, 1869–1874 (1997).
Ng, A. V., Callister, R., Johnson, D. G. & Seals, D. R. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21, 498–503 (1993).
Iwase, S., Mano, T., Watanabe, T., Saito, M. & Kobayashi, F. Age-related changes of sympathetic outflow to muscles in humans. J. Gerontol. 46, M1–M5 (1991).
Matsukawa, T., Sugiyama, Y., Watanabe, T., Kobayashi, F. & Mano, T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 275, R1600 (1998).
Dinenno, F. A., Dietz, N. M. & Joyner, M. J. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation 106, 1349–1354 (2002).
Hart, E. C., Wallin, B. G., Barnes, J. N., Joyner, M. J. & Charkoudian, N. Sympathetic nerve activity and peripheral vasodilator capacity in young and older men. Am. J. Physiol. Circ. Physiol. 306, H904–H909 (2014).
Studinger, P., Goldstein, R. & Taylor, J. A. Age- and fitness-related alterations in vascular sympathetic control. J. Physiol. 587, 2049–2057 (2009).
Greaney, J. L., Stanhewicz, A. E., Kenney, W. L. & Alexander, L. M. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J. Appl. Physiol. 117, 648–657 (2014).
Ng, A. V., Callister, R., Johnson, D. G. & Seals, D. R. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am. J. Physiol. Hear. Circ. Physiol. 267, H344 (1994).
Brandfonbrener, M., Landowne, M. & Shock, N. W. Changes in cardiac output with age. Circulation 12, 557–566 (1955).
Ng, A. V., Callister, R., Johnson, D. G. & Seals, D. R. Endurance exercise training is associated with elevated basal sympathetic nerve activity in healthy older humans. J. Appl. Physiol. 77, 1366–1374 (1994).
Sinoway, L. et al. Forearm training attenuates sympathetic responses to prolonged rhythmic forearm exercise. J. Appl. Physiol. 81, 1778–1784 (1996).
Ray, C. A. Sympathetic adaptations to one-legged training. J. Appl. Physiol. 86, 1583–1587 (1999).
Gratze, G., Mayer, H. & Skrabal, F. Sympathetic reserve, serum potassium, and orthostatic intolerance after endurance exercise and implications for neurocardiogenic syncope. Eur. Heart J. 29, 1531–1541 (2008).
Byrne, E. A., Fleg, J. L., Vaitkevicius, P. V., Wright, J. & Porges, S. W. Role of aerobic capacity and body mass index in the age-associated decline in heart rate variability. J. Appl. Physiol. 81, 743–750 (1996).
Stein, P. K., Barzilay, J. I., Chaves, P. H. M., Domitrovich, P. P. & Gottdiener, J. S. Heart rate variability and its changes over 5 years in older adults. Age Ageing 38, 212–218 (2009).
Levy, W. C. et al. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am. J. Cardiol. 82, 1236–1241 (1998).
Albinet, C. T., Boucard, G., Bouquet, C. A. & Audiffren, M. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur. J. Appl. Physiol. 109, 617–624 (2010).
Yataco, A. R., Fleisher, L. A. & Katzel, L. I. Heart rate variability and cardiovascular fitness in senior athletes. Am. J. Cardiol. 80, 1389–1391 (1997).
Da Silva, D. F., Verri, S. M., Nakamura, F. Y. & Machado, F. A. Longitudinal changes in cardiac autonomic function and aerobic fitness indices in endurance runners: a case study with a high-level team. Eur. J. Sport Sci. 14, 443–451 (2014).
Almeida-Santos, M. A. et al. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch. Gerontol. Geriatr. 63, 1–8 (2016).
Tan, J. P. H., Beilharz, J. E., Vollmer-Conna, U. & Cvejic, E. Heart rate variability as a marker of healthy ageing. Int. J. Cardiol. 275, 101–103 (2019).
Murrell, C. et al. Alterations in autonomic function and cerebral hemodynamics to orthostatic challenge following a mountain marathon. J. Appl. Physiol. 103, 88–96 (2007).
Chin, M. S. & Kales, S. N. Is there an optimal autonomic state for enhanced flow and executive task performance?. Front. Psychol. 10, 1716 (2019).
Ziemssen, T. & Siepmann, T. The investigation of the cardiovascular and sudomotor autonomic nervous system—a review. Front. Neurol. 10, 53 (2019).
Acknowledgements
This research was financially supported by the Ministry of Science and Higher Education (Poland) / Wroclaw Medical University.
Author information
Authors and Affiliations
Contributions
B.P., R.S. conceived and designed the experiments; B.P., R.S., M.W., A.N.C., W.Ł., D.A., S.W. performed the experiments; B.P., R.S., M.W., A.N.C., W.Ł., D.A., S.W. analysed the data; B.P., S.W., D.M., P.C., M.K., K.M., K.C., D.P., M.A., J.C., P.P., B.P. interpreted the results; B.P. drafted and edited paper; all authors critically revised paper and approved the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paleczny, B., Seredyński, R., Wyciszkiewicz, M. et al. Low ventilatory responsiveness to transient hypoxia or breath-holding predicts fast marathon performance in healthy middle-aged and older men. Sci Rep 11, 10255 (2021). https://doi.org/10.1038/s41598-021-89766-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89766-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.