Abstract
Selenoproteins are a group of selenocysteine-containing proteins with major roles in cellular antioxidant defense and thyroid hormone metabolism. Selenoprotein expression is determined by hierarchical mechanisms that result in tissue-specific levels. Current data inadequately explain the abundance of various selenoproteins under normal and pathological conditions, including in pancreatic β-cells. Selenocysteine insertion sequence binding protein 2 (SBP2) is a critical protein in selenoprotein translation that also plays an essential role in stabilizing selenoprotein transcripts by antagonizing nonsense-mediated decay (NMD). Importantly, dysfunctional SBP2 is associated with endocrine disorders in humans. Here we describe the impact of induced Sbp2 deficiency in pancreatic β-cells on selenoprotein transcript profiles in the pancreatic islets of C57BL/6J mice. Sex differences were noted in control mice, in which female islets showed 5 selenoproteins decreased and one increased versus male islets. Induced Sbp2 deficiency in pancreatic β-cells altered expression of only 3 selenoprotein transcripts in male islets, whereas 14 transcripts were reduced in female islets. In all cases, decreased transcription was observed in genes known to be regulated by NMD. The differential impact of Sbp2 deletion on selenoprotein transcription between sexes suggests sex-specific hierarchical mechanisms of selenoprotein expression that may influence islet biology and consequentially metabolic disease risk.
Similar content being viewed by others
Introduction
The pancreatic islets of Langerhans proteome plays a central role in the homeostatic regulation of glucose metabolism. A subset of this proteome consists of selenoproteins, a family of proteins in which selenium is incorporated into the protein structure as the unique amino acid selenocysteine (Sec). These selenoproteins comprise a group of functionally diverse molecules found in all major life forms. There are 24 and 25 known genes encoding selenoproteins in mouse and humans, respectively1. These proteins perform varied functions related to thyroid hormone metabolism, redox biology, and immune response among others. Despite the importance of these proteins in multiple aspects of cellular biology, the role of selenoproteins has not been fully elucidated in islet or β-cell biology. This is relevant as cellular antioxidant functions of selenoproteins are a critical component of antioxidant defense2,3, and oxidative stress is thought to play a key role in the β-cell dysfunction that contributes to the pathogenesis of diabetes4.
During selenoprotein translation, an in-frame UGA stop codon in the selenoprotein mRNA is recoded as the selenium-containing amino acid Sec, which is incorporated into the protein structure by a complex multicomponent machinery. Key to this process are the Sec-insertion sequence (SECIS) element in the 3′-untranslated region (UTR), a Sec-specific elongation factor (EFsec), and the SECIS-binding protein-2 (SBP2)2,5,6,7. Binding of SBP2 to SECIS elements on selenoprotein mRNA and the coordinated events that follow are essential for protecting selenoprotein mRNA from premature degradation mediated by nonsense-mediated decay (NMD), a native post-transcriptional process that orchestrates the degradation of premature stop codon-containing mRNA transcripts8,9. Thus, in addition to its role in selenoprotein translation, SBP2 is essential for the upstream stabilization of selenoprotein mRNA.
Because of their essential roles in regulating cellular oxidative homeostasis, it is likely that disruptions in selenoprotein synthesis will lead to cellular dysfunction and consequential adverse health effects. Those precise effects, however, are likely tissue-specific and dependent upon the precise functions of the complement of selenoproteins expressed in that specific tissue. For example, deficiencies of the micronutrient selenium, a limiting factor for selenoprotein synthesis, is implicated in the development of human cardiomyopathy and other adverse health effects7,10,11; moreover, it has also been suggested to impact insulin secretion and islet function in rodents while contributing to insulin resistance in humans12,13. Further evidence that disruptions in selenoprotein synthesis cause human disease comes from evidence of humans with dysfunctional SBP2, which is associated with thyroid dysfunction14 and neurocognitive deficits15 as well as azoospermia, muscular dystrophy, photosensitivity, and immune dysfunction16.
Considerable variation in selenium demand and selenoprotein expression has been reported across different tissues. Furthermore, plasma selenium status affects tissue levels of selenoproteins differentially; while some selenoproteins are affected by selenium deprivation, others are not3,17,18. Additionally, the complex nature and diversity of selenoprotein function complicates assignment of adverse health effects to specific selenoproteins17. Thus, understanding the precise complement of selenoproteins in a specific tissue and the regulation of their expression levels is critical for understanding how these essential proteins regulate function at the level of specific tissues. A critical role of SBP2 in selenoprotein biosynthesis is determined by the differential binding affinity to various SECIS elements of selenoprotein mRNAs6, which influences the variable tissue expression of selenoproteins. Consequently, β-cell-specific deletion of Sbp2 may illuminate vital information about the selenoprotein expression hierarchy that may be essential for pancreatic β-cell function and glucose homeostasis. Previously, mouse models conditionally deficient in Sbp2 gene expression have been proposed as models to study the tissue-specific function of individual selenoproteins19, here we present the selenoprotein gene expression profiles in pancreatic islets from mice we developed that have an inducible β-cell-specific Sbp2 gene deletion and discuss the sex-specific differential gene expression of selenoproteins in murine islets.
Methods
Development of conditional knockout mice and induction of β-cell-specific Sbp2 deficiency
C57BL/6J mice with β-cell-specific, tamoxifen-inducible deletion of the Sbp2 gene (Sbp2 βCKO mice) were generated by cross-breeding mice homozygous for the floxed Sbp2 allele (Sbp2 flox/flox (Sbp2 fl/fl)) with Sbp2 fl/fl mice that are also hemizygous for the Mip-Cre-ERT transgene, expressing Cre recombinase driven by the mouse Ins-1 promoter (Mip-Cre-ERT/+; Sbp2 fl/fl). Sbp2 fl/fl mice were generated by cross-breeding heterozygous Sbp2 floxed mice (Sbp2+/fl) previously reported by our team20. Mip-Cre-ERT mice were procured from Jackson Laboratories (Bar Harbor, ME). Male and female littermate mice at 15 weeks of age were used in the study. Sbp2 deficiency was induced by intraperitoneal administration of 3 mg tamoxifen dissolved in corn oil for 5 consecutive days, after which mice were allowed to recover for 3.5 weeks prior to analysis of Sbp2 gene and protein deletion as well as selenoprotein gene expression in isolated pancreatic islets of Langerhans. Mice were group housed under 14-h/10-h light/dark cycles at 22.2 °C ± 1.1 °C with ad libitum access to a selenium-replete chow diet (Teklad 7912) and water. The recommended selenium requirement for adult laboratory mice is 0.15 mg/kg of diet21; Teklad 7912 diet contains 0.16 mg/kg of selenium. All animal protocols were approved by the Institutional Care and Use Committee at the University of Illinois at Chicago (Protocols ACC 16-211 and ACC 19-198), and all experiments were conducted in accordance with the National Institutes of Health Guide for the Care of Laboratory Animals.
Isolation of pancreatic islets
Mouse pancreatic islets were isolated using methods previously described22,23. Briefly, collagenase P (Roche) at a final concentration of 0.375 mg/ml in HBSS buffer containing HEPES (10 mM) at pH 7.2 was perfused through the pancreas by cannulation of the common bile duct starting at the gall bladder while the ampulla remained clamped preventing perfusate flow into the duodenum; pancreata were digested at 37 °C for 14 min. The digested pancreas was then gently dispersed in cold wash buffer (HBSS-HEPES pH 7.2 containing 0.01% BSA) and washed thrice before handpicking the dispersed islets under stereomicroscopy.
RNA isolation and qPCR
Total islet RNA was isolated using the EZNA Total RNA Kit2 (Omega Biotech). Islets from each mouse were homogenized by passage through a 23-gauge needle, and total RNA was isolated according to the manufacturer’s instructions. RNA was further purified by digesting genomic DNA using DNAse 1 (Invitrogen). A total of 0.4 μg RNA was reverse transcribed using qScript cDNA Super Mix (Quanta Bio). The abundance of mRNA was measured by real-time quantitative PCR (qPCR) using the Bio-Rad CFX Connect real-time system and SYBR green detector dye. Primer sequences for genes analyzed in the study are listed in Table 1. All primers were validated and found to have optimal efficiency (E = 90–110%, R2 = > 0.98, slope = − 3.58 to − 3.1). Gene expression was normalized to the expression of the house-keeping gene Hprt.
SDS-PAGE and immunoblotting
Tissue proteins were extracted in RIPA buffer supplemented with complete Mini-Protease Cocktail (ROCHE). Primary antibodies against Sbp2, Gpx3, Dio1, and Txnrd2 (all from Proteintech) as well as Selenop (Santa Cruz) were used to quantify select selenoprotein expression by immunoblotting after separation by SDS-PAGE; β-actin (Cell Signaling) was used as the internal protein loading control. Clarity ECL (Bio-Rad) was used for chemiluminescent detection of protein bands with band intensity quantified using Image J software.
Statistics
All data are presented as means ± standard deviations (SD). Statistical significance was tested using two-tailed, unpaired Student’s t tests to compare control and Sbp2 knockout groups. All statistical analyses and image construction were performed using GraphPad Prism and Adobe Illustrator; p < 0.05 was considered statistically significant.
Results
Mice with tamoxifen-induced conditional Sbp2 deficiency in pancreatic β-cells
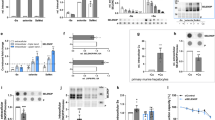
Constitutive Sbp2 gene deletion is embryonically lethal in mice; therefore, we generated transgenic mice (Sbp2 βCKO) with tamoxifen-inducible Sbp2 gene deletion specifically targeting pancreatic β-cells. This approach avoids the potential effect of congenital absence of Sbp2 on β-cell development. Sbp2 βCKO mice (Mip-Cre-ERT/+; Sbp2 fl/fl) harbor the mouse insulin promoter (Mip)-driven Cre-ER transgene that expresses the fusion protein Cre recombinase–estrogen receptor (ER). Binding of tamoxifen to the ER translocates the Cre recombinase into the nucleus24 where the homologous recombination of the LoxP sites flanking exon 14 of the Sbp2 gene results in its deletion (Fig. 1a). Exon 14 was targeted for deletion as it encodes part of the C terminus functional domains that are highly conserved across species and required for SECIS binding, ribosome binding, and Sec incorporation. It is present in all known isoforms encoded by this locus, and when deleted, the resultant transcript between exons 13 and 15 is out of frame, ensuring a nonfunctional transcript and a null allele. Sbp2-floxed mice were originally developed by our team20 and Sbp2 βCKO were then generated by crossing Mip Cre-ERT mice with Sbp2 floxed mice as described. Tamoxifen was administered at 15 weeks of age to both Sbp2 βCKO and littermate Sbp2 fl/fl control mice lacking Cre. Profiling of mRNA expression of the mouse islets showed 75% and 79% Sbp2 gene deletion in female and male Sbp2 βCKO mice, respectively (Fig. 1b,d). SBP2 protein was similarly decreased in both male and female Sbp2 βCKO mice (Fig. 1c). To ascertain whether β-cell-specific Cre expression had nonspecific effects in other tissues, we assessed Sbp2 gene expression in liver and kidney and found that there was no detectable deletion of Sbp2 in these tissues (Fig. 1b,d). Importantly, β-cells constitute approximately 60–80% of the cellular composition of murine islets, with an additional 15–20% being α-cells and the rest of the islet composed of δ- and PP-cells25. With targeted deletion of Sbp2 in β-cells, the extent of Sbp2 deletion reflects the β-cell composition of the pancreatic islets at both the mRNA and protein level in both male and female mice (Fig. 1). Of note, recombination was largely uniform in all the mice with none yielding less than 65% Sbp2 gene deletion (data not shown). Importantly, tamoxifen administration and gene deletion did not induce overt adverse health effects in the mice. Taken together our results show that our model successfully induces Sbp2 deficiency in pancreatic β-cells.
Sbp2 gene targeting and characterization in Sbp2 βCKO mice. (a) Graphical representation of genetic combinations used to breed Sbp2 βCKO mice. The mouse Sbp2 gene contains 17 exons. Sbp2 βCKO mice are hemizygous for the Mip-Cre-ERT transgene and homozygous for the floxed Sbp2 gene (exon 14 flanked by LoxP sites). Specific expression of Cre-recombinase in β-cells is induced upon tamoxifen binding, which triggers LoxP recombination and excision of exon 14. The Frt site that remained after Flp-induced excision of the NeoR cassette during the making of Sbp2 floxed mice is also excised along with exon 14 upon Cre-Lox recombination in the Sbp2 βCKO mice as shown. Sbp2 qPCR primer-binding locations within exon 14 are also shown. Male (b) and female (d) Sbp2 βCKO mouse islets, liver, and kidney were analyzed for Sbp2 mRNA expression by qPCR and compared to control Sbp2 fl/fl mice lacking the Mip-Cre-ERT transgene (Sbp2 fl/fl) but also treated with tamoxifen. (c) Representative immunoblots showing Sbp2 protein expression for islets, liver, and kidney in Sbp2 βCKO and Sbp2 fl/fl controls in both male (top panels) and female (bottom panels) mice; lanes 1 and 2 are from control mice (Sbp2 fl/fl), and lanes 3 and 4 are from Sbp2 βCKO mice; β-actin was used as the protein loading control. Corresponding quantitation of protein levels was normalized to β-actin expression in arbitrary units and is shown at the right. Between group differences were analyzed by unpaired, two-tailed Student’s t test. N = 6 mice per group; p < 0.05 was considered statistically significant with individual p values as shown.
Transcriptional analysis of selenoproteome in male and female murine islets
To characterize the islet selenoprotein profile, we performed transcriptional analyses of all 24 mouse selenoprotein genes in the pancreatic islets of Sbp2 βCKO mice and their control littermates (Sbp2 fl/fl). Sexual dimorphism in selenoprotein expression has been reported in murine tissues and plasma that may explain several sex-specific metabolic functions in mouse29,30. However, data is lacking regarding such sex specific selenoprotein expression in the pancreatic islets. We found that mRNA expression of Selenom, Selenow, Gpx1, Gpx4, and Selenon (all NMD-susceptible) were decreased while Selenoi, a non-NMD-susceptible selenoprotein was significantly increased in the female control mouse (Sbp2 fl/fl) islets compared to their male counterparts (Fig. 2).
Selenoprotein gene expression analysis in Sbp2 fl/fl control male and female mouse islets. Quantitative real time-PCR analysis (qPCR) of selenoprotein mRNA abundance in male and female control mice (Sbp2 fl/fl) is shown. Individual selenoprotein mRNA expression is displayed compared relative to the expression of Gpx3, which was the most abundantly expressed selenoprotein mRNA in both male and female islets. Expression was normalized to the housekeeping gene Hprt. Genes susceptible to NMD are marked with the following symbol: ‡. These are selenoprotein pre-mRNAs with a UGA stop codon at least 50–55 nucleotides upstream of an intron. Between group differences were analyzed by unpaired, two-tailed Student’s t test. N = 6 mice per group; p < 0.05 was considered statistically significant with individual p values as shown.
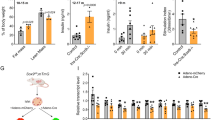
Importantly, SBP2 induces recoding of a UGA stop codon in selenoprotein pre-mRNA5, and SBP2 is essential for prevention of nonsense-mediated decay (NMD) of these transcripts8,9. NMD in mammals is activated when a pre-mRNA contains a stop codon (UGA) at least 50–55 nucleotides upstream of an intron8,9; 14 of the 24 mouse selenoprotein pre-mRNAs are known to be susceptible to NMD9. In male Sbp2 βCKO mice with greater than 70% deletion of Sbp2 (Fig. 3a), the expectation was to see similar reductions in mRNA levels of the full complement of NMD-susceptible selenoproteins. Surprisingly, islet-selenoprotein mRNA expression was largely unchanged by Sbp2 deletion with only marginal reductions in the expression of Selenow, Selenon, and Dio1, all of which are susceptible to NMD (Fig. 3a). In contrast to male islets, however, 14 of the 24 selenoproteins were decreased in islets from female Sbp2 βCKO mice; all of which are NMD-susceptible (Fig. 3b). Gpx3 and Selenop were the most highly expressed selenoproteins in both male and female islets, while Txnrd2 and Txnrd3 were the least expressed selenoproteins. Dio2, Dio3, and Selenov expression were not detected in islets from either male or female mice.
Selenoprotein gene expression analysis in Sbp2-deficient male and female mouse islets. Quantitative real time-PCR analysis (qPCR) of selenoprotein mRNA in male (a) and female (b) Sbp2 βCKO islets compared with that of control mouse islets (Sbp2 fl/fl). Individual selenoprotein mRNA expression is displayed compared relative to the expression of Gpx3. Expression was normalized to the housekeeping gene Hprt. Genes susceptible to NMD are marked with the following symbol: ‡. Representative immunoblots showing Gpx3, Selenop, Dio1, and Txnrd2 protein expression in male (c) or female (d) islets of Sbp2 βCKO and control (Sbp2 fl/fl) mice are shown; lanes 1 and 2 are representative islets from control mice (Sbp2 fl/fl), and lanes 3 and 4 are representative islets from Sbp2 βCKO islets; β-actin was used as the protein loading control. Corresponding quantification expressed in arbitrary units is shown at the right normalized to β-actin expression. (See also Supplementary Information 1.) Gene expression of the Selenop receptors Lrp1 and Lrp8 in male and female Sbp2 βCKO islets compared with that of control mouse islets (Sbp2 fl/fl) is shown in (e). Between group differences were analyzed by unpaired, two-tailed Student’s t test. N = 6 mice per group; p < 0.05 was considered statistically significant with individual p values as shown.
Protein expression of major selenoproteins (Fig. 3c,d), however, did not strictly reflect mRNA abundance. There were also notable sex differences. In islets from male mice, Sbp2 deletion did not affect Gpx3 mRNA or protein expression, however, in islets from female mice, Sbp2 deletion reduced Gpx3 at both the gene and protein levels. This suggests possible post-translational mechanisms influencing protein expression that are sex-specific, as well as unknown sex-specific NMD control mechanisms that may be operative. Potential mechanisms for these sex specific differences in selenoprotein gene expression levels upon Sbp2 deletion are not clearly understood.
Lrp1, Lrp2 and Lrp8 are known receptors for Selenop31,32, the major selenium transporter protein. Expression of Lrp8 mRNA was previously shown to be strongly upregulated in Selenop-deficient mouse tissues, suggesting one potential compensatory mechanism to ameliorate the selenium deficiency in those tissues31. Similar to these studies, Lrp8 was highly expressed in both male and female Sbp2 βCKO mice islets compared to Sbp2 fl/fl controls (Fig. 3e). In contrast, no changes in Lrp1 mRNA levels in male Sbp2 βCKO mice islets were observed; however, female Sbp2 βCKO mice islets showed reduced Lrp1 expression relative to Sbp2 fl/fl controls (Fig. 3e). Lrp2 mRNA was not expressed in mouse islets. Taken together our data indicate that Sbp2 deletion in pancreatic β-cells does not broadly impact selenoprotein mRNA levels in male islets, while many female selenoprotein transcripts are significantly decreased, indicating sexually dimorphic effects of Sbp2 deletion in β-cells.
Discussion
Selenium and several selenoproteins are known to play diverse roles in the health and function of pancreatic islets12,13,33,34. Understanding the functions of the selenoprotein complement on pancreatic β-cell and islet function is essential for appreciating the importance of this family of proteins for metabolic health as well as their potential role in the pathogenesis of diabetes. Selenoproteins are expressed hierarchically in a tissue-specific pattern that is often based upon the availability of selenium and other physiological factors26. Moreover, some tissues preferentially retain selenium relatively independent of systemic selenium availability26,27. For example, selenium levels in endocrine tissues such as the rat pituitary and thyroid remain relatively unchanged in the face of dietary selenium deficiency in order to preserve essential selenoproteins28. The unique tissue-specific expression of selenoproteins suggests that protein and mRNA expression profiles across the selenoproteome may shed light on tissue-specific selenium needs and the precise selenoprotein expression hierarchy that regulates health and mediates disease risk. Importantly, however, these data are generally lacking.
Pancreatic islets of Langerhans secrete several hormones essential for metabolic homeostasis, and disruptions in this critical endocrine organ promote the development of metabolic disease. SBP2 expression is critical for mammalian selenoprotein expression; however, little is known about the selenoprotein expression hierarchy in β-cells and the role of SBP2 in this critical cell population. We found a 70–80% decrease in Sbp2 gene expression in mouse islets in our model of β-cell-specific Sbp2 deficiency upon tamoxifen induction. Of a potential 24 selenoproteins expressed in murine tissue, only 21 were expressed in isolated pancreatic islets; Dio2, Dio3, and Selenov were not expressed. Selenoprotein mRNA profiles have been described in SBP2-deficient mouse liver19. Seeher et al. reported results from a mouse with liver-specific Sbp2 deletion in which Sbp2 was deleted embryonically by albumin-driven Cre expression. While selenoproteins such as Dio1, Gpx1, Selenop, Selenow, Selenoh, SephS2, and Selenos were significantly downregulated in both male and female Sbp2-deficient mouse livers, others showed sexual dimorphism with respect to selenoprotein expression changes, including Gpx4, Txnrd1, Selenot, Msrb1, Selenon, Selenof, Selenom, Txnrd1, Selenoi, Selenok, and Selenoo19.
In contrast, our studies aimed to induce Sbp2 deficiency during adulthood selectively in pancreatic β-cells by tamoxifen-mediated Cre induction. This approach has the advantage of bypassing effects on β-cell development and potential secondary effects mediated by life-long alterations in β-cell function. While only Dio1, Selenom, and Selenow were down regulated in male Sbp2 βCKO islets, 14 transcripts were downregulated in female islets: Gpx3, Selenop, Selenof, Selenot, Selenom, Selenow, Gpx1, Dio1, Gpx4, Selenon, Sephs2, Selenoh, Txnrd2, and Txnrd3. Additionally, compared to their male littermates, islets from control female mice (Sbp2 fl/fl) exhibited reduced expression of 5 NMD-susceptible selenoproteins, while expression of the non-NMD-susceptible selenoprotein Selenoi was increased. Less clear, however, are the mechanisms responsible for the marked sex-specificity of our findings. It is known that NMD mechanisms vary in potency in a tissue-specific manner35; however, whether sex hormones and/or sex steroid receptors play a role in this process is not understood.
Glutathione peroxidases include Gpx1, Gpx2, Gpx3, Gpx4, and Gpx6; these are the best characterized selenoproteins with known roles in islet biology5,36. Global overexpression of Gpx is known to protect pancreatic β-cells from oxidative stress and improve insulin sensitivity36,37. In our studies, Gpx3 was the most abundant selenoprotein mRNA expressed in islets, while Gpx2 and Gpx4 were comparatively very lowly expressed. Gpx3 mRNA and protein expression remained unchanged in male Sbp2 βCKO mice; however, both mRNA and the protein levels were decreased in female Sbp2 βCKO mice. Several mechanisms may explain the preservation of Gpx3 in islets from male mice. In thyrocytes, GPX3 is known to selectively accumulate extracellularly in the colloid of the thyroid gland, where it is effectively isolated and shielded from circulation; this protects the protein from degradation and renders it resistant to changes in selenium status26. Such hierarchical mechanisms may operate in male islets as well. For example, in rats fed a selenium-deficient diets for several generations, GPx activities were found decreased to undetectable levels in several tissues, and male rats were found infertile compared to females; however, levels of selenium in the testis, brain, and endocrine tissue remained unchanged26,27,28.
Selenop (Sepp or Selenoprotein P) may play a role in regulating pancreatic β-cell function while also modulating insulin sensitivity in type 2 diabetes38. Selenop serves primarily as a selenium transport protein responsible for roughly 50% of plasma selenium39. Selenop is unique in that it carries more than one selenocysteine (Sec) residue in its structure; the number of which varies across species. For example, human SELENOP has 10 residues, whereas the zebrafish protein has 175. Selenop mRNA was down-regulated significantly in female Sbp2 βCKO murine islets; however, expression in male mice was unchanged. Interestingly, Selenop protein levels did not differ in the Sbp2 βCKO mice in either male or female mice, indicating a role for other post-translational mechanisms of regulation. Curiously, despite preservation of Selenop protein levels, gene expression of the Selenop receptor Lrp8 was robustly increased in both sexes, while Lrp1 expression was reduced selectively in female Sbp2 βCKO mice. The implications of these findings with regard to intracellular selenium sensing and transport require further investigation.
Thyroid hormones play significant roles in β-cell development and function40, and tissue deiodinases regulate the activity of thyroid hormone signaling by locally modulating the levels of the active thyroid hormone T3 versus inactive metabolites. While Dio1 and Dio2 (deiodinase 1 and 2, respectively) primarily convert T4 to T3, Dio3 inactivates both T4 and T340. Only Dio1 was expressed in murine islets in our model. In contrast, Medina et al. previously reported high expression of Dio3 in male murine β-cells with a mixed genetic background (129/Sv/C57BL/6)41. Interestingly, despite Dio1 mRNA being downregulated in both male and female Sbp2 βCKO mice, protein expression was found to be unchanged.
The thioredoxin reductases are another group of proteins important in islet biology42. Thiroredoxin reductases are integral components of the thioredoxin system in which thioredoxins catalyze the reduction of disulfide bonds in target proteins, with oxidized thioredoxin in turn being reversibly reduced by thioredoxin reductases42. Thioredoxins play significant roles in antioxidant defense as they contribute to the disulfide reductions in peroxiredoxins that are involved in the reduction of hydrogen peroxide and peroxides43. Thioredoxin reductases 2 and 3 (Txnrd2 and Txnrd3, respectively) were the least expressed selenoprotein mRNAs in both male and female murine islets. Both Txnrd2 and Txnrd3 mRNA were downregulated in female Sbp2 βCKO islets but not in male islets. Protein levels of Txnrd2 were not influenced by Sbp2 deletion.
While the present studies provide new insights into the selenoprotein complement of a critical metabolic tissue, these studies have several limitations. β-cell-specific deletion of Sbp2 in murine islets affects only 60–80% of islets cells25. Because gene transcription was assessed in islets, the apparent impact of Sbp2 gene deletion may have been diluted by the presence of non-β-cells in the tissue, which we might expect to have their own unique selenoprotein transcript expression profiles, distributions, and NMD susceptibilities. Further study of the role of SBP2 using purified β-cells or β-cell lines may clarify its role in selenoprotein transcription and translation. In addition, studies in α-cells may clarify potential compensatory changes in selenoprotein expression in islet tissue. Stability of protein expression despite Sbp2 deletion and reduced mRNA levels points to a role for additional mechanisms that may operate post-translationally, and these require clarification. These mechanisms may contribute to the selenoprotein expression hierarchy in islets under normal conditions. Additional research is required to show how selenoprotein mRNA expression or protein levels change in pathological states such as diabetes and metabolic dysfunction. Further work is also required to interrogate the mechanisms responsible for the observed sex-specific effects on mRNA expression. Importantly, our studies employed Mip-Cre-ERT mice in which pancreatic β-cell-specific Cre expression is driven by the mouse Ins1 promoter; other β-cell-targeting Cre lines, such as those driven by the rat insulin promoter, are available and have been used to interrogate the impact of disrupting various aspects of selenoprotein biology on metabolic function44,45. Comparative application of these models and approaches may further illuminate the role of the selenoproteome in regulating metabolic health. We have limited our discussion to just Gpx3, Selenop, Dio1, and Txnrd2 as these are the best studied selenoproteins with regard to pancreatic β-cell function and metabolic physiology. Other selenoproteins may play significant roles as well. For example, while not an NMD-susceptible selenoprotein, SELENOS is a suggested therapeutic target for diabetes46. In our studies Selenos mRNA expression is unchanged by Sbp2 deletion in pancreatic islets from both sexes. As a major regulator of selenoprotein mRNA synthesis and mRNA decay47, our data from pancreatic islets of Langerhans add to existing information with regard to the role of SBP2 in NMD and in establishing the hierarchy of selenoprotein expression. However, further work is required to precisely clarify the role of this important protein in selenoprotein synthesis and its relative contribution to mRNA stability versus protein translation given evidence of preserved Sec insertion into proteins in Sbp2-deficient cells47.
Our report is the first to examine the complete sex-specific selenoprotein transcript profiles in β-cells and the influence of Sbp2 deletion on transcription. Given the essential role of β-cells in maintaining glucose homeostasis, further studies examining islets from models of diabetes or those subjected to other metabolic stressors will clarify the dynamics of selenoprotein expression and their role in maintaining β-cell function and overall metabolic health.
References
Kryukov, G. V. et al. Characterization of mammalian selenoproteomes. Science 300, 1439–1443. https://doi.org/10.1126/science.1083516 (2003).
Bellinger, F. P., Raman, A. V., Reeves, M. A. & Berry, M. J. Regulation and function of selenoproteins in human disease. Biochem. J. 422, 11–22. https://doi.org/10.1042/BJ20090219 (2009).
Driscoll, D. M. & Copeland, P. R. Mechanism and regulation of selenoprotein synthesis. Annu. Rev. Nutr. 23, 17–40. https://doi.org/10.1146/annurev.nutr.23.011702.073318 (2003).
Gerber, P. A. & Rutter, G. A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal 26, 501–518. https://doi.org/10.1089/ars.2016.6755 (2017).
Labunskyy, V. M., Hatfield, D. L. & Gladyshev, V. N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 94, 739–777. https://doi.org/10.1152/physrev.00039.2013 (2014).
Bubenik, J. L. & Driscoll, D. M. Altered RNA binding activity underlies abnormal thyroid hormone metabolism linked to a mutation in selenocysteine insertion sequence-binding protein 2. J. Biol. Chem. 282, 34653–34662. https://doi.org/10.1074/jbc.M707059200 (2007).
Papp, L. V., Lu, J., Holmgren, A. & Khanna, K. K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal 9, 775–806. https://doi.org/10.1089/ars.2007.1528 (2007).
de Jesus, L. A. et al. Nuclear assembly of UGA decoding complexes on selenoprotein mRNAs: A mechanism for eluding nonsense-mediated decay?. Mol. Cell Biol. 26, 1795–1805. https://doi.org/10.1128/MCB.26.5.1795-1805.2006 (2006).
Squires, J. E., Stoytchev, I., Forry, E. P. & Berry, M. J. SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Mol. Cell Biol. 27, 7848–7855. https://doi.org/10.1128/MCB.00793-07 (2007).
Rayman, M. P. The importance of selenium to human health. Lancet 356, 233–241. https://doi.org/10.1016/S0140-6736(00)02490-9 (2000).
Schweizer, U., Dehina, N. & Schomburg, L. Disorders of selenium metabolism and selenoprotein function. Curr. Opin. Pediatr. 23, 429–435. https://doi.org/10.1097/MOP.0b013e32834877da (2011).
Campbell, S. C. et al. Selenium stimulates pancreatic beta-cell gene expression and enhances islet function. FEBS Lett. 582, 2333–2337. https://doi.org/10.1016/j.febslet.2008.05.038 (2008).
Fontenelle, L. C. et al. The role of selenium in insulin resistance. Braz. J. Pharm. Sci. 54, 20 (2018).
Dumitrescu, A. M. et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat. Genet. 37, 1247–1252. https://doi.org/10.1038/ng1654 (2005).
Azevedo, M. F. et al. Selenoprotein-related disease in a young girl caused by nonsense mutations in the SBP2 gene. J. Clin. Endocrinol. Metab. 95, 4066–4071. https://doi.org/10.1210/jc.2009-2611 (2010).
Schoenmakers, E. et al. Mutations in the selenocysteine insertion sequence–binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J. Clin. Investig. 120, 4220–4235. https://doi.org/10.1172/JCI43653 (2010).
Conrad, M. & Schweizer, U. Unveiling the molecular mechanisms behind selenium-related diseases through knockout mouse studies. Antioxid. Redox Signal 12, 851–865. https://doi.org/10.1089/ars.2009.2912 (2010).
Bermano, G. et al. Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem. J. 311(Pt 2), 425–430. https://doi.org/10.1042/bj3110425 (1995).
Seeher, S. et al. Secisbp2 is essential for embryonic development and enhances selenoprotein expression. Antioxid. Redox Signal 21, 835–849. https://doi.org/10.1089/ars.2013.5358 (2014).
Fu, J., Fujisawa, H., Follman, B., Liao, X.-H. & Dumitrescu, A. M. Thyroid hormone metabolism defects in a mouse model of SBP2 deficiency. Endocrinology 158, 4317–4330. https://doi.org/10.1210/en.2017-00618 (2017).
Council, N. R. Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 1995 (The National Academies Press, London, 1995).
Marzorati, S. & Ramirez-Dominguez, M. In Islets of Langerhans (ed. Md Shahidul, I.) 83–107 (Springer, Netherlands, 2015).
Yasuda, T. et al. Rim2alpha determines docking and priming states in insulin granule exocytosis. Cell Metab. 12, 117–129. https://doi.org/10.1016/j.cmet.2010.05.017 (2010).
Jahn, H. M. et al. Refined protocols of tamoxifen injection for inducible DNA recombination in mouse astroglia. Sci. Rep. https://doi.org/10.1038/s41598-018-24085-9 (2018).
Steiner, D. J., Kim, A., Miller, K. & Hara, M. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets 2, 135–145. https://doi.org/10.4161/isl.2.3.11815 (2010).
Schomburg, L. & Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta Gener. Subj. 1790, 1453–1462. https://doi.org/10.1016/j.bbagen.2009.03.015 (2009).
Behne, D. & Höfer, T. Effects of a low selenium status on the distribution and retention of selenium in the rat. J. Nutr. 114, 1289–1296. https://doi.org/10.1093/jn/114.7.1289 (1984).
Behne, D., Hilmert, H., Scheid, S., Gessner, H. & Elger, W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim. Biophys. Acta Gener. Subj. 966, 12–21. https://doi.org/10.1016/0304-4165(88)90123-7 (1988).
Seale, L. A., Ogawa-Wong, A. N. & Berry, M. J. Sexual dimorphism in selenium metabolism and selenoproteins. Free Radic. Biol. Med. 127, 198–205. https://doi.org/10.1016/j.freeradbiomed.2018.03.036 (2018).
Riese, C. et al. Selenium-dependent pre- and posttranscriptional mechanisms are responsible for sexual dimorphic expression of selenoproteins in murine tissues. Endocrinology 147, 5883–5892. https://doi.org/10.1210/en.2006-0689 (2006).
Pietschmann, N. et al. Selenoprotein P is the essential selenium transporter for bones. Metallomics 6, 1043–1049. https://doi.org/10.1039/c4mt00003j (2014).
Misu, H. et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 23, 508–516. https://doi.org/10.1038/nm.4295 (2017).
Zhou, J., Huang, K. & Lei, X. G. Selenium and diabetes—evidence from animal studies. Free Radic. Biol. Med. 65, 1548–1556. https://doi.org/10.1016/j.freeradbiomed.2013.07.012 (2013).
Labunskyy, V. M. et al. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid. Redox Signal. 14, 2327–2336. https://doi.org/10.1089/ars.2010.3526 (2011).
Zetoune, A. B. et al. Comparison of nonsense-mediated mRNA decay efficiency in various murine tissues. BMC Genet. 9, 83. https://doi.org/10.1186/1471-2156-9-83 (2008).
Brigelius-Flohé, R. & Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta Gener. Subj. 1830, 3289–3303. https://doi.org/10.1016/j.bbagen.2012.11.020 (2013).
Robertson, R. P. & Harmon, J. S. Pancreatic islet β-cell and oxidative stress: The importance of glutathione peroxidase. FEBS Lett. 581, 3743–3748. https://doi.org/10.1016/j.febslet.2007.03.087 (2007).
Saito, Y. Selenoprotein P as a significant regulator of pancreatic β cell function. J. Biochem. https://doi.org/10.1093/jb/mvz061 (2019).
Burk, R. F. & Hill, K. E. Selenoprotein P: An extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu. Rev. Nutr. 25, 215–235. https://doi.org/10.1146/annurev.nutr.24.012003.132120 (2005).
Chen, C., Xie, Z., Shen, Y. & Xia, S. F. The roles of thyroid and thyroid hormone in pancreas: Physiology and pathology. Int. J. Endocrinol. 1–14, 2018. https://doi.org/10.1155/2018/2861034 (2018).
Medina, M. C. et al. The thyroid hormone-inactivating type III deiodinase is expressed in mouse and human β-cells and its targeted inactivation impairs insulin secretion. Endocrinology 152, 3717–3727. https://doi.org/10.1210/en.2011-1210 (2011).
Tinkov, A. A. et al. The role of the thioredoxin/thioredoxin reductase system in the metabolic syndrome: Towards a possible prognostic marker?. Cell. Mol. Life Sci. 75, 1567–1586. https://doi.org/10.1007/s00018-018-2745-8 (2018).
Kallis, G. B. & Holmgren, A. Differential reactivity of the functional sulfhydryl groups of cysteine-32 and cysteine-35 present in the reduced form of thioredoxin from Escherichia coli. J. Biol. Chem. 255, 10261–10265 (1980).
Yagishita, Y. et al. Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep. 18, 2030–2044. https://doi.org/10.1016/j.celrep.2017.01.064 (2017).
Prevost, G. et al. The PACAP-regulated gene selenoprotein T is abundantly expressed in mouse and human β-cells and its targeted inactivation impairs glucose tolerance. Endocrinology 154, 3796–3806. https://doi.org/10.1210/en.2013-1167 (2013).
Yu, S.-S. & Du, J.-L. Selenoprotein S: A therapeutic target for diabetes and macroangiopathy?. Cardiovasc. Diabetol. https://doi.org/10.1186/s12933-017-0585-8 (2017).
Fradejas-Villar, N. et al. The RNA-binding protein Secisbp2 differentially modulates UGA codon reassignment and RNA decay. Nucleic Acids Res. 45, 4094–4107. https://doi.org/10.1093/nar/gkw1255 (2017).
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences (R01 ES028879; P30 ES027792; R21 ES030884), National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK110322), and the American Diabetes Association (1-17-JDF-033).
Author information
Authors and Affiliations
Contributions
B.C. conceptualized the study, conducted the experiments, acquired and analyzed the data, generated visual representations of the data, and wrote the manuscript; L.Z. acquired data, provided methodological support, and reviewed the manuscript; M.L. created the model and reviewed the manuscript; C.M.C. provided methodological and analytical support and reviewed the manuscript; A.M.D. created the model and edited the manuscript; and R.M.S. supervised the study, interpreted the data, wrote and edited the manuscript, and acquired funding.
Corresponding author
Ethics declarations
Competing interests
RMS declares he has received honoraria from the American Medical Forum and CVS/Health. These relationships are unrelated to the present work. The rest of the authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chellan, B., Zhao, L., Landeche, M. et al. Selenocysteine insertion sequence binding protein 2 (Sbp2) in the sex-specific regulation of selenoprotein gene expression in mouse pancreatic islets. Sci Rep 10, 18568 (2020). https://doi.org/10.1038/s41598-020-75595-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75595-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.