Abstract
Predicting hearing outcomes in idiopathic sudden sensorineural hearing loss (ISSNHL) is still challenging. We hypothesized that assessment of the semicircular canal (SCC) function via the video head impulse test (vHIT) might provide prognostic information. The medical records of patients diagnosed with ISSNHL from January 2015 to December 2018 were retrospectively reviewed. The prognostic values of the vHIT and other previously known factors in predicting hearing recovery were analyzed using a logistic regression model. A total of 148 patients with normal contra-lesional hearing were analyzed. Fifty-seven patients exhibited low gain (<0.7) on the vHIT in at least one SCC, more than the number of patients complaining of dizziness. Multivariable analysis revealed that non-recovery of normal hearing was associated with older age (OR 1.040), worse canal paresis on the caloric test (OR 1.023), worse initial hearing thresholds (OR 1.045) and abnormal vHIT result in the posterior SCC (OR 3.670). Low vHIT gain in the posterior SCC had specificity of 94.4% and positive predictive value of 85.7% in predicting non-recovery of normal hearing. In conclusion, abnormal vHIT gain in the posterior SCC appears to be a specific prognostic factor for incomplete hearing recovery in ISSNHL.
Similar content being viewed by others
Introduction
Sudden sensorineural hearing loss (SSNHL) is defined as sensorineural hearing loss of 30 dB or more over at least three consecutive frequencies occurring within 72 hours1,2. In the majority of patients there is no specific identifiable cause of the hearing loss, and these cases are classified as idiopathic SSNHL (ISSNHL)1. Various etiologic backgrounds have been proposed, such as viral infection, vascular insufficiency and immunologic reaction3,4. ISSNHL has been treated with empirical therapies such as high dose steroid, antivirals, hyperbaric oxygen, etc1,5. Even if managed promptly, the outcomes of ISSNHL vary from non-recovery of previous hearing to complete recovery.
One of the most challenging aspects of ISSNHL for both clinician and patient is its uncertainty in terms of etiology, prognosis and treatment. It is difficult to predict hearing outcomes, as well as to identify the causes of individual hearing loss. Efforts have been made to establish prognoses, and known prognostic indicators include severity of initial hearing loss, age at onset, presence of vertigo, shape of audiogram and early treatment1. The prevalence of dizziness or imbalance in SSNHL has been reported to be about 30%, and is related to poor prognosis1,4. Because vertigo is a subjective symptom, unlike the other indicators, a variety of studies have been performed to elucidate the clinical significance of vestibular function tests in SSNHL. Previous reports have focused on the value of the caloric test and vestibular evoked myogenic potentials (VEMP), which reflect the functioning of the horizontal semicircular canals (SCC) and otolith organs6,7,8,9. Theoretically, however, various types of vestibulocochlear involvement can be expected in ISSNHL. In terms of anatomic position, the closest vestibular organ from the cochlea is the saccule. The endolymphatic fluid spaces of cochlea and saccule are connected via ductus reunions, and their innervating nerve fibers are close together (Fig. 1A). In terms of blood supply of the inner ear, the anterior vestibular branch of the labyrinthine artery feeds all the vestibular organs except the posterior SCC and part of the saccular macula, which are supplied by branches of the common cochlear artery – the posterior vestibular artery - without collaterals (Fig. 1B)10,11.
Schematic drawing of a transverse section through the internal auditory canal (A) showing the relationships between the facial nerve, the cochlear nerve and the vestibular nerves innervating the saccule, utricle and semicircular canals. Simplified drawing of the blood supply to the inner ear (B). SVN; superior vestibular nerve, IVN; inferior vestibular nerve, ASCC; anterior semicircular canal, HSCC; horizontal semicircular canal, PSCC; posterior semicircular canal, a.; artery.
Accordingly, we aimed to evaluate localized dysfunction of the vestibular organ in ISSNHL. We hypothesized that each of the components of the vestibular organ, especially the SCCs, can be involved variously in ISSNHL and might have distinct prognostic implications. Since subjective symptoms are not always correlated with objective vestibular function test results9, we evaluated all ISSNHL patients, including those who did not complain of dizziness. The functioning of each SCC and saccule was assessed using the video Head Impulse Test (vHIT), bi-thermal caloric test and air-conducted cervical VEMP. The aim of the study was to evaluate the clinical significance of SCC involvement, as assessed by the vHIT, in predicting hearing outcomes of ISSNHL.
Materials and Methods
Patients and study design
The medical records of patients diagnosed with unilateral ISSNHL from January 2015 to December 2018 were retrospectively reviewed. The diagnosis of SSNHL was based on sudden hearing loss of more than 30 dB at a minimum of 3 consecutive frequencies over a period of 72 hours or less12. At the time of diagnosis, otoendoscopic examination, audiometry and vestibular function tests including the caloric test, vHIT and cVEMP, were routinely performed in all patients. Subjects with pre-existing hearing loss in the contra-lesional normal ear exceeding 25 dB HL were excluded to avoid possible debate about the assessment of outcomes. Those with retrocochlear pathology or other ear diseases - vestibular schwannoma, Meniere’s disease, inner ear anomaly, perilymphatic fistula, conductive hearing loss, or AICA infarction on MRI - were also excluded.
According to the uniform treatment protocol, patients were initially treated with high dose oral steroid (prednisone 1 mg/kg daily for 7 days followed by 4 days of tapering). Thereafter, salvage intratympanic dexamethasone (5 mg/mL) injections (4 times/2 weeks) were performed in those whose hearing thresholds did not reach serviceable level (<40 dB HL) within a week.
Recovery of hearing was determined at 3 months from onset according to Siegel’s criterion, which is widely used to report hearing gain in SSNHL13. Complete recovery was defined as a final hearing threshold <25 dB HL. Partial recovery was defined as a final hearing threshold of 26–45 dB HL and >15 dB of hearing gain. Slight improvement referred to a final hearing threshold >46 dB HL and <than 15 dB of hearing gain, and no improvement meant a final hearing threshold >76 dB HL or <15 dB of hearing gain.
In this study, the complete recovery group according to Siegel’s criterion, namely patients with final hearing threshold <25 dB HL, was the group of interest. The other groups were combined as the incomplete recovery group.
Ethical issues
This investigation was approved by the ethics review board of Hanyang University Guri Hospital (IRB #2019-11-016) and performed in accordance with the Declaration of Helsinki and good clinical practice guidelines. Informed consent was waived because of the retrospective nature of the study, and the analysis used anonymous clinical data after approval of ethics review board of Hanyang University Guri Hospital.
The video head impulse test
The video head impulse test (vHIT) was performed at presentation to evaluate the high-frequency vestibulo-ocular reflex (VOR) in each SCC plane, using an ICS Impulse (GN Otometrics, Taastrup, Denmark). To enhance test reliability, two experienced examiners performed the test, using the standard protocol proposed by Halmagy14. Head impulses were given at least 10 times at the low amplitude of 10 degrees and a consistent peak velocity (100–300/second). Eye and head velocities were recorded. A calculated VOR gain of <0.7 was considered abnormal.
The caloric and cVEMP tests
The bi-thermal caloric test was performed using binaural alternative instillation of 8 liters of cold (24 °C) and warm (50 °C) air for 60 seconds15,16. The induced nystagmus was recorded by video-nystagmography (ICS Medical, Schaumburg, IL, USA) until it had decayed to the null position. The maximum slow phase velocities of the corresponding stimuli were assessed and the asymmetry of vestibular function was calculated using Jongkees’ formula15,17. Canal paresis >25% was defined as horizontal semi-circular dysfunction16,18.
The cVEMP test was performed with a Biologic Navigator Pro (Biologic System Corp., IL, USA). The reference electrode, ground electrode and two active electrodes were placed at the sternoclavicular notch, center of the forehead and the middle third of each sternocleidomastoid muscle, respectively19,20. Using a tone-burst sound stimulus of 500 Hz and 90 dB nHL via an EA-3 insert ear phone, the amplitudes of evoked potentials (P13-N23) on the same side of the sternocleidomastoid muscle were recorded19,20. cVEMP abnormality was defined as an asymmetry ratio of p13-n23 amplitude >35%, or no significant P13-N23 waveform19,20,21.
Statistical analysis
Data were analyzed with IBM SPSS Statistics, version 24.0 for Windows (IBM Corp., NY, USA). Descriptive data are expressed as means and standard deviations. A logistic regression model was used to evaluate the effects of prognostic factors on incomplete hearing recovery. Each parameter was assessed by univariable logistic regression analysis, and statistically significant factors were included in a subsequent multivariable analysis. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the vHIT for predicting non-recovery of normal hearing – incomplete recovery – were calculated. P values <0.05 were considered to indicate statistical significance. Figures were drawn with Microsoft Powerpoint 2016 MSO (Microsoft Corp., WA, USA).
Results
Demographics
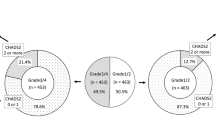
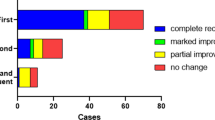
A total of 148 consecutive patients (mean age 49.7 ± 13.9 years) with ISSNHL were analyzed. The average initial hearing threshold was 62.2 ± 29.5 dB HL (Table 1). Of the 148 patients, 49 (33.1%) complained of dizziness at their first visit, and 95 (64.2%) yielded abnormal results in more than one of the vestibular function tests (Table 1, Fig. 2). Canal paresis on the caloric test was revealed in 43 cases (29.1%) and abnormal cVEMP in 60 patients (40.5%). vHIT abnormalities of the anterior, horizontal and posterior SCCs were observed in 12.8%, 16.9%, and 18.9% of enrolled patients, respectively. The mean hearing gain in the 3 months from onset was 23.8 ± 23.7 dB HL. Complete recovery of normal hearing was observed in 71 patients (48.0%) (Table 1).
Known prognostic factors for hearing recovery
Univariable analyses of previously-suggested prognostic factors for hearing outcomes are shown in Table 2. Younger age and lower initial hearing threshold were associated with complete recovery (P = 0.012, P < 0.001). Hearing recovery was negatively related to canal paresis (P = 0.001) and to vertigo (P = 0.003). Other parameters including sex, underlying disease and cVEMP abnormalities did not show any significant relation to recovery.
Prognostic value of vHIT abnormality for hearing recovery
Abnormal vHIT gain in any of the SCCs was seen in fifty-one patients (34.5%) (Table 1). Hearing outcomes according to the vHIT results are shown in Fig. 3. Of the 97 patients with normal vHIT results, 54 (55.7%) recovered normal hearing (Fig. 3A) whereas none of the patients with vHIT abnormalities in all SCCs recovered normal hearing (Fig. 3H).
Type of semicircular canal involvement in the video head impulse test and corresponding hearing outcomes. *The striped area indicates that the gain of vHIT in the corresponding canal decreased (e.g. abnormal gain in the posterior SCC). vHIT; video head impulse test, Patients; number of patients, SCC; semicircular canal.
Univariable analyses showed that abnormal vHIT results in the horizontal SCC (P = 0.031) and posterior SCC (P < 0.001) were significantly related to poor recovery (Table 2).
In a multivariable analysis including the objective clinical factors that were statistically significant in the univariable analysis, significant predictive indicators for incomplete recovery were age (OR 1.040, P = 0.011), poorer initial hearing level (OR 1.045, P < 0.001), canal paresis on the caloric test (OR 1.023, P = 0.023) and abnormal vHIT gain in the posterior SCC (OR 3.690, P = 0.047) (Table 2). Low vHIT gain in the posterior SCC had specificity of 94.4% and PPV of 85.7% in predicting incomplete recovery of hearing (Table 3).
Discussion
In the present study, we assessed the prognostic value of SCC involvement in ISSNHL using the vHIT. The results can be summarized as follows: (1) Various patterns of vHIT abnormality with SCC involvement were observed with or without subjective dizziness, (2) Multivariable analysis showed that incomplete recovery of hearing was significantly associated with decreased vHIT gain in the posterior SCC (OR 3.690, P = 0.047), worse initial hearing level at onset (OR 1.045, P < 0.001), age at onset (OR 1.040, P = 0.011), and canal paresis on the caloric test (OR 1.023, P = 0.023), (3) In our preliminary data, decreased vHIT gain in the posterior SCC had specificity of 94.4%, sensitivity of 31.2%, PPV of 85.7% and NPV of 56.7% in predicting incomplete recovery.
Since its introduction in 1988 by Halmagyi and Curthoys, the head impulse test has been used to evaluate the functioning of SCCs22. The video method of examination, vHIT, is a noninvasive and quantitative method assessing each of the six SCCs individually14. In the vHIT, the adequacy of the VOR is usually measured by the gain, the ratio of the area under the eye velocity curve to the area under the head velocity curve during small, fast, passive unpredictable head impulses14. A VOR gain of <0.7 is usually considered as identifying a deficient SCC14. The vHIT has been widely applied to evaluate SCC function, especially in peripheral vestibular disorders and some central lesions14,23,24,25,26,27,28. In this study, we used it to assess vestibular involvement in ISSNHL.
Prognostic indicators for ISSNHL have been widely investigated1,29. The well-known factors include age at onset, severity of initial hearing loss and time to initial treatment1. In our study, older age and poor initial hearing level were significantly associated with incomplete recovery of hearing (Table 2), in agreement with previous findings. Time to onset of treatment did not have any significant effect because the majority of our patients (140/148, 94.6%) were seen within 2 weeks of onset, which is considered the responsive period for treatment2. Average time to onset of treatment was 5.2 days (Table 1).
Vestibular involvement in SSNHL has been an interesting issue7,9,30. Subjective imbalance or vertigo is associated with poor hearing recovery1,30. In our study, a third of the patients complained of dizziness (Table 1, Fig. 2), and this was significantly related to incomplete recovery (Table 2) We noted, however, that not all patients who showed decreased vestibular function in the tests complained of vertigo, and those with subjective dizziness did not always have vestibular function abnormalities (Fig. 2).
In terms of evaluation of vestibular function, Yu et al., in their systemic review and meta-analysis, reported that about half of SSNHL patients had abnormal vestibular function in more than one of the tests including the caloric test, cVEMP and oVEMP30. They also postulated that damage to the horizontal SCC (caloric test) and saccule (cVEMP) was an important factor for hearing recovery, and that the extent of vestibular damage was related to the prognosis of hearing loss30. In addition, Fujimoto et al. reviewed the results of vestibular function tests in ISSNHL patients with vertigo to assess the extent of vestibular lesions7. They showed that the vestibular end organs close to the cochlea tended to be preferentially affected: there was involvement of saccule in 64%, of the horizontal SCC in 52% and of the utricle in 43% of the cases7. In our study, about 2/3 of the patients gave abnormal results in vestibular function tests. As in previous reports, the saccule was most often affected (60/148, 40.5%) followed by the horizontal SCC, based on abnormal caloric responses (43/148, 29.1%) (Table 1). Worse canal paresis (%) in the caloric test was significantly associated with incomplete hearing recovery (OR 1.023, P = 0.023) (Table 2) as in previous work6,7,8. However, the extent of SCC damage was not closely correlated with hearing outcome, especially in patients with partial involvement (Fig. 3). In terms of horizontal SCC involvement, there were discrepancies between abnormalities in the caloric test (43/148, 29.1%) and the vHIT (25/148, 16.9%) (Table 1). These could be explained by the contribution of the vertical canals to the caloric response, as shown in previous research31.
Interestingly, low vHIT gain in the posterior SCC was found to be an independent parameter affecting hearing recovery (OR 3.690, P = 0.047) (Table 2). Among the patients who showed complete recovery, sixty-seven (67/71, 94.4%) had normal vHIT gains in the posterior SCC (Table 3), whereas of those with abnormal vHIT results in the posterior SCC, only four (4/28, 14.3%) recovered completely (Fig. 2). In addition, eleven of those with abnormal vHIT results (11/28, 39.3%) had normal caloric responses, and eight (8/28, 28.6%) did not complain of dizziness, which means that those patients may be considered as having normal vestibular function if vHIT was not performed.
In terms of the inflammatory origin of ISSNHL, the involvement of adjacent vestibular organs can be explained by the flow of endolymphatic fluid within the inner ear, as well as the proximity of the vestibulocochlear nerve, and vestibular involvement can vary between individual patients. Empirical corticosteroid therapy may be expected to play a crucial role in such cases, as well as where viral infection and autoimmunity are implicated.
It has also been suggested that certain patterns of vestibular involvement point to a vascular etiology for ISSNHL, as opposed to an inflammatory cause. In particular, compromise of the common cochlear artery can induce sudden hearing loss with isolated posterior SCC hypofunction (Fig. 1)9. Rambold et al. described a subgroup of patients with a distinct lesion pattern specifically involving the posterior SCC and cochlea9. In our study, twelve patients (12/148, 8.1%) showed this pattern (Fig. 3D). To compare this specific group with others showing anterior or horizontal SCC involvement (Fig. 3B,C), a post-hoc analysis was performed. Hearing outcomes were notably poorer in patients with posterior SCC involvement compared to involvement of other individual canals (P = 0.028 by Fisher’s exact test). Based on the results of an animal study showing that ischemia of 30 minutes or longer induces irreversible cochlear damage32, the vulnerability of the cochlea to ischemia might offer a possible explanation for this outcome.
In the present study, multivariable analysis showed that risk factors for incomplete recovery were older age, low initial hearing level, canal paresis on the caloric test and abnormal vHIT gain in the posterior SCC (Table 2). Posterior SCC involvement had the highest odds ratio of the clinical factors considered (OR = 3.690). It seems that the functioning of the posterior SCC reflects the severity of cochlear damage better than other indicators. Vascular supply, as mentioned above, as well as the close proximity of the cochlear nerve to the vestibular nerve fibers innervating the posterior SCC, may provide a rationale for this result (Fig. 1).
To the best of our knowledge this is the first study assessing the prognostic value of SCC involvement in ISSNHL using the vHIT. We assessed all ISSNHL patients, regardless of subjective dizziness. In addition, the inclusion criteria included normal contra-lesional hearing to alleviate possible debate about outcome determination. The evaluation of vestibular function, including each SCC, provided distinctive results pointing to a new prognostic indicator of hearing recovery. Unfortunately, oVEMP, which assesses utricular function, was not included in our analysis due to a lack of reliable data. Although we reviewed consecutive ISSNHL patients who were managed with a uniform treatment protocol during the enrollment period, there remains a possibility that biases may have arisen from the retrospective nature of the study. Hopefully, a future prospective study including all available vestibular function tests in a large number of patients will give further insight into the role of vestibular function in ISSNHL. Apart from providing prognostic information, we expect future studies to help develop individual treatment strategies to improve outcomes in ISSNHL.
Conclusion
Abnormal vHIT gain in the posterior SCC is probably a specific prognostic factor indicating incomplete hearing recovery in ISSNHL.
Data availability
Anonymized data will be shared on request from any qualified investigator for the purpose of replicating procedures and results.
References
Kuhn, M., Heman-Ackah, S. E., Shaikh, J. A. & Roehm, P. C. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends in amplification 15, 91–105, https://doi.org/10.1177/1084713811408349 (2011).
Chandrasekhar, S. S. et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery 161, S1–S45, https://doi.org/10.1177/0194599819859885 (2019).
El-Saied, S., Joshua, B. Z., Segal, N., Kraus, M. & Kaplan, D. M. Sudden hearing loss with simultaneous posterior semicircular canal BPPV: possible etiology and clinical implications. American journal of otolaryngology 35, 180–185, https://doi.org/10.1016/j.amjoto.2013.08.021 (2014).
Chung, J. H. et al. Clinical significance of arterial stiffness in idiopathic sudden sensorineural hearing loss. The Laryngoscope 126, 1918–1922, https://doi.org/10.1002/lary.25853 (2016).
Eryigit, B., Ziylan, F., Yaz, F. & Thomeer, H. The effectiveness of hyperbaric oxygen in patients with idiopathic sudden sensorineural hearing loss: a systematic review. Eur Arch Otorhinolaryngol 275, 2893–2904, https://doi.org/10.1007/s00405-018-5162-6 (2018).
Shih, C. P. et al. Analysis of caloric test responses in sudden hearing loss. Ear, nose, & throat journal 96, 59–64, https://doi.org/10.1177/014556131709600207 (2017).
Fujimoto, C. et al. Involvement of vestibular organs in idiopathic sudden hearing loss with vertigo: an analysis using oVEMP and cVEMP testing. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology 126, 1033–1038, https://doi.org/10.1016/j.clinph.2014.07.028 (2015).
Iwasaki, S. et al. Extent of lesions in idiopathic sudden hearing loss with vertigo: study using click and galvanic vestibular evoked myogenic potentials. Archives of otolaryngology–head & neck surgery 131, 857–862, https://doi.org/10.1001/archotol.131.10.857 (2005).
Rambold, H. et al. Differential vestibular dysfunction in sudden unilateral hearing loss. Neurology 64, 148–151, https://doi.org/10.1212/01.wnl.0000148599.18397.d2 (2005).
Yao, Q., Xu, C., Wang, H., Shi, H. & Yu, D. Video head impulse test results suggest that different pathomechanisms underlie sudden sensorineural hearing loss with vertigo and vestibular neuritis: Our experience in fifty-two patients. Clinical otolaryngology: official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 43, 1621–1624, https://doi.org/10.1111/coa.13196 (2018).
Pogson, J. M. et al. Vertigo with sudden hearing loss: audio-vestibular characteristics. Journal of neurology 263, 2086–2096, https://doi.org/10.1007/s00415-016-8214-0 (2016).
Stachler, R. J. et al. Clinical practice guideline: sudden hearing loss. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery 146, S1–35, https://doi.org/10.1177/0194599812436449 (2012).
Siegel, L. G. The treatment of idiopathic sudden sensorineural hearing loss. Otolaryngologic clinics of North America 8, 467–473 (1975).
Halmagyi, G. M. et al. The Video Head Impulse. Test. Frontiers in neurology 8, 258, https://doi.org/10.3389/fneur.2017.00258 (2017).
Recommended procedure. Br J Audiol 33, 179–185, https://doi.org/10.3109/03005369909090097 (1999).
Shepard, N. T. & Jacobson, G. P. The caloric irrigation test. Handbook of clinical neurology 137, 119–131, https://doi.org/10.1016/b978-0-444-63437-5.00009-1 (2016).
Jongkees, L. B., Maas, J. P. & Philipszoon, A. J. Clinical nystagmography. A detailed study of electro-nystagmography in 341 patients with vertigo. Practica oto-rhino-laryngologica 24, 65–93 (1962).
Kim, H. J., Kim, D. Y., Hwang, J. H. & Kim, K. S. Vestibular Neuritis With Minimal Canal Paresis: Characteristics and Clinical Implication. Clinical and experimental otorhinolaryngology 10, 148–152, https://doi.org/10.21053/ceo.2016.00948 (2017).
Rosengren, S. M., Colebatch, J. G., Young, A. S., Govender, S. & Welgampola, M. S. Vestibular evoked myogenic potentials in practice: Methods, pitfalls and clinical applications. Clin Neurophysiol Pract 4, 47–68, https://doi.org/10.1016/j.cnp.2019.01.005 (2019).
Rosengren, S. M., Welgampola, M. S. & Colebatch, J. G. Vestibular evoked myogenic potentials: past, present and future. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology 121, 636–651, https://doi.org/10.1016/j.clinph.2009.10.016 (2010).
Iwasaki, S., Takai, Y., Ito, K. & Murofushi, T. Abnormal vestibular evoked myogenic potentials in the presence of normal caloric responses. Otol Neurotol 26, 1196–1199, https://doi.org/10.1097/01.mao.0000194890.44023.e6 (2005).
Halmagyi, G. M. & Curthoys, I. S. A clinical sign of canal paresis. Arch Neurol 45, 737–739 (1988).
Bartolomeo, M. et al. Value of the video head impulse test in assessing vestibular deficits following vestibular neuritis. Eur Arch Otorhinolaryngol 271, 681–688, https://doi.org/10.1007/s00405-013-2451-y (2014).
Choi, J. Y., Kim, H. J. & Kim, J. S. Recent advances in head impulse test findings in central vestibular disorders. Neurology 90, 602–612, https://doi.org/10.1212/WNL.0000000000005206 (2018).
Taylor, R. L. et al. Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. Journal of neurology 262, 1228–1237, https://doi.org/10.1007/s00415-015-7697-4 (2015).
Castellucci, A., Malara, P., Delmonte, S. & Ghidini, A. A Possible Role of Video-Head Impulse Test in Detecting Canal Involvement in Benign Paroxysmal Positional Vertigo Presenting With Positional Downbeat Nystagmus. Otol Neurotol, https://doi.org/10.1097/MAO.0000000000002500 (2019).
Cordero-Yanza, J. A. et al. Comparative study between the caloric vestibular and the video-head impulse tests in unilateral Meniere’s disease. Acta Otolaryngol 137, 1178–1182, https://doi.org/10.1080/00016489.2017.1354395 (2017).
Tarnutzer, A. A., Bockisch, C. J., Buffone, E. & Weber, K. P. Association of posterior semicircular canal hypofunction on video-head-impulse testing with other vestibulo-cochlear deficits. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology 128, 1532–1541, https://doi.org/10.1016/j.clinph.2017.04.029 (2017).
Byun, H. et al. The clinical value of 4-hour delayed-enhanced 3D-FLAIR MR images in sudden hearing loss. Clinical otolaryngology: official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 44, 336–342, https://doi.org/10.1111/coa.13305 (2019).
Yu, H. & Li, H. Vestibular Dysfunctions in Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-analysis. Frontiers in neurology 9, 45, https://doi.org/10.3389/fneur.2018.00045 (2018).
Bohmer, A., Straumann, D., Henn, V., Arai, Y. & Suzuki, J. Effects of semicircular canal plugging on caloric nystagmus recorded in three dimensions. Acta Otolaryngol Suppl 520(Pt 1), 178–180, https://doi.org/10.3109/00016489509125221 (1995).
Tsuji, S., Tabuchi, K., Hara, A. & Kusakari, J. Long-term observations on the reversibility of cochlear dysfunction after transient ischemia. Hearing research 166, 72–81 (2002).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2017R1D1A1B03033051, NRF-2018R1D1A1B07048796). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit this manuscript for publication.
Author information
Authors and Affiliations
Contributions
Dr. Jae Ho Chung and Hayoung Byun had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis and interpretation of data: All authors. Drafting of the manuscript: Hayoung Byun. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Hayoung Byun and Jae Ho Chung. Obtained funding: Jae Ho Chung and Hayoung Byun. Administrative, technical and material support: Seung Hwan Lee. Supervision: Jae Ho Chung.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Byun, H., Chung, J.H. & Lee, S.H. Clinical implications of posterior semicircular canal function in idiopathic sudden sensorineural hearing loss. Sci Rep 10, 8313 (2020). https://doi.org/10.1038/s41598-020-65294-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65294-5
This article is cited by
-
A prognostic value of estimated pulse wave velocity in idiopathic sudden sensorineural hearing loss
European Archives of Oto-Rhino-Laryngology (2024)
-
Vestibular mapping in Ramsay-Hunt syndrome and idiopathic sudden sensorineural hearing loss
European Archives of Oto-Rhino-Laryngology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.