Abstract
The use of neonatal hearing screening has enabled the identification of congenital unilateral sensorineural hearing loss (USNHL) immediately after birth, and today there are several intervention options available to minimize potential adverse effects of this disease, including cochlear implantation. This study aims to analyze the characteristics of the inner ear of a homogeneous group of congenital non-syndromic USNHL to highlight the features of the inner ear, which can help in clinical, surgical, and rehabilitative decision-making. A retrospective chart review was carried out at a tertiary referral center. Systematic diagnostic work-up and rigorous inclusion–exclusion criteria were applied to 126 children with unilateral hearing impairment, leading to a selection of 39 strictly congenital and non-syndromic USNHL cases, undergoing computed tomography (CT) and magnetic resonance (MR) imaging studies. The frequency and type of malformations of the inner ear in USNHL and unaffected contralateral ears were assessed, with an in-depth analysis of the deficiency of the cochlear nerve (CND), the internal auditory canal (IAC) and the cochlear aperture (CA). Inner ear anomalies were found in 18 out of 39 (46%) of the USNHL patients. In 1 subject, the anomalies were bilateral, and the CND resulted in the predominant identified defect (78% of our abnormal case series), frequently associated with CA stenosis. Only 3 out of 14 children with CND presented stenosis of the IAC. CND and CA stenosis (and to a much lesser extent IAC stenosis) are a frequent association within congenital and non-syndromic USNHL that could represent a distinct pathological entity affecting otherwise healthy infants. In the context of a diagnostic work-up, the evaluation with CT and MRI measurements should take place in a shared decision-making setting with thorough counseling. Both imaging techniques have proven useful in differentiating the cases that will most likely benefit from the cochlear implant, from those with potentially poor implant performance.
Similar content being viewed by others
Introduction
Estimates of the universal newborn hearing screening programs suggest that around 1–2 in 1000 infants have significant hearing loss, with a unilateral deficit representing one-third of all the children born with permanent hearing loss1. Interest in the management of childhood unilateral sensorineural hearing loss (USNHL) has significantly increased in recent years. The reasons stem primarily from the growing awareness that functional binaural hearing requires sound input for both ears2. Furthermore, thanks to the widely adopted universal newborn hearing screening programs, the diagnosis of congenital USNHL can now be made in the first few months of life instead of being detected in preschool and school-age3,4, allowing early treatment. However, another incentive for USNHL treatment arises from the technological advancements of hearing devices5,6.
Gordon and Papsin (2019) described the binaural hearing as a function that provides a map of our environment in all the directions, which we use to separate and group auditory streams7. Adults with USNHL experience difficulties in localizing sounds and understanding speech, competing with environmental noise8. If the USNHL is congenital or occurs in the first years of life, the localization skills seem to be less compromised, possibly due to the successful use of monoaural cues through developmental compensation9. However, many papers highlighted the negative consequences of USNHL on children’s development, which include delayed or impaired auditory skills10, increased risk of receptive and expressive language problems11, behavioral and educational challenges12, and reduced hearing-related quality of life13. The etiology of congenital USNHL is much less defined than bilateral sensorineural hearing loss, and although specific causative agents are not often recognized14,15, the literature indicates a high frequency of inner ear malformations in congenital USNHL cases16,17,18. Temporal bone anomalies are identified in 29–40% of pediatric USNHL when assessed by CT. In 10–25% of the cases, MR detects anomalies of the cochlear, vestibular, or facial nerves, of the cerebellopontine angle structures, or auditory pathways16,19. It is essential to recognize an inner ear malformation to clarify the underlying pathology, to offer critical information in terms of possible hearing loss progression or contralateral involvement, as well as to provide tips for counseling and treatment. Indeed, rehabilitation management has been variably provided to children with USNHL, with a range of options available but, so far, no evidence of the superiority of one approach to another20. In the past, the most conventional approach to congenital USNHL was watchful waiting and monitoring of auditory-language development21. There are now several devices available for USNHL prosthetic treatment: acoustic hearing aids, contralateral routing of signal hearing aids, bone conduction hearing implants and cochlear implants22. Cochlear implant has recently sparked increasing interest as a treatment option23,24, described as the most likely tool for improving binaural hearing for adults and children affected by severe-to-profound USNHL25. A systematic review established that no firm conclusions can be drawn on the efficacy of cochlear implant in children with USNHL, due to the heterogeneous findings and small sample sizes26. The significant variability of the outcomes can be attributed to heterogeneous etiologies and times of the onset of USNHL (i.e., congenital and acquired, with or without hearing experience or auditory deprivation27).
This study aims to analyze the characteristics of the inner ear of a homogeneous group of congenital non-syndromic USNHL in order to highlight the anatomical features that can help in clinical, surgical, and rehabilitative decision-making. We focused on:
-
(a)
Characteristics of inner ear malformations on the affected side;
-
(b)
Anomalies on the contralateral unaffected side;
-
(c)
Cochlear nerve deficiency (CND), cochlear aperture (CA) stenosis and internal auditory canal (IAC) stenosis.
Material and methods
Subjects
This retrospective chart review involves all children who have been diagnosed with a permanent USNHL and assessed using imaging techniques from 2012 to 2019 at the Department of Otorhinolaryngology and Audiology of the Institute for Maternal and Child Health IRCCS “Burlo Garofolo”, Trieste, Italy. USNHL was defined as bone conduction pure tone threshold average of 500, 1000 and 2000 Hz, > 40 dB hearing level (HL) in the affected ear and < 15 dB HL in the contralateral unaffected ear. Hearing loss degree was classified as moderate (41–60 dB HL), severe (61–80 dB HL) or profound (> 80 dB HL). The identified children (n = 126) underwent a systematic diagnostic work-up, which included ear, nose and throat examination, ophthalmologic evaluation, kidney ultrasonography, electrocardiogram, and inner ear imaging, mainly aimed at verifying the presence of syndromes or inner ear malformations. Cytomegalovirus infection was ruled out after the failure of the universal newborn hearing screening. In the case of older infants with late USNHL diagnostic work-up, a detailed brain MR review was performed, to highlight abnormalities possibly involved with a congenital cytomegalovirus infection syndrome. Audiological tests confirmed a hearing loss and classified its type, laterality and degree. In order to gather a homogeneous population of congenital USNHL, strict inclusion and exclusion criteria have been applied (see Table 1).
Thirty-nine children (20 males, 19 females) who met the inclusion and exclusion criteria, as reported in Table 1, were included for the analysis. The mean age at diagnosis was 4.4 years (3.4 standard deviation); 11 out of 39 subjects underwent the universal newborn hearing screening. USNHL was present on the left side in 27 children (69.2%) and on the right side in 12 children (30.8%). The hearing loss’ degree was profound in 29 out of 39 cases, severe in 5 cases and moderate in the last 5 cases.
Imaging evaluation
Both temporal bone CT and cerebral MR were performed in the same session during natural sleep or sedation, if necessary. Temporal bone CT: patients were examined by high-resolution CT Philips Brilliance 40, with a slice thickness of 0.67 mm, slice increment of 0.33 mm, collimation 16 × 0.625 mm, time of rotation 1 s., amperage 140 kV, mAs 400mAs, CTDI 93.7 mGy, DLP mGy*cm 503.16, the scale of the length of 52.8 mm and field of view of 155 mm. Temporal bone MR: patients were examined with Philips Ingenia 1.5 T with DRIVE 3D HR sequences: TE 198 ms; TR 1500 ms; ETL 40; the field of view of 160 mm; isotropic voxel of 0.6 × 0.6 × 0.6 mm, interpolate 50%; matr. 252 × 265; scan percentage 80%; slice thickness 0.9 mm.
The measurements were reconstructed on axial, coronal, and oblique-sagittal views using the Picture Archiving and Communication System. The measures were reported in millimeters; dimensions from 1 to 6 were measured on axial, while 8–10 on the coronal plane. The standard range values for the basal and middle turn of the cochlea, the cochlear height, the bone island width of the semicircular canals, and the coronal width of the internal auditory canal were calculated based on the average measurement of our normal controls minus/plus 2 standard deviations-SD28. The average range values were directly selected from the previously published literature for other parameters such as the cochlear aperture, vestibular aqueduct, cochlear length, cochlear nerve29,30,31,32. Precisely, this study was based on the following parameters and standard range measurement:
-
(1)
The cochlear aperture (CA) was measured in its mid-portion at a mid-modiolar level and was classified as “normal”, “aplastic” (when completely ossified) or “stenotic” (≤ 1.2 mm), based on Lim et al.31 who provided a cut off elaborated with an explicit methodology;
-
(2)
The cochlear basal turn was measured from the bone cochlea near the oval window to the furthest end of the basal turn (range: mean value in normal controls ± 2 SD; 8.1–9.7 mm)28;
-
(3)
The cochlear middle turn was measured in its largest part (range: mean value in normal controls ± 2 SD; 3.8–4.6 mm)28;
-
(4)
The cochlear height was measured from the base of the modiolus, through the modiolus, to the cochlear apex30 (range: mean value in normal controls ± 2 SD; 3.8–5 mm)28;
-
(5)
The vestibular aqueduct was measured at the midpoint between the common crus and the operculum32. It has been defined “enlarged” if greater than 2 mm;
-
(6)
The bony island width of the posterior semicircular canal was measured in axial view at its greatest diameter (range: mean value in normal controls ± 2 SD; 4.3–6.3 mm)28;
-
(7)
The bony island width of the lateral semicircular canal was measured in coronal view at its greatest diameter (range: mean value in normal controls ± 2 SD; 2.4–4.8 mm)28;
-
(8)
The bony island width of the superior semicircular canal was measured in coronal view at its greatest diameter (range: mean value in normal controls ± 2 SD; 4.4–6 mm)28;
-
(9)
The cochlear length was measured from the medial point of the round window (air-perilymph interface) to the more external bony point, passing through the modiolus (range: 8.1–9.59 mm)30;
-
(10)
The coronal width of the internal auditory canal (IAC) was drawn perpendicular to an imaginary line from the transverse crest to the mid-point of the porus. (range: mean value in normal controls ± 2 SD; 3.9–6.7 mm)28.
MR measurements:
-
(11)
The cochlear nerve was evaluated on axial and especially on sagittal-oblique images. It was classified as “aplastic,” “hypoplastic,” or “normal” according to its size compared to the ipsilateral facial nerve29 and contralateral cochlear nerve diameter. The terms of aplasia and hypoplasia are also referred as cochlear nerve deficiency (CND);
-
(12)
The endolymphatic sac was defined as “not visible” or “visible,” although not included in the statistical comparison.
A dedicated pediatric radiologist, a temporal bone imaging expert, with no information on the affected child's ear, examined and compared the imaging.
Inner ear malformations were documented according to Sennaroğlu Classification33.
Normal controls
We evaluated 14 children (23 ears) not affected by sensorineural hearing loss who performed audiometry, CT and MR for other reasons, mainly of chronic ear surgery. In the case of cholesteatoma history, the affected ear was ruled out for the possible presence of cochleovestibular anomalies in this type of pathology34.
Statistics
Given the small group of subjects and the non-normal distribution of the data samples (Shapiro–Wilk normality tests, p < 0.05), we decided to obtain a statistical indication using a nonparametric test. We described variables with medians and quartiles. Comparisons between groups were made using Wilcoxon rank-sum test for continuous variables, and Fisher’s exact test for categorical variables. P values < 0.05 were considered significant. Data were analyzed and visualized using Excel (version 16.23) and R Commander (version 3.6.0 GUI 1.70)35.
Ethical approval
The study was conducted according to the 1964 Helsinki declaration and its later amendments, under the framework of the research project 17/17 approved by the institutional review board and by the Italian Ministry of Health-CCM (project 12/2013).
Informed consent
Informed consent was obtained from a parent and/or legal guardian of all participants included in the study.
Results
Table 2 summarizes the identified anomalies with the associated hearing loss degree. Of 39 cases analyzed, 18 (46%) had an inner ear malformation on the USNHL side with several overlapping conditions. The only case with bilateral malformation presented a defective apical part of the modiolus on both sides (similarly to the incomplete partition type 2, IP2, but without the enlarged vestibular aqueduct, EVA), with a milder expression on the normal hearing side.
The CND, classified as hypoplasia (n = 7) or aplasia (n = 7), was the more represented pathological picture (35.9%). All aplasia cases showed a profound hearing loss, while hypoplasia was associated with a variable degree of hearing loss. Two CNDs were found in the right ear, while the other 12 CND cases belonged to left ears. In 10 out of 14 USNHL cases, the CND was associated with CA stenosis (Fisher’s exact test, p = 0.001), and in only 3 CNDs a stenotic IAC was found. The 4 CNDs without CA stenosis were associated with an EVA in 2 cases, with an IP2 in 1 case, while the last CND case (cochlear nerve aplasia) presented only with an isolated stenotic IAC.
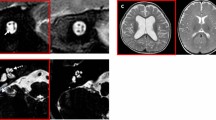
Examples of inner ear CT scans with measures of inner auditory canal, cochlear height, cochlear aperture, and MR images of cochlear nerve are provided in Fig. 1.
Inner ear CT scans with measures of inner auditory canal, cochlear height, cochlear aperture, and MR images of cochlear nerve. (A) Inner auditory canal (CT coronal view, right ear, normal); (B) Cochlear height (CT axial view, right ear, normal); (C) Cochlear aperture (CT axial view, right ear, normal); (D) Severe cochlear aperture stenosis (CT axial view, left ear); (E) Normal cochlear nerve, the bony canal is well visible and comparable with the facial nerve bony canal; (MR oblique-sagittal view); (F) Hypoplastic cochlear nerve, the bony canal is almost completely ossified, (MR oblique-sagittal view).
A total of 6 EVAs were identified: apart from the 2 already mentioned as associated with CND, 3 EVAs were isolated, 1 was associated with IP type 1 malformation (infrequent association). In 4 out of 6 EVAs, the endolymphatic sac was visible on MR images. In 1 case, it was possible to visualize the endolymphatic sac without the appreciation of a vestibular aqueduct diameter > 2 mm.
The anomalies of the bony island of the lateral semicircular canal (SCC) did not appear to be related explicitly to USNHL: we detected reduced bony island dimensions in 3 unaffected ears and 2 USNHL sides. Only in one subject of our series, the lateral SCC bony island anomaly was bilateral: the USNHL side harbored the IP 1 malformation with lack of lateral SCC bony island, and the contralateral normal hearing side presented a lateral SCC bony island < 2.4 mm.
Statistical comparisons of the measured parameters are presented in Table 3: the only significant differences between affected and unaffected sides of USNHL subjects and normal controls regards the CA and the cochlear height dimensions. As also represented in Fig. 2, the CA showed a significantly smaller dimension in the affected side in comparison to both the contralateral side (W = 438.5, p < 0.01) and normal controls (W = 587, p = 0.05). Overall, also cochlear height measurements were bilaterally smaller in USNHL children, even though only 3 out of 39 in the affected side and 4 in the unaffected side subjects resulted below our normal range values. (All measured parameters values resulting outside the normal range can be viewed in the Supplementary Table S1 online).
The box-plot A represents the comparisons of the cochlear aperture’s dimension in millimeters between normal controls, affected side, and unaffected side. The box-plot B represents the comparison of the cochlear height’s dimensions between the same groups. In the y-axis, the values represent the measurement in millimeters35.
Finally, the dispersion graphs on Fig. 3 compare CND ears14 with ears that present a normal cochlear nerve25. The Fig. 3A highlights that ears with CND have lower CA dimension compared with ears without CND. This difference is more evident when the CND is restricted to only aplasia cases (Fig. 3B).
Dispersion graphs35 of IAC and CA measures, indicating that the size of CA is a more accurate indicator of CND, especially in case of cochlear nerve aplasia. A. Comparison between the affected ears with CND (aplasia and hypoplasia, 14 cases, blue area) and affected ears with normal cochlear nerve (25 cases, pink area). CA measures are represented on x-axis, while the IAC measures are represented in the y-axis. The cut offs to define the IAC or CA stenosis are indicated in red. The central point of the blue area represents the average dimensions of IAC and CA in patients with CND (IAC = 4.7 mm, SD = 1.2; CA = 1.2 mm, SD = 0.7), while the central point of the pink area represents the average dimensions of the same parameters but in patients with normal CN (IAC = 5.6 mm, SD = 1.1; CA = 2.1 mm, SD = 0.7). For both areas, the internal ellipse represents 1 standard deviation, while the external one represents 2 standard deviations. B. The blue area focuses on CND cases with nerve aplasia7, after exclusion of hypoplasia cases (central point: IAC = 4.4 mm, SD = 1.1; CA = 0.9 mm, SD = 0.7).
Discussion
Our study confirms that individuals with congenital USNHL are more likely to have inner ear malformations than those with congenital bilateral hearing loss36. Anomalies are rarely present on both sides, only in 1 case of our series. A CND characterizes a large portion (35.9%) of our population, most frequently associated with CA stenosis. Both of these anomalies have been previously reported in the literature, although more often individually, or described as a possible feature within more heterogeneous populations33,37,38.
Unlike most studies describing inner ear anomalies in USNHL patients38,39,40,41, we adopted strict exclusion and inclusion criteria to focus on a strictly selected population. Besides, only a few reports have analyzed USNHL functionality with a double imaging technique (CT and MRI) on a significant number of patients with non-syndromic congenital USNHL. A multi-institutional retrospective imaging study review was conducted by Shah et al.39 on 219 patients with USNHL aged 0–18 years that underwent CT and/or MRI. The overall prevalence of abnormal radiological findings associated with USNHL was 40%. EVA was the most common positive finding (15%) identified on CT imaging, while CND was the most common anomaly (52%) in 16 out of 31 patients who underwent MRI only. Similarly, to our description, there were many patients with more than one positive finding on imaging. This study did not include a measurement of the CA on CT images, and therefore it was not possible to relate CA stenosis and CND in these patients. By applying stricter inclusion criteria, Lim et al.31 analyzed the medical records and temporal bone CT scans of 42 patients under the age of 13 years with a diagnosis of USNHL with unknown etiology (ototoxic, sudden hearing loss, trauma, ear infection, syndromes, craniofacial anomalies, ear surgery were excluded). A CA < 1.2 mm was detected in 52.4% of the total population. Even this study did not include MR images nor a normal control group. Vilchez-Madrigal et al.42 compared the CT imaging studies of 36 children with unilateral CA stenosis (< 1.0 mm), with controls without temporal bone injury. Contralateral ears had smaller CA (p < 0.00) and cochlea (p < 0.00) than controls, although to a lesser extent than on the stenotic side.
Thanks to the comprehensive anatomical imaging study of the inner ear structures in a homogeneous population, our work has linked CA stenosis and CND as a specific and frequent pathological profile in congenital non-syndromic USNHL. Contrary to general clinical opinion, aplasia/hypoplasia is not systemically or frequently associated with IAC stenosis in congenital USNHL. Only 2 cases of aplasia out of 14 CND showed the co-presence of a stenotic CA and an IAC stenosis, and only in 1 case out of 14 CND the stenotic IAC was isolated. As indicated by Tahir et al.37, the detection of IAC stenosis alone may be insufficient to indicate a CND: therefore, the CA is also required to predict cochlear nerve status. It is worth observing that there is no general agreement regarding how to measure the IAC width37, and the CA’s diameter can therefore represent a more reliable measure. Further morphological and genetic studies could, in the future, define whether the heterogeneity of the presence of the three anomalies described (CND, CA stenosis, and IAC stenosis) belongs to a continuum of the pathology of the inner ear.
The interest in CA is aroused by the fact that a narrow cochlear aperture probably indicates an anatomical or functional deficiency in the cochlear nerve. Consequently, a CA stenosis appeared to be a criterion for a negative outcome of the cochlear implant surgery, indicating that patients with stenotic CA demonstrate poorer cochlear implants results than patients with normal CA. Chung et al.43 compared the post-cochlear implant speech performances among 3 groups of various CA diameters (< 1.4 mm; 1.4–2.0 mm; > 2.0 mm). CND occurred more frequently in the group with a diameter of < 1.4 mm which, also showed a worse post-cochlear implant outcome.
The observations on the imaging of our work are relevant in today's perspective of the audiological management of congenital USNHL. Today's advantage in identification times and observations of our study stress the importance of adopting a very comprehensive diagnostic work-up in order to characterize the USNHL critical elements that can lead to the most rational and timely therapeutic approach.
The literature has discussed the need for early imaging in USNHL44. The main issue is exposure to CT radiations. We believe that a comprehensive imaging evaluation should take place in a shared decision-making setting with thorough counseling, taking into account that inner ear malformations are associated with congenital USNHL and an increased risk of developing bacterial meningitis in the pediatric population17. This was the case of one child in our series, who presented a cochleovestibular cystic malformation (IP-1), known to be associated with a higher risk of spontaneous cerebrospinal fluid fistula and recurrent meningitis33. An early temporal bone CT in the diagnosis of USNHL could be 17.4 to 80.3 times more likely to help prevent meningitis than to be associated with a subsequent malignant abnormality due to exposure to CT radiations18.
Interventions in pediatric USNHL include surgical and non-surgical devices that can be divided into 2 broad categories: in one approach, the intent is to transmit auditory stimuli that come from the impaired side to the healthy ear45; the second, aims to recover binaural hearing through traditional amplification or cochlear implantation. Contralateral routing of signal hearing aid, bone conduction hearing implants or cochlear implants are indicated for USNHL in the range of single-sided deafness with the abovementioned differential approach: contralateral routing of signal hearing aid and the bone conduction hearing implant deliver the stimuli to the normal hearing ear, while the only chance to restore a real binaural hearing in single side deafness is a cochlear implant. Bone conduction hearing implant can offer more advantages than contralateral routing of signal hearing aid amplification for USNHL46. However, there is still no compelling evidence that one of these strategies is superior to others in terms of hearing outcomes and cost-utility47,48. As regards the pediatric population, studies of cochlear implant results frequently include non-congenital USNHLs, i.e., children with some binaural hearing experience and short hearing deprivation27. Cochlear implants are still to be considered an emerging treatment option for children with congenital USNHL, and even the most recent literature reports only limited descriptions of the benefit of cochlear implant in these cases20,49. Therefore, evidence-based decisions regarding the most appropriate management in USNHL children are still lacking. In this perspective, our study shows that over a third of congenital and non-syndromic "idiopathic" USNHLs affecting otherwise healthy infants may not be suitable for current cochlear implant technology. In fact, among other factors known to influence cochlear implant outcomes, the choice (and benefits) of a cochlear implant depends on neuronal health and the presence of an inner ear malformation. Aplasia or hypoplasia of the auditory nerve is the primary concern, due to the possibility of compromising nerve stimulation through electrical impulses delivered by the cochlear implant. The literature reports that children with CND are generally considered poor candidates for cochlear implant surgery, and only a minority of children implanted with CND achieve good results50.
The present study has some limitations. The retrospective nature of data collection makes it subject to possible bias. Despite the strict inclusion and exclusion criteria, it is sometimes difficult to ascertain the congenital origin of the USNHL. Only 11 of the 39 USNHL children investigated were detected through the UNHS program, which became mandatory in our region in 2012, and in Italy, in 2017. Similarly, although all children performed a brain MR whose analysis excluded subjects with signs of congenital cytomegalovirus infection syndrome51, it is only with the recent introduction of cytomegalovirus testing on urine samples of infants in their first weeks of life that is possible to exclude a congenital cytomegalovirus -related hearing loss52.
Another issue is the cochlear nerve measurements based on comparison with a neighboring nerve rather than a range of reference values of the nerve diameter. Furthermore, the non-visualization of cochlear nerve in MR does not necessarily imply the complete absence of nerve fibers due to the difficulty of detecting small nerves53. The visibility of the nerve depends on the intensity of the magnetic field. The hypoplasia/aplasia ratio would increase if conducted using a device with higher magnetic field. Future prospective studies together with an accurate diagnostic work-up and strict imaging criteria will allow obtaining more details on the prevalence and clinical distinction of the different pathogenetic profiles in congenital USNHL. However, this study’s strength is to rely on a blind assessment of inner ear imaging performed by a dedicated radiologist who was aware of the clinical and audiological picture of the patients. Other authors have pointed out that the increase in clinical records using a multicentric collaboration, significantly increases the simultaneous risk of higher variability in imaging criteria and reports39.
Conclusion
Congenital non-syndromic USNHL is frequently linked to inner ear malformations, which occurs, almost always, only on the affected side and is, in most cases, represented by CND combined with CA stenosis. CT and MRI are needed to identify specific abnormalities that could shed light on the pathogenesis of congenital USNHL, affecting the therapeutic approach and the expected benefits of any cochlear implant surgery in about half of infants with non-syndromic congenital USNHL.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding Author on reasonable request.
Abbreviations
- USNHL:
-
Unilateral sensorineural hearing loss
- CT:
-
Computed tomography
- MR:
-
Magnetic resonance
- CND:
-
Cochlear nerve deficiency
- IAC:
-
Inner auditory canal
- CA:
-
Cochlear aperture
- HL:
-
Hearing level
- SD:
-
Standard deviation
- IP:
-
Incomplete partition
- EVA:
-
Enlarged vestibular aqueduct
- SCC:
-
Semicircular canal
References
Barsky-Firkser, L. & Sun, S. Universal newborn hearing screenings: a three-year experience. Pediatrics 99(6), E4 (1997).
Kral, A., Hubka, P. & Tillein, J. Strengthening of hearing ear representation reduces binaural sensitivity in early single-sided deafness. Audiol. Neurootol. 20(Suppl 1), 7–12 (2015).
Morton, C. C. & Nance, W. E. Newborn hearing screening–a silent revolution. N. Engl. J. Med. 354(20), 2151–2164 (2006).
American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 120(4), 898–921 (2007).
Hempel, J. M., Simon, F. & Müller, J. M. Extended applications for cochlear implantation. Adv. Otorhinolaryngol. 81, 74–80 (2018).
Appachi, S. et al. Auditory outcomes with hearing rehabilitation in children with unilateral hearing loss: a systematic review. Otolaryngol. Head Neck Surg. 157, 565–571 (2017).
Gordon, K. A. & Papsin, B. C. Special edition on unilateral deafness and hearing loss: an introduction and overview. Hear. Res. 372, 1–2 (2019).
Wie, O. B., Pripp, A. H. & Tvete, O. Unilateral deafness in adults: effects on communication and social interaction. Ann. Otol. Rhinol. Laryngol. 119, 772–781 (2010).
Nelson, E., Reeder, R. M., Holden, L. K. & Firszt, J. B. Front-and rear-facing horizontal sound localization results in adults with unilateral hearing loss and normal hearing. Hear. Res. 372, 3–9 (2019).
Kishon-Rabin, L., Kuint, J., Hildesheimer, M. & Ari-Even Roth, D. Delay in auditory behaviour and preverbal vocalization in infants with unilateral hearing loss. Dev. Med. Child Neurol. 57, 1129–1136 (2015).
Fitzpatrick, E. M. et al. Auditory and language outcomes in children with unilateral hearing loss. Hear. Res. 372, 42–51 (2019).
Lieu, J. E., Tye-Murray, N. & Fu, Q. Longitudinal study of children with unilateral hearing loss. Laryngoscope 122, 2088–2095 (2012).
Roland, L. et al. Quality of life in children with hearing impairment: systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 155, 208–219 (2016).
Dodson, K. M. et al. Etiology of unilateral hearing loss in a national hereditary deafness repository. Am. J. Otolaryngol. 33, 590–594 (2012).
Howell, J. B., Appelbaum, E. N., Armstrong, M. F., Chapman, D. & Dodson, K. M. An analysis of risk factors in unilateral versus bilateral hearing loss. Ear Nose Throat J. 98, 330–333 (2019).
van Beeck Calkoen, E. A. et al. High prevalence of abnormalities on CT and MR imaging in children with unilateral sensorineural hearing loss irrespective of age or degree of hearing loss. Int. J. Pediatr. Otorhinolaryngol. 97, 185–191 (2017).
Muzzi, E., Battelino, S., Gregori, M., Pellegrin, A. & Orzan, E. Life-threatening unilateral hearing impairments. Review of the literature on the association between inner ear malformations and meningitis. Int. J. Pediatr. Otorhinolaryngol. 79, 1969–1974 (2015).
Muzzi, E., Gregori, M. & Orzan, E. Inner ear malformations and unilateral sensorineural hearing loss-The elephant in the room. JAMA Otolaryngol. Head Neck Surg. 145, 874 (2019).
McClay, J. E., Booth, T. N., Parry, D. A., Johnson, R. & Roland, P. Evaluation of pediatric sensorineural hearing loss with magnetic resonance imaging. Arch. Otolaryngol. Head Neck Surg. 134, 945–952 (2008).
Huttunen, K., Erixon, E., Löfkvist, U. & Mäki-Torkko, E. The impact of permanent early-onset unilateral hearing impairment in children: a systematic review. Int. J. Pediatr. Otorhinolaryngol. 120, 173–183 (2019).
Lieu, J. E. Permanent unilateral hearing loss (UHL) and childhood development. Curr. Otorhinolaryngol. Rep. 6, 74–81 (2018).
Choi, J. E. et al. A comparison between wireless CROS/BiCROS and soft-band BAHA for patients with unilateral hearing loss. PLoS ONE 14, e0212503 (2019).
Arndt, S. et al. Cochlear implantation in children with single-sided deafness: Does aetiology and duration of deafness matter?. Audiol. Neurotol. 20(suppl 1), 21–30 (2015).
Zeitler, D. M. & Dorman, M. F. Cochlear implantation for single-sided deafness: a new treatment paradigm. J. Neurol. Surgery B Skull Base. 80, 178–186 (2019).
Hassepass, F. et al. Unilateral deafness in children: audiologic and subjective assessment of hearing ability after cochlear implantation. Otol. Neurotol. 34, 53–60 (2013).
Peters, J. P., Ramakers, G. G., Smit, A. L. & Grolman, W. Cochlear implantation in children with unilateral hearing loss: a systematic review. Laryngscope 126, 713–721 (2016).
Rohlfs, A. K. et al. Unilateral hearing loss in children: a retrospective study and a review of the current literature. Eur. J. Pediatr. 176, 475–486 (2017).
Yi, J. S. et al. Proportion of bony cochlear nerve canal anomalies in unilateral sensorineural hearing loss in children. Int. J. Pediatr. Otorhinolaryngol. 77, 530–533 (2013).
Cinar, B. C., Batuk, M. O., Tahir, E., Sennaroglu, G. & Sennaroglu, L. Audiologic and radiologic findings in cochlear hypoplasia. Auris Nasus Larynx 44, 655–663 (2017).
Liu, Y. K. et al. The diagnostic value of measurement of cochlear length and height in temporal bone CT multiplanar reconstruction of inner ear malformation. Acta Otolaryngol. 137, 119–126 (2017).
Lim, C. H. et al. Bony cochlear nerve canal stenosis in pediatric unilateral sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 106, 72–74 (2018).
Ascha, M. S. et al. Vestibular aqueduct midpoint width and hearing loss in patients with an enlarged vestibular aqueduct. JAMA Otolaryngol. Head Neck Surg. 143(6), 601–608 (2017).
Sennaroğlu, L. & Bajin, M. D. Classification and current management of inner ear malformations. Balkan Med. J. 34, 397–411 (2017).
Propst, E. J. et al. Cochleovestibular anomalies in children with cholesteatoma. Laryngoscope. 118, 517–521 (2008).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. (2016).
Masuda, S. & Usui, S. Comparison of the prevalence and features of inner ear malformations in congenital unilateral and bilateral hearing loss. Int. J. Pediatr. Otorhinolaryngol. 125, 92–97 (2019).
Tahir, E., Bajin, M. D., Atay, G., Mocan, B. Ö. & Sennaroğlu, L. Bony cochlear nerve canal and internal auditory canal measures predict cochlear nerve status. J. Laryngol. Otol. 131, 676–683 (2017).
Gruber, M., Brown, C., Mahadevan, M. & Neeff, M. Concomitant imaging and genetic findings in children with unilateral sensorineural hearing loss. J. Laryngol. Otol. 131(8), 688–695 (2017).
Shah, J. et al. Evaluating diagnostic yield of computed tomography (CT) and magnetic resonance imaging (MRI) in pediatric unilateral sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 115, 41–44 (2018).
Friedman, A. B. et al. Risk analysis of unilateral severe-to-profound sensorineural hearing loss in children. Int. J. Pediatr. Otorhinolaryngol. 77(7), 1128–1131 (2013).
Paul, A. et al. Unilateral sensorineural hearing loss: medical context and etiology. Audiol. Neurootol. 22(2), 83–88 (2017).
Vilchez-Madrigal, L. D. et al. Children with unilateral cochlear nerve canal stenosis have bilateral cochleovestibular anomalies. Laryngscope 129, 2403–2408 (2019).
Chung, J. et al. Does the width of the bony cochlear nerve canal predict the outcomes of cochlear implantation?. Biomed Res. Int. 2018, 1–9 (2018).
Ropers, F. G. et al. Assessment of the clinical benefit of imaging in children with unilateral sensorineural hearing loss: a systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 145(5), 431–443 (2019).
Wazen, J. J. et al. Transcranial contralateral cochlear stimulation in unilateral deafness. Otolaryngol Head Neck Surg. 129, 248–254 (2003).
Lin, L. M. et al. Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol. Neurotol. 27, 172–182 (2006).
Peters, J. P. et al. CINGLE-trial: cochlear implantation for siNGLE-sided deafness, a randomised controlled trial and economic evaluation. BMC Ear Nose Throat Disord. 15, 3 (2015).
Marx, M. et al. Cochlear implantation as a treatment for single-sided deafness and asymmetric hearing loss: a randomized controlled evaluation of cost-utility. BMC Ear Nose Throat Disord. 19, 1 (2019).
Thomas, J. P., Neumann, K., Dazert, S. & Voelter, C. Cochlear implantation in children with congenital single-sided deafness. Otol. Neurotol. 38, 496–503 (2017).
Isaiah, A. et al. Clinical outcomes following cochlear implantation in children with inner ear anomalies. Int. J. Pediatr. Otorhinolaryngol. 93, 1–6 (2017).
Manara, R. et al. Brain magnetic resonance findings in symptomatic congenital cytomegalovirus infection. Pediatr. Radiol. 41, 962–970 (2011).
Rawlinson, W. D. et al. Neonates with congenital Cytomegalovirus and hearing loss identified via the universal newborn hearing screening program. J. Clin. Virol. 102, 110–115 (2018).
van Wieringen, A., Boudewyns, A., Sangen, A., Wouters, J. & Desloovere, C. Unilateral congenital hearing loss in children: challenges and potentials. Hear. Reas. 372, 29–41 (2019).
Acknowledgements
The Authors thank Dr. Martina Bradaschia for the English revision of the manuscript and Dr. Amanda Saksida for the support in the statistical analysis of the data.
Funding
The authors deny having received funds for the study.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiment: E.O., G.P. Collected data: G.P., M.G., R.M. Analysed data: L.T., G.P. Wrote the paper: E.O. Provided critical review of the paper: E.M., L.T. All Authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orzan, E., Pizzamiglio, G., Gregori, M. et al. Correlation of cochlear aperture stenosis with cochlear nerve deficiency in congenital unilateral hearing loss and prognostic relevance for cochlear implantation. Sci Rep 11, 3338 (2021). https://doi.org/10.1038/s41598-021-82818-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82818-9
This article is cited by
-
Evaluation of auditory pathway by EABR before cochlear implantation and the postoperative effect analysis
European Archives of Oto-Rhino-Laryngology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.