Abstract
The objective of this study was to determine if 15 weeks of resistance training (RT) can alter the levels of blood lipids, body iron status, and oxidative stress in postmenopausal women with vasomotor symptoms. Postmenopausal women enrolled in a randomised controlled trial were allocated to either a sedentary control group (n = 29) or a RT group (n = 26). Blood samples were taken at week-0 and week-15 for all participants. Blood lipids and iron status were measured via routine clinical analyses. Immunoassays were used to measure oxidative stress markers. The RT group, with good compliance, was associated with significant reductions in ferritin, total cholesterol, low-density lipoprotein, and non-high-density lipoprotein cholesterol. Moreover, ferritin was positively correlated with atherogenic lipids while negatively correlated with high-density lipoprotein in RT women. This occurred without alterations in serum iron, transferrin, transferrin-saturation, C-reactive protein and oxidative stress markers. No differences were found in control women. This study suggests that RT in postmenopausal women both reduces levels of ferritin and counteracts atherogenic lipid profiles independent of an apparent oxidative mechanism. RT may be a beneficial intervention in postmenopausal women via an interaction between ferritin and lipids; however, further investigation in a larger cohort is essential.
Similar content being viewed by others
Introduction
The risk for cardiovascular disease (CVD) approximately doubles in women during the 10 years after menopause, and physical inactivity contributes to this risk1,2. The increase in CVD risk is partially attributed to the reduced levels of oestrogens (oestradiol and oestrone) that increase high-density lipoprotein (HDL) levels and decrease low-density lipoprotein (LDL)3. Oestrogens also increase production of the vasodilator nitric oxide by means of nitric oxide-synthase activation, and possess antioxidant properties reducing levels of reactive oxygen species3. Oxidative stress levels are a risk factor for CVD and known to be greater in postmenopausal women, compared to women of fertile age, with higher levels of 4-hydroxynonenal (HNE), malondialdehyde, and oxidised LDL4. In addition, postmenopausal women have an increased body iron burden, in comparison to premenopausal women, that can contribute to iron-driven oxidative stress5. Ferritin, an iron storage protein, is increased in postmenopausal women and associated with the metabolic syndrome, subclinical coronary atherosclerosis6, and carotid atherosclerosis in postmenopausal women7.
Physical inactivity increases the risk for the development of CVD and all-cause mortality in postmenopausal women8. A comparative study between sedentary postmenopausal women and postmenopausal women who perform regular aerobic exercise showed a significantly lower level of iron burden, including serum ferritin, iron and transferrin saturation, and oxidative stress levels in the active women9. Aerobic exercise has also shown beneficial effects on lipid profiles in postmenopausal women, with reduced levels of total cholesterol, LDL and triglyceride, and a simultaneous increase in HDL10,11. Resistance training (RT) is a form of anaerobic physical activity, whereby muscle actions are designed to improve muscular fitness. Moderate aerobic exercise and RT have both shown beneficial effect in lowering inflammatory activity12. However, it is unknown whether RT causes a correlative change in lipid profile, body iron status, and oxidative stress in postmenopausal women.
The aim of this study was to determine if a RT based 15 weeks regime of physical exercise can alter the blood lipid profile, body iron burden and oxidative stress in a cohort of postmenopausal women. The hypothesis for this study is that 15 weeks of RT in postmenopausal women will improve the blood lipid profile by reducing LDL and increasing HDL, and to reduce body iron burden and oxidative stress levels.
Material and Methods
Study design and population
This study was an analysis of secondary outcomes of a randomised controlled intervention trial (registered as ID: NCT01987778), with a published protocol13, conducted at Linköping University Hospital, Sweden. The research protocol was approved by the Regional Ethical Review Board in Linköping (2013/285-31 and 2013/338-32), and the study was performed in accordance to the Declaration of Helsinki and applicable sections of Good Clinical Practice. Written informed consent was obtained from all participants prior entry to the trial. The primary outcome of this trial was to assess the effect of RT on reducing hot flush frequency in postmenopausal women, and has recently been published including full details on the study recruitment, randomisation, sample size, and intervention14. In this sub-analysis, investigations into RT-associated effects on blood lipids, iron status, and oxidative stress were assessed.
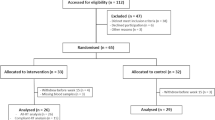
Postmenopausal women (n = 65) with vasomotor symptoms, describing hot flashes/flushing and night sweats, were recruited and randomly assigned to either control (sedentary) or intervention (RT) groups for a study period of 15 weeks (Fig. 1). Block randomisation and allocation concealment was performed by an independent statistician, and allocations were blinded to researchers performing analyses until the conclusion of the study. Postmenopausal status was confirmed in all participants, and full inclusion/exclusion criteria have previously been published13,14, and described in the Supplementary Materials and Methods. Anthropometric parameters, age, body mass index (BMI), blood pressure, haemoglobin levels, were recorded at the start of the 15 weeks RT program for all participants and presented in Table 1. Relevant medication use was recorded (Table 1), the medication use represented stable usage that had been used by the women for at least three months prior entry to the trial (Table 1). Active smoking status was also recorded, with one smoking participant in each group (Table 1). No significant differences were recorded at baseline between the control and RT groups of postmenopausal women.
Study flow chart. Postmenopausal women were recruited and randomly allocated to the intervention (n = 33) or control group (n = 32). Blood lipid and iron status analyses were included into the trial after initiation, thus fewer samples were available for these analyses. Two series of analyses were perfomed in the resistance training (RT) intervention group, all-RT and compliant-RT, based on the participants good compliance to the resistance training regime.
Intervention
The RT regime was comprised of 15 weeks structured RT, targeting all major muscles groups, performed three times per week. The regime consisted of six exercises in seated resistance machines and two body-weight exercises in the following order; chest press, leg press, seated row, leg curl, latissimus dorsi pull-down, leg extension, crunches and back raises. The seated exercises were performed in 15–20 repetitions in two sets week 1–3, and 8–12 repetitions in two sets week 4–15. Body-weight exercises were performed until exhaustion in two sets13,14. A physiotherapist instructed every woman at baseline and supervised one session per week to assess fidelity to the training schedule, which was adapted to each individual with gradually increased loads during the trial.
Personal physical activity logs, together with logs from the electronic card system at the designated gym, were recorded to track compliance. Established study criteria stipulated good compliance as an average of two or more RT sessions per week, excluding time-off due to illness13. Participants in the control group were instructed to remain sedentary, and not change their level of physical activity for the study period.
A total of 58 women completed the study, 29 controls and 29 in the RT group, with seven women being excluded due to withdrawal criteria or lack of compliance. In addition, plasma samples were missing for another three women in the RT group, giving a total of 26 in RT group. Within the RT group, the number of participants with good compliance to the RT protocol was 15 out of 26 women (58%), whereas 11 women had an average of below two RT sessions per week. The average number of RT sessions for all-RT, compliant-RT and non-compliant-RT women were 2.2, 2.6 and 1.7 sessions per week, respectively.
Fasting blood samples were taken at week-0 and again at week-15 of the study period for each participant. Within 1 hour of collection blood samples were centrifuged, 10 minutes at 1500 G, and plasma (EDTA) was immediately frozen and stored at −70 °C for later analysis of oxidative stress markers. Fasting blood samples, for a subset of participants, were also taken for direct analysis of blood lipids and iron status.
Blood lipids and iron status
A subset of participants (n = 21 for both control and RT groups) were available for analysis of blood lipids and iron status. Plasma was used for the analysis of blood lipids and iron status, including; cholesterol, LDL, HDL, triglycerides, apolipoprotein A1, apolipoprotein B, ferritin, iron, transferrin, and transferrin saturation. All analyses were performed via routine clinical analysis in an accredited laboratory (ISO/IEC 17025; Diagnostikcentrum i Östergötland, Linköping, Sweden). Methodological principles are described in the Supplementary Materials and Methods.
Oxidative stress markers
Total antioxidant capacity (TAC) was measured using the OxiSelect TAC Assay Kit (Cell Biolabs, San Diego, CA, USA). This assay measures the capacity of biomolecules within a sample to neutralise radicals, via a single electron transfer mechanism, based on the reduction of copper (II) to copper (I). The assay was performed according to manufacturer guidelines. Briefly, 20 µL of plasma samples were added to a 96-well plate. Initial absorbance was then measured at 490 nm. Copper (II) ions were then added to initiate the reaction, and after 2 minutes the absorbance was measured again at 490 nm. TAC was then determined by comparison with a set of uric acid standards.
HNE, a natural by-product of lipid peroxidation, was assayed using the OxiSelect HNE Adduct Competitive ELISA Kit (Cell Biolabs) according to manufacturer guidelines. Briefly, 50 µL of plasma samples were added to HNE conjugate coated 96-well plates and incubated at room temperature for 10 minutes. Antibody probing was performed using primary anti-HNE antibody, followed by secondary HRP-conjugated antibody, both of which were incubated sequentially at room temperature for one hour. Absorbance was measured spectrophotometrically at a wavelength of 450 nm. HNE concentration was determined using a standard curve of predetermined HNE-bovine serum albumin samples.
Protein carbonyl derivatives, common products of protein oxidation, were assayed using the OxiSelect Protein Carbonyl ELISA Kit (Cell Biolabs) according to manufacturer guidelines. Briefly, plasma samples containing 10 µg/mL of protein were absorbed onto a 96-well plate over two hours at 37 °C. Protein carbonyls in the samples were then derivatised to 2,4-dinitrophenylhydrazine (DNPH) via incubation at room temperature in the dark for 45 minutes. Antibody probing was performed using primary anti-DNP antibody, followed by secondary HRP-conjugated antibody, both of which were incubated sequentially at room temperature for 1 hour. Absorbance was measured spectrophotometrically at a wavelength of 450 nm. Protein carbonyl concentration was determined using a standard curve of predetermined reduced and oxidised bovine serum albumin samples.
Statistics
Data are expressed as either mean ± standard deviation (SD) or median ± quartile range, dependent on data distribution, unless otherwise stated. All statistical analyses were performed in SPSS v.24.0 (IBM, Portsmouth, UK). Two series of statistical analyses were performed, based on: (1) results from all RT participants who had measurements fom the baseline to 15 weeks regardless of the level of compliance (herein termed “all-RT ”), and (2) results from only RT participants with good compliance (herein termed “compliant-RT ”). Data comparisons between week-0 and week-15 in either control or RT groups were performed using non-parametric Wilcoxon-signed rank statistics. Comparisons between control and RT groups after 15 weeks were performed using non-parametric Mann-Whitney U test, by using a percentage of the week-15 values to the corresponding week-0 values (percentage change from baseline). Correlations between ferritin and blood lipids were calculated using Spearman’s correlation test. Correlations were run for both time-points individually, either week-0 or week-15, and also for values representing the change across the study period (week-15 minus week-0). A p-value < 0.05 was considered significant.
Results
RT resulted in significantly reduced levels of atherogenic lipids
A subset of participants was available for blood lipids and iron status analysis (n = 21 for both control and RT groups). No significant changes were observed from week-0 to week-15 regarding any of the lipid parameters in either control or in all-RT group (Table 2). However, significant decreases were observed in the compliant-RT group in total cholesterol, LDL, and non-HDL cholesterol (Table 2). At week-0, no statistical differences were seen between the groups (Supplementary Table S1). Among the non-complaint-RT women, there was no significant differences in lipid profiles (Supplementary Table S2).
RT significantly reduced the levels of plasma ferritin
Ferritin decreased significantly between week-0 and week-15 in the all-RT group (p = 0.01) and compliant-RT groups (p = 0.02, Table 2). Additionally, ferritin levels decreased significantly more in the all-RT and compliant-RT groups when compared to the control group (Fig. 2A) using values normalised to baseline (% of corresponding week-0 values). No significant differences were present for iron, transferrin or transferrin saturation between controls and RT groups at 0-weeks or 15-weeks or regarding change over the 15 weeks (Table 2 and Fig. 2B–D). There was no statistical difference in ferritin levels between control and RT groups at week-0 (Supplementary Table S1). Among the non-compliant-RT women, no significant differences were observed (Supplementary Table S2). Additionally, CRP levels were not found to differ significantly in all groups after 15 weeks (8.7 µg/mL for control, 8.6 µg/mL for all-RT, and 9.3 µg/mL for compliant-RT group).
15-weeks resistance training (RT) was associated with decreases in plasma ferritin levels but not the other iron status parameters in postmenopausal women. Postmenopausal women were randomised into either control (n = 21) or RT groups, presented as both all-RT (n = 21) and compliant-RT (n = 15). Body iron burden parameters were measured at week-0 and week-15 of the study period. Values were presented as percentage of corresponding week-0 values, median ± IQR. (A) ferritin. (B) iron, (C) transferrin and (D) transferrin saturation.
RT did not affect oxidative stress markers
The complete study cohort was available for oxidative stress analysis (n = 29 for control and n = 26 for RT). To compare controls with RT group after 15 weeks, the week-15 values were presented as a percentage of the corresponding 0-weeks values. We found no statistical difference between study groups in TAC, HNE or protein carbonyl (Supplementary Fig. S1).
Ferritin positively correlated with atherogenic lipid profiles and negatively correlated with HDL in RT women
Correlation analyses between ferritin and blood lipids were performed for control and both RT groups. Significant negative correlations were found between ferritin and HDL, in the all-RT group at week-0, and in all participant groups when analysing the change between week-0 and week-15 (Table 3). In the all-RT group, there were significant positive correlations between ferritin and triglycerides, non-HDL, and apolipoprotein-B when analysing the change between week-0 and week-15 (Table 3).
Discussion
Physical activity interventions are known to reduce the risk of developing CVD in postmenopausal women, likely by altering lipid profiles or improving antioxidant capacity and decreasing body iron burden9,10,11. However, it is unclear whether RT in postmenopausal women can lead to correlative changes in iron metabolism, lipid profile, and oxidative stress, which have been separately linked to the ferritin related cardiometabolic conditions. Our study shows that 15 weeks of RT not only significantly reduced the levels of ferritin, but also levels of total cholesterol, LDL cholesterol, and non-HDL cholesterol. Moreover, among RT women correlative changes were found between ferritin levels and atherogenic lipid profiles.
Physical inactivity and onset of menopause are known risk factors for CVD in women15,16. It has previously been shown that exercise with 10 weeks Nordic walking or a 3-month high-intensity aerobic training have beneficial effects on lipid profiles amongst postmenopausal women10,11. The majority of RT trials in postmenopausal women have primarily investigated overweight/obese postmenopausal women, and results regarding lipid profiles are contradictory with some trials showing the benefits of RT17, whilst others show no benefit18 or no additional benefit in combined aerobic and RT trials19. The present study demonstrated that 15 weeks of RT with good compliance had beneficial effects on lipid profiles of total cholesterol, LDL cholesterol, and non-HDL cholesterol in the overall population.
Ferritin has been associated with several cardiometabolic conditions including dyslipidaemia, hypertension, obesity, and insulin resistance, although the mechanisms by which they are associated are still not fully understood. Ferritin is an iron storage protein and increased serum ferritin levels have been associated with onset of coronary heart disease20, myocardial infarction, and coronary artery disease related mortality21. High levels of serum ferritin was found to be associated with increased risk of stroke in postmenopausal women22, as well as with metabolic syndrome23. In addition, ferritin is also recognised as an acute phase marker protein and is potentially higher during infection or inflammation. Ferritin was found to be positively associated with CRP in patients with type 2 diabetes24. In the present study, we found that RT trained women had reduced levels of ferritin, but no change in other parameters associated with iron metabolism (iron, transferrin and transferrin saturation), nor in the levels of CRP and oxidative stress markers. This may suggest that the RT-associated reduction in ferritin levels are unrelated to its function in iron metabolism or oxidative stress mechanisms. However, RT-associated effects on low grade inflammation via lowering ferritin cannot be excluded and warrant further study.
Ferritin levels have been associated with atherogenic lipid profiles in previous studies. It has been reported a negative correlation between levels of serum ferritin and HDL-cholesterol, while positive correlations between levels of serum ferritin and total cholesterol, triglyceride, and LDL-cholesterol25. In the present study, for the first time, we demonstrate that ferritin in postmenopausal women participating in RT over 15 weeks was positively correlated with atherogenic lipids including triglycerides, non-HDL and apolipoprotein-B, while negatively correlated with HDL. These results may indicate a mechanistic link between RT mediated changes of iron and lipid metabolisms among postmenopausal women.
The molecular mechanism behind the association between increased ferritin and metabolic syndrome and CVD is not yet fully understood. A possible mechanism is that iron serves as a catalyst for free radical reactions in oxidative stress in atherogenesis and metabolic syndrome26. Moderate exercise and an active lifestyle have been demonstrated to be beneficial for not only prevention of oxidative stress but also protection of cardiovascular related morbidity27, type II diabetes, metabolic syndrome28, and Alzheimer’s disease29. However, inconsistent findings have been reported frequently in the area. A recent study showed that both aerobic exercise and RT can increase lipid peroxidation30. In contrast, another study on aerobic exercise documented a decrease in lipid peroxidation31. In the present study, we found no significant difference in levels of studied markers of oxidative stress after 15 weeks RT exercise. This suggests that effect of RT seen in the present study may depend on other functions of ferritin in the development of cardiometabolic disorders, possibly energy metabolism7. However, the muscle specific response could be different. It is known that skeletal muscle fibres produce free-radicals during exercise32. Previous correlations between malondialdehyde, a pro-oxidative stress marker, and RT-induced changes in skeletal muscle have been seen in healthy older women33. Thus, the oxidative stress mechanisms in the local muscle environment should not be ruled out, and be considered for future studies.
There are limitations in the current study that need to be considered. The primary outcome for this trial was to investigate the effects of RT on vasomotor symptoms in postmenopausal women, and as such power calculations were based on this primary outcome. Thus, the investigations into RT-associated effects on iron metabolism, oxidative stress, and lipid profiles were not the primary outcome in this clinical cohort, rather a pilot clinical trial. Therefore, the participant number included is relatively low. Participants’ dietary intake were not recorded that may affect our results, particularly on the oxidative stress markers with respect to levels of iron and antioxidants consumed. Thus, additional studies with a larger cohort including recording of dietary intake of iron and antioxidants are warranted.
Conclusions
The present study shows that postmenopausal women who engaged in supervised RT over a 15-week period had significantly lowered levels of ferritin and, with good compliance, atherogenic lipids. The correlations of ferritin with atherogenic lipids or HDL may suggest a mechanistic link between lipid and ferritin function mediated by RT in postmenopausal women. The RT-associated changes in ferritin levels were not associated with changes in iron, CRP and oxidative stress levels. The results indicate that RT in postmenopausal women may have beneficial outcomes in preventing atherosclerosis and reducing CVD risk. However, these findings need to be confirmed in a large-scale cohort.
Data availability
All data are summarised in this published article, and the raw datasets are available from the corresponding authors or reasonable request.
References
Lisabeth, L. & Bushnell, C. Menopause and Stroke: An Epidemiologic Review. Lancet neurol. 11, 82–91, https://doi.org/10.1016/S1474-4422(11)70269-1 (2012).
Mosca, L. et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update. Circulation 123, 1243 (2011).
Stice, J. P., Lee, J. S., Pechenino, A. S. & Knowlton, A. A. Estrogen, aging and the cardiovascular system. Future cardiol. 5, 93–103, https://doi.org/10.2217/14796678.5.1.93 (2009).
Signorelli, S. S. et al. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas 53, 77–82, https://doi.org/10.1016/j.maturitas.2005.03.001 (2006).
Yuan, X. M. & Li, W. The iron hypothesis of atherosclerosis and its clinical impact. Ann. Med. 35, 578–591, https://doi.org/10.1080/07853890310016342 (2003).
Seo, S. K. et al. Association of serum ferritin levels with metabolic syndrome and subclinical coronary atherosclerosis in postmenopausal Korean women. Clin. Chim Acta 438, 62–66, https://doi.org/10.1016/j.cca.2014.07.042 (2015).
Ma, H. et al. Serum ferritin levels are associated with carotid atherosclerosis in Chinese postmenopausal women: the Shanghai Changfeng Study. Br. J. Nutr. 114, 1064–1071, https://doi.org/10.1017/S0007114515001944 (2015).
Oguma, Y., Sesso, H., Paffenbarger, R. & Lee, I. Physical activity and all cause mortality in women: a review of the evidence. Br. J. Sports Med. 36, 162–172, https://doi.org/10.1136/bjsm.36.3.162 (2002).
Bartfay, W. & Bartfay, E. A Case–Control Study Examining the Effects of Active Versus Sedentary Lifestyles on Measures of Body Iron Burden and Oxidative Stress in Postmenopausal Women. Biol. Res. Nurs. 16, 38–45, https://doi.org/10.1177/1099800413501717 (2013).
Hagner-Derengowska, M. et al. Effects of Nordic Walking and Pilates exercise programs on blood glucose and lipid profile in overweight and obese postmenopausal women in an experimental, nonrandomized, open-label, prospective controlled trial. Menopause 22, 1215–1223, https://doi.org/10.1097/gme.0000000000000446 (2015).
Mandrup, C. M. et al. Effects of high-intensity training on cardiovascular risk factors in premenopausal and postmenopausal women. Am. J. Obstet. Gynecol. 216, 384.e381–384.e311, https://doi.org/10.1016/j.ajog.2016.12.017 (2017).
Soares, F. H. R. & de Sousa, M. B. C. Different Types of Physical Activity on Inflammatory Biomarkers in Women With or Without Metabolic Disorders: A Systematic Review. Women & Health 53, 298–316, https://doi.org/10.1080/03630242.2013.782940 (2013).
Berin, E., Hammar, M. L., Lindblom, H., Lindh-Åstrand, L. & Spetz Holm, A.-C. E. Resistance training for hot flushes in postmenopausal women: Randomized controlled trial protocol. Maturitas 85, 96–103, https://doi.org/10.1016/j.maturitas.2015.12.015 (2016).
Berin, E. et al. Resistance training for hot flushes in postmenopausal women: A randomised controlled trial. Maturitas 126, 55–60, https://doi.org/10.1016/j.maturitas.2019.05.005 (2019).
Humphries, K. H. et al. Sex differences in cardiovascular disease – Impact on care and outcomes. Front. Neuroendocrinol. 46, 46–70, https://doi.org/10.1016/j.yfrne.2017.04.001 (2017).
Elhakeem, A. et al. Physical Activity, Sedentary Time, and Cardiovascular Disease Biomarkers at Age 60 to 64 Years. J. Am. Heart Assoc. 7, e007459–e007459, https://doi.org/10.1161/JAHA.117.007459 (2018).
Wooten, J. S. et al. Resistance Exercise and Lipoproteins in Postmenopausal Women. Int. J. Sports Med. 32, 7–13, https://doi.org/10.1055/s-0030-1268008 (2011).
Mohanka, M. et al. Serum Lipoproteins in Overweight/Obese Postmenopausal Women: A One-Year Exercise Trial. Med. Sci. Sports Exerc. 38, 231–239, https://doi.org/10.1249/01.mss.0000184584.95000.e4 (2006).
Rossi, F. E. et al. Combined Training (Aerobic Plus Strength) Potentiates a Reduction in Body Fat but Demonstrates No Difference on the Lipid Profile in Postmenopausal Women When Compared With Aerobic Training With a Similar Training Load. J. Strength Cond. Res. 30, 226–234, https://doi.org/10.1519/jsc.0000000000001020 (2016).
Ahluwalia, N. et al. Iron Status Is Associated with Carotid Atherosclerotic Plaques in Middle-Aged Adults. J. Nutr. 140, 812–816, https://doi.org/10.3945/jn.109.110353 (2010).
Salonen, J. T. et al. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 86, 803–811, https://doi.org/10.1161/01.cir.86.3.803 (1992).
van der, D. L. et al. Serum Ferritin Is a Risk Factor for Stroke in Postmenopausal Women. Stroke 36, 1637 (2005).
Jehn, M., Clark, J. M. & Guallar, E. Serum Ferritin and Risk of the Metabolic Syndrome in U.S. Adults. Diabetes Care 27, 2422 (2004).
Alam, F., Fatima, F., Orakzai, S., Iqbal, N. & Fatima, S. S. Elevated levels of ferritin and hs-CRP in type 2 diabetes. J. Pak. Med. Assoc. 64, 1389–1391 (2014).
Ellidag, H. Y. et al. The relationship between serum ferritin levels and serum lipids and HDL function with respect to age and gender. Ukr. Biochem. J. 88, 76–85 (2016).
Yuan, X.-M. & Li, W. Iron Involvement in Multiple Signaling Pathways of Atherosclerosis: A Revisited Hypothesis. Curr. Med. Chem. 15, 2157–2172, https://doi.org/10.2174/092986708785747634 (2008).
Orkaby, A. R. & Forman, D. E. Physical activity and CVD in older adults: an expert’s perspective. Expert Rev. Cardiovasc. Ther. 16, 1–10, https://doi.org/10.1080/14779072.2018.1419062 (2018).
Church, T. Exercise in Obesity, Metabolic Syndrome, and Diabetes. Progress in Cardiovasc. Dis. 53, 412–418, https://doi.org/10.1016/j.pcad.2011.03.013 (2011).
DeWeerdt, S. Prevention: Activity is the best medicine. Nature 475, S16, https://doi.org/10.1038/475S16a (2011).
Carteri, R. B. et al. Acylated Ghrelin and Circulatory Oxidative Stress Markers Responses to Acute Resistance and Aerobic Exercise in Postmenopausal Women. J. Phys. Act. Health 13, 632–639, https://doi.org/10.1123/jpah.2015-0413 (2016).
Karolkiewicz, J. et al. Response of oxidative stress markers and antioxidant parameters to an 8-week aerobic physical activity program in healthy, postmenopausal women. Arch. Gerontol. Geriatr. 49, e67–e71, https://doi.org/10.1016/j.archger.2008.09.001 (2009).
Bailey, D. M. et al. Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free Radic. Res. 41, 182–190, https://doi.org/10.1080/10715760601028867 (2007).
Carru, C. et al. Markers of oxidative stress, skeletal muscle mass and function, and their responses to resistance exercise training in older adults. Exp. Gerontol. 103, 101–106, https://doi.org/10.1016/j.exger.2017.12.024 (2018).
Acknowledgements
The work was supported by grants from the Swedish Research Council (VR), Olle Engkvist Foundation, Swedish Gamla Tjänarinnor Foundation, and Linköping University Hospital Research Fund. The authors would like to thank research nurses Åsa Rydmark Kersley and Linda Shosholli for their support with blood sampling and meta-data. The authors would also like to thank Associate Professor Xi-Ming Yuan for his feedback and input in critiquing the manuscript. Open access funding provided by Linköping University.
Author information
Authors and Affiliations
Contributions
M.H., L.L.Å., M.R., A.C.S.H. and W.L. conceived idea and conception. L.J.W. and W.L. contributed to the design of the experiments. H.L. supervised the intervention. E.B. and H.L. curated participant data. L.J.W. performed experiments and data acquisition. L.J.W. and W.L. performed statistical analyses. L.J.W. and W.L. wrote the first draft of the manuscript. L.J.W., M.H., L.L.Å., E.B., H.L., M.R., A.C.S.H. and W.L. edited and revised manuscript, and approved the final version of the manuscript. M.H. and W.L. ensured financial support for the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ward, L.J., Hammar, M., Lindh-Åstrand, L. et al. Does resistance training have an effect on levels of ferritin and atherogenic lipids in postmenopausal women? – A pilot trial. Sci Rep 10, 3838 (2020). https://doi.org/10.1038/s41598-020-60759-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60759-z
This article is cited by
-
Resistance training decreases plasma levels of adipokines in postmenopausal women
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.