Abstract
Neonatal sepsis is characterised by dysregulated immune responses. Lipid mediators (LMs) are involved in the regulation of inflammation. Human recombinant thrombomodulin (rhTM), an anticoagulant, has anti-inflammatory effects and might be useful for sepsis treatment. A stock caecal slurry (CS) solution was prepared from adult caeca. To induce sepsis, 1.5 mg/g of CS was administered intraperitoneally to 4 d-old wild-type FVB mouse pups. Saline (Veh-CS) or rhTM (3 or 10 mg/kg; rhTM3-CS or rhTM10-CS) was administered subcutaneously 6 h prior to sepsis induction, and liver LM profiles at 3 and 6 h post-sepsis induction and survival up to 7 days were examined. Mortality was significantly lower (47%) in the rhTM3-CS group and significantly higher (100%) in the rhTM10-CS group, compared with the Veh-CS group (79%, p < 0.05). Eleven LMs (12-HEPE, EPA, 14-HDHA, DHA, PD1, PGD2, 15d-PGJ2, 12S-HHT, lipoxin B4, 12-HETE, AA) were significantly increased at 3 h, and five LMs (5-HEPE, 15-HEPE, 18-HEPE, 17-HDHA, PD1) were significantly increased at 6 h post-sepsis induction. Increased EPA, DHA, 12S-HHT, lipoxin B4, and AA were significantly suppressed by rhTM pre-treatment. rhTM was protective against neonatal sepsis. This protective effect might be mediated via LM modulation. Further post-sepsis studies are needed to determine clinical plausibility.
Similar content being viewed by others
Introduction
Neonatal sepsis is characterised by systemic bacterial invasion followed by a massive inflammatory response. The mortality rate of neonatal sepsis is variable and was reported to be ≥35%1. Of note, the mortality rate of sepsis in preterm infants is two-fold higher than that in term infants. In addition, preterm sepsis was reported to be associated with adverse neurodevelopmental outcomes, such as cerebral palsy, hearing impairment, and neurodevelopmental impairment1. Although the pathophysiology of neonatal sepsis is not fully elucidated, altered premature immune responses have been hypothesised to play a crucial role2,3. In addition to the well-characterised functions of inflammatory cytokines and chemokines, bioactive lipid mediators (LMs) were recently discovered to be key signalling molecules that regulate the inflammatory profile4. LMs are biosynthesised through specific metabolic pathways and exert various bioactive effects through their specific receptors, which have two opposite functions: inflammatory and anti-inflammatory5,6. The anti-inflammatory function of LMs is reportedly associated with the resolution of inflammation, and the dysregulated balance of LMs has attracted attention as a pathway involved in the pathogenesis of sepsis4.
Currently, there are guidelines for the treatment of sepsis in adults and children, including term newborns; however, there are no established guidelines for the treatment of sepsis in preterm infants1. Single antibiotic therapy for sepsis in very low birth weight infants is ineffective, and adjunctive immunotherapy, such as granulocyte stimulating factor and immunoglobulin treatment, did not improve the long-term outcome of preterm sepsis7. In adult sepsis, novel therapeutic options such as interleukin-22, anti-high mobility group box 1 (HMGB1) monoclonal antibody, vascular endothelial growth factor receptor monoclonal antibodies, and recombinant human thrombomodulin (rhTM) are the focus of preclinical investigation8. Furthermore, low-dose corticosteroids9, orally administered protease inhibitors10, and mesenchymal stem cell therapy11 are currently being investigated in clinical trials. However, no such trials have been initiated for neonatal sepsis.
The biological agent rhTM was approved and is used clinically for disseminated intravascular coagulation (DIC) treatment in Japan12,13. The effects of rhTM on DIC were previously established in a multicentre randomised controlled trial14, which showed that the beneficial effect was associated with a reduction in mortality in adult sepsis patients with DIC12. To exert its anticoagulant effect, rhTM forms a complex with thrombin to inhibit clotting activity, and the thrombin-rhTM complex itself converts protein C (PC) to activated protein C (APC), which selectively inactivates activated factor V (Va) or activated factor VIII (VIIIa). This results in the inhibition of further thrombin formation15. Recently, the pleiotropic and anti-inflammatory effects of rhTM have attracted attention. These include inhibition of intercellular adhesion molecule-1 expression (ICAM-1), activation of protease activated receptor-1 (PAR-1)15, neutralisation of endotoxins, and adsorptive dissolution of HMGB-1 proteins16. Because rhTM has anti-coagulative and anti-inflammatory effects, it is regarded as a useful therapeutic agent for sepsis. Importantly, Shirahata et al. reported post-marketing surveillance data regarding the use of rhTM in neonatal DIC in Japan; these data showed that its safety and effectiveness were similar to those in paediatric and adult patients with DIC17.
However, the efficacy of rhTM for the treatment of sepsis in preterm infants has not been determined. In addition, although the target of LMs is presumed to be the pathology of sepsis, there has been no human or animal study on the effects of LMs in neonatal sepsis.
Therefore, to clarify the effect of rhTM on sepsis in preterm infants, we investigated the protective effect of subcutaneous rhTM administration in a non-surgical preterm sepsis mouse model, and investigated the dynamics of LMs in this model.
Methods
Animals
Adult FVB/NJcl mouse breeders were obtained from CLEA Japan, Inc. (Tokyo, Japan) and provided a standard rodent diet and water ad libitum. All pups were kept with their mothers throughout the course of the study. The pups were randomised on an individual basis within each litter for each experiment. At least three different litters were used for each experimental group to eliminate any litter bias effects. For identification, the back of each pup was labelled using a Sharpie marker. This study was carried out in accordance with the ARRIVE guidelines and performed as approved by the Kobe University Institutional Animal Care and Use Committee (Protocol P160608).
Bacterial viability of the caecal slurry (CS) stock
As described previously2, a single stock CS solution was prepared from adult caeca, and then stored at −80 °C in 1-mL aliquots until use. At each sepsis induction, an aliquot of stock CS was thawed at room temperature, and 50 µl was plated onto 1.5% agar containing brain/heart infusion (BHI) broth18. Agar plates were then incubated at 37 °C for 24 h and colony-forming units (CFUs) were counted. For the entire study, the mean CFU count was 5.2 ± 1.2 × 105 CFU/mL.
Sepsis induction
To induce sepsis, 4-day-old mouse pups, which are immunologically equivalent to human preterm infants2,3, were given various doses of CS intraperitoneally (IP), and then closely monitored daily for health and survival for up to 7 days, based on the method used in a previous report18. For the following experiment, we used a CS dose of LD80 for sepsis induction, based on the approach in our previous studies2,3.
Recombinant human thrombomodulin (rhTM) treatment
To determine the safety of rhTM in neonatal pups, we administered 10.0 mg/kg of rhTM subcutaneously (SC) to 4-day-old mice and monitored survival for 7 days. Injection of 10.0 mg/kg of rhTM SC to 4-day-old mice did not induce mortality for up to 7 days (100% survival, n = 10). At 6 h before-sepsis induction, we administered 3.0 mg/kg of rhTM (rhTM3-CS), 10.0 mg/kg of rhTM (rhTM10-CS), or an equivalent volume of normal saline (Veh-CS) SC to 4-day-old mice, based on the method used in a previous report19. The timing of rhTM SC administration was determined based on a pharmacokinetic study to achieve a peak concentration at sepsis induction. In a pharmacokinetic study of adult rats, a single subcutaneous injection of 3.0 mg/kg of rhTM increased blood concentration slowly, such that it reached sufficient levels between 3 and 9 hours after administration20. At 6 h after rhTM administration, sepsis was induced by IP administration of 1.5 mg/g body weight (BW) CS; BW changes and survival were then monitored for 7 days. Blood gas and liver LM analysis was performed at 3 and 6 h post-sepsis induction based on the methods used in previous reports21,22.
Blood gas parameters
At 3 h post-sepsis induction, pups were sacrificed by decapitation under room air and 30 to 80 µL of blood pooled from two or three pups was immediately collected in capillary blood collection tubes containing lithium heparin (Capiject®; Terumo Medical Corporation, Tokyo, Japan). All measurements were performed using an ABL 90 FLEX blood gas analyser (Radiometer Medical ApS, Copenhagen, Denmark).
Measurement of lipid mediators
At 3 and 6 h post-sepsis induction, pups were sacrificed and approximately 5 × 5 × 1 mm pieces from freshly harvested livers were placed in liquid nitrogen and then stored at −80 °C until use. The liver samples were homogenised in ice-cold methanol. Then, the deuterated internal standards d4-leukotriene B4 (d4-LTB4), d8-5-hydroxyeicosatetraenoic acid (d8-5-HETE), d4-prostaglandin E2 (d4-PGE2), and d5-resolvin D2 (d5-RvD2), which represented each chromatographic region of the identified LMs, were added to the samples (500 pg each) to facilitate quantification. Samples underwent solid phase extraction (SPE) on C18 columns and were subjected to LC-MS/MS. The system consisted of a Qtrap 6500 (Sciex, Framingham, MA, USA) equipped with a Shimadzu LC-30AD HPLC system. A ZORBAX Eclipse Plus C18 column (100 mm × 4.6 mm, 3.5 µm, Agilent Technologies, Santa Clara, CA, USA) was used with a gradient of methanol/water/acetic acid from 55:45:0.01 (v/v/v) to 98:2:0.01 at a flow rate of 0.4 ml/min. A multiple reaction monitoring (MRM) method was developed for the signature ion pairs Q1 (parent ion)/Q3 (characteristic fragment ion) of each molecule to monitor and quantify the levels of targeted LMs. The LMs were identified using published criteria, including LC retention time, specific fragmentation patterns, and diagnostic fragmentation ions. The samples were quantified based on the peak area of the MRM chromatograph, and linear calibration curves were obtained with authentic standards for each compound5,23.
Statistical analyses
Statistical analyses were performed using log-rank tests for Kaplan–Meier survival curves, unpaired Student’s two-tailed t-test, Mann–Whitney U-test or Chi-square test for comparisons between two groups. Statistical analyses were performed using GraphPad Prism 7.0 software (Graphpad Software, Inc., San Diego, CA, USA). Differences were deemed statistically significant when p < 0.05.
Results
Effect of rhTM on the severity of sepsis
We randomly assigned mouse pups into four groups: non-septic control (vehicle [Veh]-pretreated normal saline IP administration, Veh-Veh) group, Veh-treated septic (Veh-CS) group, and rhTM pretreated septic (rhTM3-CS or rhTM10-CS) groups, based on the method used in a prior study24.
BW Change
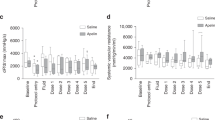
When comparing the BW gain at 24 h post-sepsis induction of surviving pups only, there were no significant differences among the Veh-CS (1.7 ± 4.1%, n = 5), rhTM3-CS (1.9 ± 4.3%, n = 11), and rhTM10-CS (2.3 ± 1.8%, n = 6) groups. However, the BW gain of these groups was significantly lower than the BW gain of the non-septic Veh-Veh group (28.9 ± 4.9%, n = 7, p < 0.0001, Fig. 1).
Body weight gain at 24 h post-sepsis induction. Body weight gain of surviving pups only is shown: vehicle-treated septic (Veh-CS) group (1.7 ± 4.1%, n = 5), 3.0 mg/kg of rhTM pretreated septic (rhTM3-CS) group (1.9 ± 4.3%, n = 11), 10.0 mg/kg of rhTM pretreated septic (rhTM10-CS) group (2.3 ± 1.8%, n = 6), and non-septic control (Veh-Veh) group (28.9 ± 4.9%, n = 7). The body weight changes of Veh-CS, rhTM3-CS, and rhTM10-CS groups were significantly smaller than the body weight change of the Veh-Veh group. *p < 0.0001.
Blood gas
At 3 h post-sepsis induction, lactate levels, anion gaps, and blood glucose were significantly higher in pups of the Veh-CS group than in the Veh-Veh controls (p < 0.05, Table 1). However, blood glucose was significantly higher and blood pH indicated significantly lower alkalinity in the rhTM3-CS and rhTM10-CS groups, compared with the Veh-Veh controls (p < 0.05, Table 1). In addition, lactate levels and anion gaps were significantly lower and pCO2 was significantly higher in the rhTM10-CS group than in the Veh-CS group (p < 0.05, Table 1). There were no significant differences in blood gas parameters between the rhTM3-CS and rhTM10-CS groups.
Mortality
The mortality rates of Veh-CS-treated pups (79%, n = 11), rhTM3-CS-treated pups (47%, n = 17), and rhTM10-CS-treated pups (100%, n = 16) were significantly higher than the mortality rate of Veh-Veh-treated pups (0%, n = 7, p = 0.003, p = 0.04, and p < 0.0001, respectively). In addition, mortality was significantly lower in the rhTM3-CS group than in the Veh-CS-treated group (p = 0.03, Fig. 2). Conversely, mortality was significantly higher in the rhTM10-CS-treated group than in the Veh-CS-treated group (p = 0.02, Fig. 2).
Kaplan–Meier survival plots of 4 d-old pups pretreated with 3.0 mg/kg of rhTM (rhTM3-CS: ●, n = 17), 10.0 mg/kg of rhTM (rhTM10-CS: ▲, n = 16), or equivalent volume of normal saline (Veh-CS: ○, n = 11) subcutaneously at 6 h pre-sepsis induction. The mortality was significantly lower in the rhTM3-CS group (47%) and significantly higher in the rhTM10-CS group (100%), compared with the Veh-CS group (79%).
Effect of rhTM on lipid mediators
While the administration of 3.0 mg/kg rhTM resulted in improved survival, injection of 10.0 mg/kg did not show a protective effect; thus, the following studies were conducted using only 3.0 mg/kg rhTM. At 3 h post-sepsis induction, the livers of Veh-CS-treated pups contained two eicosapentaenoic acid (EPA)-derived LMs (12-HEPE, EPA), three docosahexaenoic acid (DHA)-derived LMs (14-HDHA, DHA, PD1), and six arachidonic acid (AA)-derived LMs (PGD2, 15 d-PGJ2, 12S-HHT, lipoxin B4, 12-HETE, AA), which were significantly increased compared with the Veh-Veh-treated group. At 6 h post-sepsis induction, three EPA-derived LMs (5-HEPE, 15-HEPE, 18-HEPE) and two DHA-derived LMs (17-HDHA, PD1) were significantly increased in livers of Veh-CS-treated pups, compared with Veh-Veh-treated pups (Table 2, Supplementary Table S1).
The significant increases of these LMs, including EPA, DHA, 12S-HHT, lipoxin B4, and AA, at 3 h post-sepsis induction were significantly suppressed by rhTM pre-treatment. Moreover, only EPA was significantly increased at 6 h post-sepsis induction in pups that received rhTM pre-treatment (Table 2, Supplementary Table S1).
Changes of LM levels compared with baseline
The change in LM level from baseline (non-septic control) was abbreviated as Δ LMs. The levels of Δ EPA, Δ DHA, Δ AA, Δ 12S-HHT, and Δ lipoxin B4 were significantly lower in the rhTM pre-treatment group than in the Veh-CS-treated group at 3 h post-sepsis induction. However, Δ EPA in the rhTM pre-treatment group was significantly higher than that in the Veh-CS-treated group at 6 h post-sepsis induction (Table 3, Supplementary Table S2).
Discussion
In this study, there were two important findings. First, subcutaneous rhTM administration attenuated sepsis severity in a non-surgical preterm sepsis mouse model. Second, levels of several inflammatory LMs were increased at 3 and 6 h post-sepsis induction, and these increases were partially suppressed by rhTM pre-treatment.
In our preterm sepsis mouse model, subcutaneous administration of 3 mg/kg of rhTM at 6 h prior to sepsis induction led to improvements in the blood gas parameters and mortality rate. In contrast, subcutaneous administration of 10 mg/kg of rhTM worsened the overall mortality, despite significant improvement of blood gas parameters at 3 h post-sepsis induction. To date, there have been several reports investigating the effect of rhTM in septic animal models. Nagato et al. reported that the intravenous administration of 1 mg/kg of rhTM 30 min prior to sepsis induction significantly improved sepsis survival, and suppressed inflammatory cytokines and HMGB1 elevation in an LPS-treated rat sepsis model16. Takehara et al. reported that the intravenous administration of 3 mg/kg of rhTM 30 min prior to sepsis induction improved sepsis survival, and suppressed the elevation of inflammatory cytokines and HMGB1 in serum and ascites in an LPS-treated mouse sepsis model25. However, these studies used adult rodent sepsis models, and there has been no report investigating the effects of rhTM in an animal model of neonatal sepsis. In addition, these studies utilised models of sepsis induced by LPS administration; however, sepsis induction using this model might not truly reflect the pathophysiology of human sepsis because LPS induces endotoxemia or systemic inflammation, but does not induce sepsis26. Furthermore, in LPS models, activation of the innate immune system can only have deleterious effects, whereby any intervention that blunts the inflammatory response can be beneficial; in contrast, sepsis in human patients is triggered by an infectious process in which immunological responses can be both beneficial and deleterious27. Thus, the caecal ligation and puncture model has been widely used because it closely resembles the progression and characteristics of human sepsis; however, newborn pups do not tolerate this surgically invasive procedure28. Here, we established a mouse model of non-surgical preterm sepsis using the CS method established by Wynn et al.29 and CS stock preparation protocol established by Starr et al.18. This is a simple technique consisting of intraperitoneal CS administration to 4-day-old mouse pups, an age immunologically equivalent to human preterm infants30. Similar to the caecal ligation and puncture model, the advantages of this model are the existence of an infection focus (i.e., an abdominal abscess) and its polymicrobial nature.
Regarding the dynamics of post-sepsis LMs, two EPA-, three DHA-, and six AA-derived LMs were increased 3 h post-sepsis induction, and six EPA- and two DHA-derived LMs were increased 6 h post-sepsis induction. Recently, the roles of LMs in maintenance of inflammation and their convergence have been reported in various inflammatory diseases. LMs exert various bioactive effects through their specific receptors, and are categorised as inflammatory LMs and anti-inflammatory LMs, which have diametrically opposite effects. Anti-inflammatory LMs are regarded as important pro-resolution mediators. The protective effect of anti-inflammatory LM administration was reported in an adult sepsis model mice31,32. Regarding LM dynamics in adult sepsis patients, Dalli et al. showed that inflammatory LMs, such as PGF2α, and anti-inflammatory LMs, including RvD5 and PD1, were significantly increased in non-survivors, compared with survivors. Based on these findings, they speculated that the interaction of inflammatory and anti-inflammatory LMs might have a key role in sepsis33. However, there have been no reports regarding the regulation and function of LM dynamics in neonatal sepsis. Here, we clarified the involvement of LMs in the pathogenesis of a preterm sepsis mouse model, and suggest that the protective effect of rhTM in this model might be mediated by LM regulation.
In human studies, rhTM administration was reported to improve the survival rate in DIC associated with sepsis in an adult population13, and this has been proven in systematic reviews34. Regarding the therapeutic use of rhTM for neonates, its efficacy and safety in neonatal DIC were similar to those of adults17. Thus, rhTM might be an option for the treatment of neonatal sepsis.
In this study, administration of 3 mg/kg of rhTM significantly suppressed increases in five LMs at 3 h post-sepsis induction, although these LMs rebounded at 6 h post-sepsis induction. These findings suggest that the protective effect of rhTM is transient and that a sufficient blood concentration cannot be maintained with a dose of 3 mg/kg of rhTM. Thus, a higher dose is needed to maintain the blood rhTM concentration at a sufficient level; accordingly, we examined the effects of a higher dose of rhTM (10 mg/kg). We found that the mortality was significantly increased, such that it was higher than the rate in the Veh-CS-treated septic group. This might be due to the coagulation abnormality caused by an excessive concentration of rhTM. Recently, there have been reports regarding a fusion protein of TM [single-chain variable fragment antibody (scFv)/TM] that targets red blood cells, resulting in extended circulation time and an enhanced TM effect35,36. scFv/TM has more potent anti-inflammatory and anticoagulant effects than soluble TM (sTM); thus, scFV/TM shows an efficacy similar to that of sTM at 50-fold lower doses37. A single systemic injection of scFv/TM showed remarkably superior outcomes, compared with those of sTM, in animal models of thrombosis and sepsis38. In particular, scFv/TM provided both prophylactic and therapeutic protective effects in a mouse model of sepsis, whereas sTM provided inferior prophylactic effects and failed to provide therapeutic effects37. Thus, further studies are needed using scFv/TM in our neonatal sepsis model; we speculate that scFV/TM might be able to suppress the inflammatory LM rebound observed at 6 h post-sepsis induction.
This study had some limitations. First, we did not measure the coagulation and fibrinolytic functions. Regarding coagulopathy, previous studies with sepsis patients reported side effects of rhTM based on the presence or absence of bleeding symptoms17,34. In this study, we did not observe bleeding, even in neonatal pups treated with a high dose (10.0 mg/kg) of rhTM. However, significant mortality was observed in our rhTM10-CS group, which might have been due to coagulation abnormalities. Thus, a future study should include measurement of clotting ability to elucidate the mechanism of this adverse effect. APC, a coagulation factor produced following rhTM administration, is involved in the cross-talk between blood coagulation and inflammation. APC was reported to exert an anti-inflammatory effect by suppressing the nuclear translocation of NF-κB and inhibiting the production of inflammatory cytokines including TNFα, IL-1β, IL-6, and IL-839. Therefore, the evaluation of coagulation factors, such as APC, is essential to clarify the mechanism of the anti-inflammatory activity of rhTM in preterm sepsis. In addition, in a clinical trial involving the use of recombinant human APC for treatment of children with severe sepsis, an increased risk of bleeding was noted in neonates after treatment40. Thus, in preterm infants, the risk of bleeding may increase due to the action of rhTM via APC; future studies should include evaluations of APC, such as the contribution of thrombin-dependent APC pathways, as well as direct quenching of HMGB-1 and other mediators via lectin-like domains.
Second, the timing of rhTM administration in this study was before the induction of sepsis, which was preventative and therefore not therapeutic. Although rhTM is administered via the intravenous route in clinical settings, it is difficult to place a venous catheter in neonatal mice; therefore, we chose subcutaneous administration in this study. Based on the pharmacological kinetic data for rhTM20, we subcutaneously administered it at 6 h prior to sepsis induction, which achieved a sufficient blood concentration in adult rats. Based on the above, we think this result reflects the protocol used for clinical rhTM therapy using the intravenous route at the onset of sepsis. However, this study investigated pre-sepsis treatment, rather than post-sepsis treatment. Thus, to determine the therapeutic efficacy of TM, further post-sepsis treatment studies are necessary using large animal models in which an intravenous route can be secured, or using scFv/TM, which has shown protective effects even when administered after the induction of sepsis in an adult mouse model of sepsis.
Third, we have performed bioassays at two very early points to elucidate the acute LM response in this model41. However, we could not determine whether the elevation of LMs at 6 h represents the true peak of the response. Thus, to characterise the detailed mechanism by which rhTM influences LM dynamics, further studies are necessary including later time points, such as 9 h or 12 h. In addition, histological evaluation was not performed in this study. Because most Veh-treated controls (71.4%) died within 48 h post-sepsis induction, it was difficult to collect specimens for histological analysis. To further elucidate the pathophysiology, we plan to conduct a study that includes histologic examination using a newly established murine model of sepsis with a prolonged disease course and high survival rate42. Although we utilised decapitation for blood collection in our study, this procedure is not ideal. Intracardiac puncture might be desirable in future studies, as it is more ethical and technically feasible.
In conclusion, we report that subcutaneous rhTM administration attenuated sepsis severity in a non-surgical preterm sepsis mouse model. Furthermore, several inflammatory LMs were increased at 3 and 6 h post-sepsis induction, and these increases were suppressed by rhTM pre-treatment only at 3 h post-sepsis induction. Thus, we concluded that LMs might be involved in the pathogenesis of preterm sepsis. scFv/TM might be a promising option to more effectively control post-septic elevation of inflammatory LMs.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Wynn, J. L. & Wong, H. R. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 37, 439–479, https://doi.org/10.1016/j.clp.2010.04.002 (2010).
Fujioka, K. et al. Induction of Heme Oxygenase-1 Attenuates the Severity of Sepsis in a Non-Surgical Preterm Mouse Model. Shock 47, 242–250, https://doi.org/10.1097/SHK.0000000000000689 (2017).
Fujioka, K., Kalish, F., Zhao, H., Wong, R. J. & Stevenson, D. K. Heme oxygenase-1 deficiency promotes severity of sepsis in a non-surgical preterm mouse model. Pediatr Res, https://doi.org/10.1038/s41390-018-0028-6 (2018).
Aziz, M., Jacob, A., Yang, W. L., Matsuda, A. & Wang, P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J. Leukoc. Biol. 93, 329–342, https://doi.org/10.1189/jlb.0912437 (2013).
Colas, R. A., Shinohara, M., Dalli, J., Chiang, N. & Serhan, C. N. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol 307, C39–54, https://doi.org/10.1152/ajpcell.00024.2014 (2014).
Buckley, C. D., Gilroy, D. W. & Serhan, C. N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327, https://doi.org/10.1016/j.immuni.2014.02.009 (2014).
Cohen-Wolkowiez, M., Benjamin, D. K. Jr. & Capparelli, E. Immunotherapy in neonatal sepsis: advances in treatment and prophylaxis. Curr. Opin. Pediatr. 21, 177–181, https://doi.org/10.1097/MOP.0b013e32832925e5 (2009).
Cohen, J. et al. Sepsis: a roadmap for future research. Lancet Infect. Dis. 15, 581–614, https://doi.org/10.1016/S1473-3099(15)70112-X (2015).
Venkatesh, B. et al. The ADRENAL study protocol: adjunctive corticosteroid treatment in critically ill patients with septic shock. Crit. Care Resusc. 15, 83–88 (2013).
DeLano, F. A., Hoyt, D. B. & Schmid-Schonbein, G. W. Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock. Sci Transl Med 5, 169ra111, https://doi.org/10.1126/scitranslmed.3005046 (2013).
Zheng, G. et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir. Res. 15, 39, https://doi.org/10.1186/1465-9921-15-39 (2014).
Yamakawa, K. et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Intensive Care Med. 39, 644–652, https://doi.org/10.1007/s00134-013-2822-2 (2013).
Yamakawa, K. et al. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study. Crit. Care 15, R123, https://doi.org/10.1186/cc10228 (2011).
Saito, H. et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J. Thromb. Haemost. 5, 31–41, https://doi.org/10.1111/j.1538-7836.2006.02267.x (2007).
Ito, T. & Maruyama, I. Thrombomodulin: protectorate God of the vasculature in thrombosis and inflammation. J. Thromb. Haemost. 9(Suppl 1), 168–173, https://doi.org/10.1111/j.1538-7836.2011.04319.x (2011).
Nagato, M., Okamoto, K., Abe, Y., Higure, A. & Yamaguchi, K. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit. Care Med. 37, 2181–2186, https://doi.org/10.1097/CCM.0b013e3181a55184 (2009).
Shirahata, A. et al. Recombinant soluble human thrombomodulin (thrombomodulin alfa) in the treatment of neonatal disseminated intravascular coagulation. Eur. J. Pediatr. 173, 303–311, https://doi.org/10.1007/s00431-013-2155-8 (2014).
Starr, M. E. et al. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS One 9, e115705, https://doi.org/10.1371/journal.pone.0115705 (2014).
Iwashita, Y. et al. Thrombomodulin protects against lung damage created by high level of oxygen with large tidal volume mechanical ventilation in rats. J. Intensive Care 2, 57, https://doi.org/10.1186/s40560-014-0057-0 (2014).
Yamada, Y. et al. Effect of thrombomodulin on the development of monocrotaline-induced pulmonary hypertension. J. Anesth. 28, 26–33, https://doi.org/10.1007/s00540-013-1663-z (2014).
Park, M., Rosario, A. L., Schettino Gde, P. & Azevedo, L. C. Hemodynamic and perfusion variables during experimental septic shock treated with goal-directed fluid resuscitation. Rev. Bras. Ter. Intensiva 23, 283–290 (2011).
Norris, P. C. et al. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Sci. Rep. 8, 18050, https://doi.org/10.1038/s41598-018-36679-4 (2018).
Tanaka, N. et al. Eicosapentaenoic Acid-Enriched High-Density Lipoproteins Exhibit Anti-Atherogenic Properties. Circ. J. 82, 596–601, https://doi.org/10.1253/circj.CJ-17-0294 (2018).
Bolognese, A. C. et al. Activation of Invariant Natural Killer T Cells Redirects the Inflammatory Response in Neonatal Sepsis. Front. Immunol. 9, 833 (2018).
Takehara, K. et al. Evaluation of the effect of recombinant thrombomodulin on a lipopolysaccharide-induced murine sepsis model. Exp. Ther. Med. 13, 2969–2974, https://doi.org/10.3892/etm.2017.4308 (2017).
Buras, J. A., Holzmann, B. & Sitkovsky, M. Animal models of sepsis: setting the stage. Nat. Rev. Drug. Discov. 4, 854–865, https://doi.org/10.1038/nrd1854 (2005).
Fink, M. P. Animal models of sepsis. Virulence 5, 143–153, https://doi.org/10.4161/viru.26083 (2014).
Dejager, L., Pinheiro, I., Dejonckheere, E. & Libert, C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 19, 198–208, https://doi.org/10.1016/j.tim.2011.01.001 (2011).
Wynn, J. L. et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28, 675–683, https://doi.org/10.1097/SHK.0b013e3180556d09 (2007).
Adkins, B., Leclerc, C. & Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4, 553–564, https://doi.org/10.1038/nri1394 (2004).
Spite, M. et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291, https://doi.org/10.1038/nature08541 (2009).
Chiang, N. et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528, https://doi.org/10.1038/nature11042 (2012).
Dalli, J. et al. Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations With Survival and Clinical Outcomes. Crit. Care Med. 45, 58–68, https://doi.org/10.1097/CCM.0000000000002014 (2017).
Yamakawa, K. et al. Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J. Thromb. Haemost. 13, 508–519, https://doi.org/10.1111/jth.12841 (2015).
Ding, B. S. et al. Anchoring fusion thrombomodulin to the endothelial lumen protects against injury-induced lung thrombosis and inflammation. Am. J. Respir. Crit. Care Med. 180, 247–256, https://doi.org/10.1164/rccm.200809-1433OC (2009).
Zaitsev, S. et al. Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood 119, 4779–4785, https://doi.org/10.1182/blood-2011-12-398149 (2012).
Carnemolla, R. et al. Targeting thrombomodulin to circulating red blood cells augments its protective effects in models of endotoxemia and ischemia-reperfusion injury. FASEB J. 31, 761–770, https://doi.org/10.1096/fj.201600912R (2017).
Villa, C. H. et al. Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv. 2, 165–176, https://doi.org/10.1182/bloodadvances.2017011734 (2018).
Foley, J. H. & Conway, E. M. Cross Talk Pathways Between Coagulation and Inflammation. Circ. Res. 118, 1392–1408, https://doi.org/10.1161/CIRCRESAHA.116.306853 (2016).
Nadel, S. et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 369, 836–843, https://doi.org/10.1016/S0140-6736(07)60411-5 (2007).
Serhan, C. N. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177, 1576–1591, https://doi.org/10.2353/ajpath.2010.100322 (2010).
Steele, A. M., Starr, M. E. & Saito, H. Late Therapeutic Intervention with Antibiotics and Fluid Resuscitation Allows for a Prolonged Disease Course with High Survival in a Severe Murine Model of Sepsis. Shock 47, 726–734, https://doi.org/10.1097/SHK.0000000000000799 (2017).
Acknowledgements
We thank Dr. Ronald J. Wong at Stanford University for invaluable advice regarding our experimental design. We thank Ryan Chastain-Gross, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was supported by the Kobe Sinryokukai Association (Japan); JSPS KAKENHI Grant Numbers 16H06971, 18K15710, and 19K17360 (Japan); the Morinaga Hoshi-kai Foundation (Japan); and The Mother and Child Health Foundation (Japan). The rhTM used in this study was provided by Asahi Kasei Pharma Corporation, Tokyo, Japan.
Author information
Authors and Affiliations
Contributions
M.A. and K.F. contributed to the conception and design of the study; M.A., K.F., K.N., S.O. and T.I. performed the animal experiments; M.S. performed the lipid mediator experiments and data analysis; M.A. and K.F. performed statistical analysis, interpretation of data, and drafting of the manuscript; M.S. and K.I. critically reviewed the manuscript. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ashina, M., Fujioka, K., Nishida, K. et al. Recombinant human thrombomodulin attenuated sepsis severity in a non-surgical preterm mouse model. Sci Rep 10, 333 (2020). https://doi.org/10.1038/s41598-019-57265-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-57265-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.