Abstract
Background
Recombinant human IGF-1/binding protein-3 (rhIGF-1/BP3) is currently being tested in phase II clinical trials in premature infants to prevent bronchopulmonary dysplasia, but its impact on the neonatal intestine remains unclear. The aim of this study was to determine whether rhIGF-1/BP3 protects against necrotizing enterocolitis (NEC) in mice and to investigate the mechanisms involved.
Methods
Neonatal mice were dam fed or injected intraperitoneally with rhIGF-1/BP3 (or vehicle) and submitted to an experimental NEC model. Serum IGF-1 was assessed by ELISA and intestinal vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR2) expression by Western blot. Intestinal endothelial cell proliferation, and enterocyte proliferation and migration were examined by immunofluorescence. Pup survival and histological intestinal injury were determined.
Results
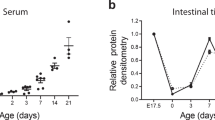
In pups exposed to experimental NEC, serum IBP3-bound IGF-1 level was decreased. Exogenous rhIGF-1/BP3 preserved VEGF and VEGFR2 protein expression, decreased vascular permeability, and preserved endothelial cell proliferation in the small intestine. Furthermore, rhIGF-1/BP3 promoted enterocyte proliferation and migration, which effects were attenuated by inhibiting VEGFR2 signaling, decreased enterocyte apoptosis and decreased systemic and intestinal inflammation. rhIGF-1/BP3 improved survival and reduced the incidence of severe intestinal injury in experimental NEC.
Conclusions
Exogenous rhIGF-1/BP3 protects neonatal mice against experimental NEC via multiple mechanisms.
Impact
-
Exogenous rhIGF-1/BP3 preserves intestinal microvascular development and integrity, promotes enterocyte proliferation and migration, decreases local and systemic inflammation, and protects neonatal mice against NEC.
-
The article adds pre-clinical evidence of a protective role for rhIGF-1/BP3 on the premature gut.
-
It provides evidence supporting the use of rhIGF1/BP3 in premature neonates to protect against NEC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Neu, J. & Walker, W. A. Necrotizing Enterocolitis. N. Engl. J. Med 364, 255–264 (2011).
Bowker, R. M. et al. Dimethyloxalylglycine Preserves the Intestinal Microvasculature and Protects against Intestinal Injury in a Neonatal Mouse Nec Model: Role of Vegf Signaling. Pediatr. Res. 83, 545–553 (2018).
Yan, X. et al. Lack of Vegfr2 Signaling Causes Maldevelopment of the Intestinal Microvasculature and Facilitates Necrotizing Enterocolitis in Neonatal Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G716–G725 (2016).
Yan, X., Managlia, E., Zhao, Y. Y., Tan, X. D. & De Plaen, I. G. Macrophage-Derived Igf-1 Protects the Neonatal Intestine against Necrotizing Enterocolitis by Promoting Microvascular Development. Commun. Biol. 5, 320 (2022).
Hellström, A. et al. Postnatal Serum Insulin-Like Growth Factor I Deficiency Is Associated with Retinopathy of Prematurity and Other Complications of Premature Birth. Pediatrics 112, 1016–1020 (2003).
Holgersen, K. et al. Supplemental Insulin-Like Growth Factor-1 and Necrotizing Enterocolitis in Preterm Pigs. Front. Pediatr. 8, 602047 (2020).
Yakar, S., Wu, Y., Setser, J. & Rosen, C. J. The Role of Circulating Igf-I: Lessons from Human and Animal Models. Endocrine 19, 239–248 (2002).
Rajaram, S., Baylink, D. J. & Mohan, S. Insulin-Like Growth Factor-Binding Proteins in Serum and Other Biological Fluids: Regulation and Functions. Endocr. Rev. 18, 801–831 (1997).
Boisclair, Y. R., Rhoads, R. P., Ueki, I., Wang, J. & Ooi, G. T. The Acid-Labile Subunit (Als) of the 150 Kda Igf-Binding Protein Complex: An Important but Forgotten Component of the Circulating Igf System. J. Endocrinol. 170, 63–70 (2001).
Jeschke, M. G. et al. Gut Mucosal Homeostasis and Cellular Mediators after Severe Thermal Trauma and the Effect of Insulin-Like Growth Factor-I in Combination with Insulin-Like Growth Factor Binding Protein-3. Endocrinology 148, 354–362 (2007).
Baregamian, N., Song, J., Jeschke, M. G., Evers, B. M. & Chung, D. H. Igf-1 Protects Intestinal Epithelial Cells from Oxidative Stress-Induced Apoptosis. J. Surg. Res. 136, 31–37 (2006).
Hunninghake, G. W. et al. Insulin-Like Growth Factor-1 Levels Contribute to the Development of Bacterial Translocation in Sepsis. Am. J. Respir. Crit. Care Med. 182, 517–525 (2010).
Ozen, S. et al. Insulin-Like Growth Factor Attenuates Apoptosis and Mucosal Damage in Hypoxia/Reoxygenation-Induced Intestinal Injury. Biol. Neonate 87, 91–96 (2005).
Wolterink-Donselaar, I. G., Meerding, J. M. & Fernandes, C. A Method for Gender Determination in Newborn Dark Pigmented Mice. Lab. Anim. 38, 35–38 (2009).
Tian, R. et al. Characterization of a Necrotizing Enterocolitis Model in Newborn Mice. Int. J. Clin. Exp. Med. 3, 293–302 (2010).
Bergmann, K. R. et al. Bifidobacteria Stabilize Claudins at Tight Junctions and Prevent Intestinal Barrier Dysfunction in Mouse Necrotizing Enterocolitis. Am. J. Pathol. 182, 1595–1606 (2013).
Bu, H. F. et al. Milk Fat Globule-Egf Factor 8/Lactadherin Plays a Crucial Role in Maintenance and Repair of Murine Intestinal Epithelium. J. Clin. Invest. 117, 3673–3683 (2007).
Yan, X., Managlia, E., Tan, X. D. & De Plaen, I. G. Prenatal Inflammation Impairs Intestinal Microvascular Development through a Tnf-Dependent Mechanism and Predisposes Newborn Mice to Necrotizing Enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G57–g66 (2019).
Díaz-Gómez, N. M., Domenech, E. & Barroso, F. Breast-Feeding and Growth Factors in Preterm Newborn Infants. J. Pediatr. Gastroenterol. Nutr. 24, 322–327 (1997).
Hellström, A. et al. Role of Insulin like Growth Factor 1 in Fetal Development and in the Early Postnatal Life of Premature Infants. Am. J. Perinatol. 33, 1067–1071 (2016).
Andersen, A. D. et al. Delayed Growth, Motor Function and Learning in Preterm Pigs During Early Postnatal Life. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R481–R492 (2016).
Cakir, B. et al. Igf1, Serum Glucose, and Retinopathy of Prematurity in Extremely Preterm Infants. JCI Insight 5, e140363 (2020).
Bowker, R. M., Yan, X. & De Plaen, I. G. Intestinal Microcirculation and Necrotizing Enterocolitis: The Vascular Endothelial Growth Factor System. Semin. Fetal Neonatal Med. 23, 411–415 (2018).
Slomiany, M. G. & Rosenzweig, S. A. Hypoxia-Inducible Factor-1-Dependent and -Independent Regulation of Insulin-Like Growth Factor-1-Stimulated Vascular Endothelial Growth Factor Secretion. J. Pharm. Exp. Ther. 318, 666–675 (2006).
Feng, J. & Besner, G. E. Heparin-Binding Epidermal Growth Factor-Like Growth Factor Promotes Enterocyte Migration and Proliferation in Neonatal Rats with Necrotizing Enterocolitis. J. Pediatr. Surg. 42, 214–220 (2007).
Cetin, S. et al. Endotoxin Inhibits Intestinal Epithelial Restitution through Activation of Rho-Gtpase and Increased Focal Adhesions. J. Biol. Chem. 279, 24592–24600 (2004).
Leaphart, C. L. et al. A Critical Role for Tlr4 in the Pathogenesis of Necrotizing Enterocolitis by Modulating Intestinal Injury and Repair. J. Immunol. 179, 4808–4820 (2007).
Fhölenhag, K., Arrhenius-Nyberg, V., Sjögren, I. & Malmlöf, K. Effects of Insulin-Like Growth Factor I (Igf-I) on the Small Intestine: A Comparison between Oral and Subcutaneous Administration in the Weaned Rat. Growth Factors 14, 81–88 (1997).
Alexander, A. N. & Carey, H. V. Oral Igf-I Enhances Nutrient and Electrolyte Absorption in Neonatal Piglet Intestine. Am. J. Physiol. 277, G619–G625 (1999).
Houle, V. M., Schroeder, E. A., Odle, J. & Donovan, S. M. Small Intestinal Disaccharidase Activity and Ileal Villus Height Are Increased in Piglets Consuming Formula Containing Recombinant Human Insulin-Like Growth Factor-I. Pediatr. Res. 42, 78–86 (1997).
Jilling, T., Lu, J., Jackson, M. & Caplan, M. S. Intestinal Epithelial Apoptosis Initiates Gross Bowel Necrosis in an Experimental Rat Model of Neonatal Necrotizing Enterocolitis. Pediatr. Res. 55, 622–629 (2004).
Zhang, W. et al. Insulin-Like Growth Factor-I Improves Mucosal Structure and Function in Transplanted Rat Small Intestine. Transplantation 59, 755–761 (1995).
Baregamian, N., Rychahou, P. G., Hawkins, H. K., Evers, B. M. & Chung, D. H. Phosphatidylinositol 3-Kinase Pathway Regulates Hypoxia-Inducible Factor-1 to Protect from Intestinal Injury During Necrotizing Enterocolitis. Surgery 142, 295–302 (2007).
Tian, F., Liu, G. R., Li, N. & Yuan, G. Insulin-Like Growth Factor I Reduces the Occurrence of Necrotizing Enterocolitis by Reducing Inflammatory Response and Protecting Intestinal Mucosal Barrier in Neonatal Rats Model. Eur. Rev. Med. Pharm. Sci. 21, 4711–4719 (2017).
Ge, R. T. et al. Insulin-Like Growth Factor-1 Endues Monocytes with Immune Suppressive Ability to Inhibit Inflammation in the Intestine. Sci. Rep. 5, 7735 (2015).
Puzik, A. et al. Insulin-Like Growth Factor-I Regulates the Neonatal Immune Response in Infection and Maturation by Suppression of Ifn-γ. Cytokine 60, 369–376 (2012).
Klevebro, S. et al. Elevated Levels of Il-6 and Igfbp-1 Predict Low Serum Igf-1 Levels During Continuous Infusion of Rhigf-1/Rhigfbp-3 in Extremely Preterm Infants. Growth Horm. IGF Res. 50, 1–8 (2020).
Sheng, X., Sun, X., Li, F., Wang, J. & Ma, J. Linear Growth Failure Induced by Systemic Inflammation Inhibiting Igf-1/Igfbp Axis in Rats with Asymptomatic Colitis. BMC Gastroenterol. 19, 96 (2019).
Hellgren, G. et al. Increased Postnatal Concentrations of Pro-Inflammatory Cytokines Are Associated with Reduced Igf-I Levels and Retinopathy of Prematurity. Growth Horm. IGF Res. 39, 19–24 (2018).
Acknowledgements
We would like to thank Marissa Docter for her technical assistance in conducting the NEC model.
Funding
This work was funded by Takeda Pharmaceuticals/Oak Hill Biol (I.G.D.P.), by the National Institute of Health Grants R01 DK116568 (I.G.D.P.), and R01s to X.D.T. including DK123826 and DK129960, US Department of Veterans Affairs Merit Review Award I01BX001690 (X.D.T.), and the Stanley Manne Children’s Research Institute of the Ann & Robert H. Lurie Children’s Hospital of Chicago (I.G.D.P.).
Author information
Authors and Affiliations
Contributions
X.Y. performed the in vivo experiments. L.M. performed the ELISA study. X.Y. and I.G.D.P. were involved in the overall design of experiments and interpretation of results. X.Y. and I.G.D.P. performed histological evaluation of tissue samples. G.C., N.B. and X.D.T. provided intellectual input for the project. X.Y. and I.G.D.P. wrote the manuscript with input from all authors. I.G.D.P. conceived and orchestrated the project.
Corresponding author
Ethics declarations
Competing interests
I.G.D.P. received a grant from Shire Human Genetic Therapies, Inc., a member of the Takeda group of companies, for testing rhIGF-1/BP3 in experimental NEC. X.Y., E.M and X.D.T. have no competing interests to declare. G.C. and N.B. were full-time employees of Takeda and stockholders of Takeda Pharmaceutical Company Limited at the time of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, X., Managlia, E., Carey, G. et al. Recombinant IGF-1/BP3 protects against intestinal injury in a neonatal mouse NEC model. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03069-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03069-8