Abstract
Novel approaches to synthesize efficient inorganic electride [Ca24Al28O64]4+(e−)4 (thereafter, C12A7:e−) at ambient pressure under nitrogen atmosphere, are actively sought out to reduce the cost of massive formation of nanosized powder as well as compact large size target production. It led to a new era in low cost industrial applications of this abundant material as Transparent Conducting Oxides (TCOs) and as a catalyst. Therefore, the present study about C12A7:e− electride is directed towards challenges of cation doping in C12A7:e− to enhance the conductivity and form target to deposit thin film. Our investigation for cation doping on structural and electrical properties of Sn- and Si-doped C12A7:e− (Si-C12A7:e, and Sn-C12A7:e−) reduced graphene oxide (rGO) composite shows the maximum achieved conductivities of 5.79 S·cm−1 and 1.75 S·cm−1 respectively. On the other hand when both samples melted, then rGO free Sn-C12A7:e− and Si-C12A7:e− were obtained, with conductivities ~280 S.cm−1 and 300 S·cm−1, respectively. Iodometry based measured electron concentration of rGO free Sn-C12A7:e− and Si-C12A7:e−, 3 inch electride targets were ~2.22 × 1021 cm−3, with relative 97 ± 0.5% density, and ~2.23 × 1021 cm−3 with relative 99 ± 0.5% density, respectively. Theoretical conductivity was already reported excluding any associated experimental support. Hence the above results manifested feasibility of this sol-gel method for different elements doping to further boost up the electrical properties.
Similar content being viewed by others
Introduction
Electrides are exotic ionic-solid materials in solid state with cavity-trapped electrons occupies crystallographic site that serve as smallest possible anions1,2,3,4,5,6,7,8,9. The cavity-trapped electrons are neither localized on specific atoms/molecules, nor delocalized as in metals, but rather occupy sites of anions (Cl−, OH− etc.)6. Two major classes of electride, organic and inorganic, have been developed so far4,6,10,11. Most organic electrides are, unfortunately, unstable at room temperature, and get degrade after exposure to air or moisture. The first inorganic high temperature stable electride in which trapped electrons counter cations occupy separate sites is C12A7:e−, named “mayenite electride”12,13.
Oxymayenite, [Ca24Al28O64]4+(2O2−) (thereafter, C12A7) is an excellent insulator, transparent oxide material (energy band gape, Eg ~7 eV) which can be converted into conducting light metal oxide, C12A7:e− 14. Since, the first room-temperature stable electride, C12A7:e− was discovered (2003), it has attracted much attention due to its unique properties. The Insulator-Metal (I-M)-conversion can get either by removal of clathrate free oxygen from the cages or by introduction of hydrogen along with illumination by UV-radiations15. First method based on removal of clathrate free oxygen from cages is very important to further explore to achieve highly conductivity C12A7:e− 16. Till now different methods are reported time by time for the conversion of insulating C12A7 into different degree of conductivity and transparency by reduction of free oxygen clathrate in cages. Each method had their own advantage and limitation, so can choose particular synthesis techniques according to application requirements16 e.g. complete removal of free oxygen, mass production, and electron-doped area7,12,17,18. It was highly desired to develop a simple, less time/energy consuming, cost-effective, and easy scale-up synthesis method for large-scale cations doping.
Microstructure control doping of C12A7:e− is also an important but less experimentally studied19. In contrast to anionic substitutions into C12A7:e− less is known about the influence of cationic substitutions on the aluminum and calcium site19. The availability of access free cages increases the possibility of storage of further extra “extrinsic free oxygen” in available empty cages in C12A7 unit cell. So, after doping C12A7, the excess extra free oxygen anions can go into an ionic crystal of the stoichiometric compound, resulting either in equivalent number of cations being oxidized or creation of vacancies to balance charge like in case of excess oxygen in UO2+x20 and metal deficit manganous Mn1−xO and ferrous oxides Fe1−xO19. Hence, after reduction of doped C12A7:e− further extra electron, rather than that of intrinsic electride (~2.3 × 1023 cm−3), can accommodate till theoretical value of ~6 × 1023 cm−3 and hence corresponding conductivity will further increased19. Huang et al. based on DFT simulations give a detail explanations about the role of cationic (Mg2+, Cu2+, Sr2+, Fe3+, Ir4+,P5+, and V5+) substitutions and also theoretically evaluate the structural and electronic features of C12A7:e− 19. Theoretical based experimental studies has been done for some of the cation substitutions in C12A7 like, Mg, Si, Ga, Fe, V etc.21,22,23,24,25. So shortly the results of all type of doping based on previously introduced synthesis methods, except synthesis method introduced by our group, causes decrease in the electronic conductivity, except Si-substitution21 where conductivity increases from 0.15 to 0.61 S·cm−1 for x = 0 to 4 and in case of Ga-doping in C12A7 the C12A7 phase is decomposed after reduction24. Further improvements in synthesis techniques are required to further boost up conductivity of C12A7:e− 5,26. New findings in the cation doped C12A7:e− electride will opened up a new frontier in TCO based electronics and fuel cells12,26,27,28,29. The advantages of mayenite are its and its low cost precursors easy availability16. It is accepted that the key in the development of doped C12A7:e− electride is low cost and large scale production. As conductivity of C12A7:e− originate from the cage conduction bands (CCB) related to orbital of the cations in the cages framework. Therefore, cation substitutions can be expected to change the framework of nano cages, affect the CCB states, and thus alter the conductivity of the material. Therefore, the present work provides guidance and insights for exploring simple and scalable synthesis method for the novel doped C12A7:e− materials with enhanced electron transport properties, via careful selection and manipulation of cations doping. Hence, we intended to apply single step facile sol-gel scheme to synthesize C12A7:e− electride nano-powder doped with different suitable cations.
Scope of Method
Synthesis and characterization of doped C12A7:e–
The academic research on the C12A7:e− material is constantly in progress to develop new fundamental science and their potential industrial applications. This new proposed modified sol-gel method will provide a new way for synthesis of C12A7:e− with various cations doping. Enhancement in conductivity was observed experimentally for doped samples similarly reported based on theoretical calculations30. Flexibility associated with this synthesizing approach for synthesis of unique cage structure single phase doped C12A7:e− should enable many applications and also presents opportunity for studies of suitable elements doping in C12A7:e− with aim of optimizing its opto-electrical properties.
Experimental scheme and synthesis
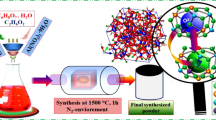
Cation doped C12A7:e− was prepared by using low cost precursors (Ca(NO3)2.4H2O and Al(NO3)3.9H2O) and using ethylene glycol (EG) as a solvent by using modified sol-gel method. First of all, stoichiometric ratios of nitrides were weighted (12:14). The raw materials for “Sn”, and “Si” doping elements used were, SnCl4·5H2O, and SiO2 respectively. The sources of doped elements were added to EG solution separately, along with nitrates at 60 °C under stirring condition to get transparent solution. This gel was kept at about 100 °C for one hour to vaporize physically absorbed water and then finally dried at 275 °C for 4 hours in a drier to vaporize extra EG to get dried gel. Before further heat treatment, the resulting dried gel was crushed into powder and was further heat treated at 500 °C for 1 h in a nitrogen environment with 5 °C/min increase rate. The product was again crushed into powder and then divided into two parts, one was pressed in pellet shape at 150 MPa and the other was directly used to get a conductive powder. Finally, the resultant powder and pellets were sintered in alumina crucible under nitrogen atmosphere with a heating rate of 4 °C/min and kept at 1550 °C for 1 h (Fig. 1). Finally we melted samples at 1700 °C for 30 min under nitrogen environment, and hence solid state migration of carbon species outside of mayenite structure occurs, hence after polishing the melted target we can got rGO free doped C12A7:e− target to deposit thin film.
Results and Discussion
X-ray powder diffraction (XRD) analysis was performed to study the obtained phase microstructures. The Raman spectra were excited by a 532 nm air-cooled argon ion laser (20 mW) to characterization/investigate the molecule structure and bonding of the mayenite electride and graphitic materials. Electrical conductivity was measured by four probe method, where Pt-past was used to further improve the contact. The simple and accurate iodometry technique was used to determine electrons concentration9. Scanning electron microscope (SEM) and Transmission Electron Microscopy (TEM) were used to study the microstructure and morphology of final products. The X-ray photoelectron spectroscopy (XPS) was used for elemental analysis along with bonding formations between those elements to verify required final material synthesis. Now we are going to discuss each characterization method results in detail1,2.
Sn-doped C12A7:e−
Crystalline phase analysis
In this section first of all we studied the single step based synthesis of Sn-doped C12A7:e−. The XRD based study of Sn-doped C12A7:e− regarding to the phase identification and crystallinity were observed with all doping levels shown in Fig. 2. All peaks are corresponding to the well crystalline C12A7 phase (JCPDS, CAS number 48–1882)1,2,8,9.
Doping didn’t change the basic structure of the lattice framework of C12A7 under high temperature treatment and no secondary impurity phase(s) was (were) observed due to decomposition of mayenite electride. That is because of the presence of carbon species which are in form of like rGO with slightly other graphitic materials properties, produced by thermal reduction of ethylene glycol. This acts as a template, which also makes this material stable at this high temperature1,2,8,9.
Microstructural analysis
The SEM images of Sn-doped C12A7:e− (C12A7-xSnx:e−, where x = 0 to 1) samples synthesized after annealing at 1550 °C are shown in Fig. 3. The Sn-doped C12A7:e− sample tended to form nano size particles. In Fig. 3, EDS mapping of Sn-doped C12A7:e− shows all expected elements, Ca, Al, O, C and Sn in synthesized Sn-doped C12A7:e− sample1,2,8,9. Now for further application point of view we are going to discuss the measured electrical properties of synthesized material.
Electrical properties study
Another important characterization is the measuring of conductivity (Fig. 4). In case of Sn-doped C12A7:e− electrical conductivity was in the range of 1.6 S·cm−1 to 5.79 S·cm−1.
The rGO free Sn-doped C12A7:e− sample melted at 1700 °C for 30 min under N2 with highest conductivity of 280 S.cm−1 and Iodometry based measured electron concentration was ~2.22 × 1021 cm−3 1,2 Hence we first time synthesized a 3 inch size electride target with relative density of 97 ± 0.5%, and it, will bring great revolution in opto-electronic industry. Hence, in this experiment, we successfully synthesized the Sn-C12A7:e− free of rGO as a pure phase and showed its increase in conductivity behavior with doping level and its stability on reduction31. Therefore we also check this synthesis method for other element to check its universality for other elements doping in C12A7:e−.
Si-doped C12A7:e−
The heat treatment at 1550 °C/1 h for all samples doped with different Si values, i.e., x = 0.25, 0.50, 0.75, and 1, under N2 gas atmosphere for 1 h were carried out. The XRD patterns of all the Si-doped samples are shown in Fig. 5. All the peaks corresponds to the well crystalline C12A7 phase (JCPDS, CAS number 48–1882) without any second impurity phase peak due to decomposition of C12A7 phase.
The reduction treatment did not change the basic structure of the lattice framework of C12A7 under high temperature treatment and with increased doping level.
The surface morphological study of Si-doped C12A7:e− showed nano sized particles. Such porous type nano size particles may further enhance the specific surface area and could be isolated completely by dissolving in some solvent followed by ultra sonication. Figure 6 shows all expected elements, Ca, Al, O C, and Sn in Si-doped C12A7:e− sample. For further particles size analysis we studied TEM of synthesized powder.
Figure 7 shows TEM image, where nano-size of synthesized particles were confirmed, and nano-size formation will further boost the industrial properties of this material, especially as electrocatalyst. Inset Fig. 7 shows HR-TEM image, which confirmed the C12A7:e− particles, was also supported by XRD. For industrial application, important point of synthesized material is to study the conductivity (Fig. 8). In case of the Si-doped C12A7:e− composite the conductivity was in range of 0.16 S·cm−1 to 1.75 S·cm−1 32 but when we melted the sample at 1700 °C for 30 minutes under nitrogen environment, the highest conductivity achieved of rGO free Si-doped C12A7:e− sample was ~300 S.cm−1, and iodometry based measured electron concentration was ~2.23 × 1021 cm−3. It is demonstrated that moderate conductivities were attributed to well crystalline morphology of Si-doped C12A7:e−. Hence, first time we synthesized rGO free, pure and stable phase of Si-doped C12A7:e− electride target of 3 inch size, with relative density of 99 ± 0.5%. As TCOs, it will bring great revolution in field of the devices, especially in display devices.
Electron Paramagnetic Resonance (EPR)
The EPR analysis was used to calculate the density and electronic structure of trapped electrons in cages of synthesized Si-doped C12A7:e− melted sample (Fig. 9). The EPR spectrum exhibits an absorption signal with at about g = 1.994, as previously reported7. Hence, the EPR investigation also provides the confirmation of synthesis of rGO free Si-doped C12A7:e− electride.
Raman Spectroscopy
Figure 10(b) shows Raman spectrum, where bands located at 200–1000 cm−1 were arose from lattice framework of C12A733. The peak between 1128 to 1164 cm−1 are assigned as the O2− stretching mode, were not observed, indicating the reduction of C12A7 into C12A7:e−1,2. G-band, 2D-band and D-band confirm the existence of carbon family and a very weak D band peak (1340 cm−1) emerges in spectra, shown defective layers1,2,8,9. As the peak intensity ratio I2D/IG is less than 1 and the FWHM of the 2D band peak is ~89 cm−1, the obtained rGO should be the stacking of multilayer sheets1,2. Comparatively small D band peak than 2D band peak in the Raman spectrum could be ascribed to the excellent reduction of rGO during applied synthesis process, in which the oxygen moieties were removed and the sp2 network was restored due to structural relaxation2,8,9,34. On the other hand, Fig. 10(a) shows rGO free pure Si-doped C12A7:e− because the D, G, and 2D bands diapered on melting. Previous study suggested the band appear at 186 cm−1 is related to the electrons present in cages and its intensity is proportional to “Ne”35.
Inset Fig. 10 shows EDX of the melted rGO free Si-doped C12A7:e−, also confirmed Raman spectroscopy results.
X-ray photoelectron spectroscopy (XPS)
XPS technique was applied for elemental analysis where we apply Shirley background correction and Gaussian-Lorentzian peak shape for curve fitting of C 1 s. Figure 11(a) show XPS wide range scan data of Si-doped C12A7:e− composite with in all required elements (Ca, Al, O, C, and Si). For further XPS based verification of the stoichiometric compositions of Si-doped C12A7:e− based on binding energies related peaks positions of C 1 s, Ca 2p, Al 2p, O 1 s, and S1 2p were studied (Fig. 11(b–f)).
Based on those results, Al 2p peak was positioned at around 70.473 eV, shows that the valence state of Al in C12A7 is similar to that of Al2O3 (Fig. 11(b))1,2. In addition, the O 1 s spectrum the peak is at about 529.15 eV (Fig. 11(c))36. Also, the Ca 2p narrow XPS spectrum, along with profiles fits shows two peaks at around 344.57 eV and 348 eV, related to Ca 2p1/2 and Ca 2p3/2, respectively (Fig. 11(f)). Those are due to spin orbit splitting of the Ca 2p in XPS spectrum. Hence, these results shows that Ca is connected with O, making CaO36. For the rGO configuration and confirmation, we investigated the C 1 s peak data (Fig. 11(d)). The resulting peak after fitting was positioned at 281.95 eV (sp2, 47%)37,38. This peak was corresponds to non-oxygenated rings of carbon atoms in a conjugated honey comb lattice, shows that this highest peak ratio is due to formation of C-C skeleton by the reduction process of oxygen-containing species39,40. Similarly, oxygenated rings peaks at 282.59 eV, and 286.05 eV could be attributed to the C in C-O or C-OH (33%), and carbonyl (C = O, 20%), respectively37,38. The C/O ratios was ~13.5, which is almost similar to chemically produced rGO41. Hence these XPS results also support the Raman spectroscopy data for the formation of rGO on surface of Si-doped C12A7:e− composite37 with some stable oxygenated functional groups remains preserved even after reduction39. Finally the positions of Si 2p along with other elements, Ca, Al, and O in XPS data, confirm the formation of Si-doped C12A7:e− nano-particles with rGO coating on it, but this rGO were removed after melting of Si-doped C12A7:e− nano-particles sample.
Thin film deposition
In this part, we deposited thin film by magnetron sputtering method using Si-doped C12A7:e− electride 3 inches target to explore its potential applications in optoelectronic devices. The sputtering process was conducted at 90 °C with the sputtering power set at 180 W for 10 mints.
First of all before we deposit the film we close the shutter and sputter the target for 10 mints to clean the surface of target and after that we started deposition by open the shutter. The UV-Vis optical transmittance + reflectance spectrum of as-deposited thin films with quartz substrate and measured thickness of grown thin film was about 390 nm and inset Fig. 12 shows optical photograph of the as-deposited thin film with highest achieved transparency. The energy band gap of electride thin film was calculated by Tauc’s formula, was about ~5.7 eV, which well fit with band gap value from cage conduction band to valence band, as previously reported9. For optoelectronic devices application, additional investigations are currently underway.
Conclusion
In this paper we successfully synthesized doped-C12A7:e− composites with different suitable elements and with/without rGO electride. In case of Sn-doped C12A7:e− composite the conductivity was in the range of 1.6 S·cm−1 to 5.79 S·cm−1 but the melted rGO free sample with highest achieved conductivity of 280 S.cm−1 and the 3 inch size electride target has a relative density of 97 ± 0.5% was achieved. In case of the Si-doped C12A7:e− composite the conductivity was in the range of 0.16 S·cm−1 to 1.75 S·cm−1, well agreed with previous results32 but melted sample with highest achieved conductivity of ~300 S.cm−1 and the 3 inch size electride target has a relative density of 99 ± 0.5%. Furthermore, transparent amorphous thin film was fabricated via magnetron sputtering, with almost zero percent losses. In conclusion, this optimized sol-gel method is suitable for doping different elements in C12A7:e− to further alter the electrical and optical properties.
References
Khan, K. et al. Facile metal-free reduction-based synthesis of pristine and cation-doped conductive mayenite. RSC Advances 8, 24276–24285, https://doi.org/10.1039/c8ra02790krsc.li/rsc-advances (2018).
Khan, K. et al. Facile synthesis of cationic doped [Ca24Al28O64]4+.(4e−) composite via rapid citrate sol-gel method. Dalton Transactions 47, 3819–3830 (2018).
Khan, K. et al. Facile synthesis of Tin doped mayenite electride compositeas a non-noble metal, durable electrocatalysts for oxygen reduction reaction (ORR) Dalton Transactions, https://doi.org/10.1039/C8DT02548G (2018).
DYE, J. L. Electrides: Early Examples of Quantum Confinement. accounts of chemical research 42, 1564–1572 (2009).
Kitano, M. et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nature chemistry 4, 934–940 (2012).
Dye, J. L. Electrons as Anions. Science 301, 607–607 (2003).
Matsuishi, S. et al. High-density electron anions in a nanoporous single crystal: [Ca24Al28064]4+(4e−). Science 301, 626–629 (2003).
Khan, K. et al. Low Temperature Synthesis of Nano Porous 12CaO∙7Al2O3 Powder by Hydrothermal Method. Journal of Wuhan University of Technology-Mater. Sci. Ed. 31, 1201–1205, https://doi.org/10.1007/s11595-016-1512-7 (2016).
Zou, W. et al. Direct Fabrication of C12A7 Electride Target and Room Temperature Deposition of Thin Films with Low Work Function. Materials Research Express 4, 1–20, https://doi.org/10.1088/2053-1591/aa63c7 (2017).
Miao, M.-s & Hoffmann, R. High-Pressure Electrides: The Chemical Nature of Interstitial Quasiatoms. Journal of American Chemecal Socity. 137, 3631–3637 (2015).
Zhang, X. et al. Two-Dimensional Transition-Metal Electride Y2C. Chemistry of Material 26, 6638–6643 (2014).
Hayashi, K., Matsuishi, S., Kamiya, T., Hirano, M. & Hosono, H. Light-induced conversion of an insulating refractory oxide into a persistent electronic conductor. Nature 419, 462–465 (2002).
Matsuishi, S. et al. High-Density Electron Anions in a Nanoporous Single Crystal: [Ca24Al28O64]4+(4e−). SCIENCE 301, 626–629 (2003).
Kim, S. W. & Hosono, H. Synthesis and properties of 12CaO.7Al2O3 electride: review of single crystal and thin film growth. Philosophical Magazine 92, 2596–2628 (2012).
Kamiya, T. et al. Field-Induced Current Modulation in Nanoporous Semiconductor, Electron-Doped 12CaO 7Al2O3. Chemestry of Material 17, 6311–6316 (2005).
Kim, S.-W. et al. Fabrication of room temperature-stable 12CaO.7Al2O3 electride: a review. Journal of Material Science and Material Electronics 18, S5–S14 (2007).
Kim, S. et al. Simple and Efficient Fabrication of Room Temperature Stable Electride: Melt-Solidification and Glass Ceramics. Journal of American Chemical Society 127, 1370–1371 (2005).
Matsuishi, S. et al. Direct Synthesis of Powdery Inorganic Electride [Ca24Al28O64]4+(e−)4 and Determination of Oxygen Stoichiometry. Chemistry of Material communication 21, 2589–2591 (2009).
Huang, J., Valenzano, L. & Sant, G. Framework and Channel Modifications in Mayenite (12CaO·7Al2O3) Nanocages By Cationic Doping. Chemestry of Material 27, 4731–4741 (2015).
Wang, J., Ewing, R. C. & Becker, U. Average structure and local configuration of excess oxygen in UO2-x. Scientific reports 2014, 1–5 (2014).
Mason, M. I. Ba. T. O. Enhanced electronic conductivity in Si-substituted calcium aluminate. Journal of applied physics 102, 113704–113701 (2007).
Matsuishi, S., Muramatsu, H. & Hosono, H. Photochemistry of Nanocage Ca12Al14-xSixO32Cl2+x (x = 0.0 and 3.4) Crystals. Chem. Lett. 43, 1371–1373 (2014).
Mason, M. I. Ba. T. O. Tunable conductivity and conduction mechanism in an ultraviolet light activated electronic conductor. Journal of applied physics 97, 103713-103711–103713-103717 (2005).
Luis Palacios, S. B. and Miguel A. G. Aranda. Structure of gallium-doped mayenite and its reduction behaviour. physica state solid b 245, 666–672 (2008).
Ebbinghaus, S. G., Krause, H., Lee, D.-K. & Janek, J. Single Crystals of C12A7 (Ca12Al14O33) Substituted with 1 mol % Iron. Crystal Growth & Design 14, 2240–2245 (2014).
Toda, Y. et al. Work Function of a Room-Temperature, Stable Electride [Ca24Al28O64]4+(e−)4. Advance Material 19, 3564–3569 (2007).
Matsuishi, S. et al. High-Density Electron Anions in a Nanoporous Single Crystal: [Ca24Al28O64] +4 (4e−). science 301, 626–629 (2003).
Buchammagari, H. et al. Room Temperature-Stable Electride as a Synthetic Organic Reagent: Application to Pinacol Coupling Reaction in Aqueous Media. Organic letters 9, 4287–4289 (2007).
Kim, S.-W., Hayashi, K., Hirano, M. & Hosono, H. Electron Carrier Generation in a Refractory Oxide 12CaO. 7Al2O3 by Heating in Reducing Atmosphere: Conversion from an Insulator to a Persistent Conductor. Journal of American Ceramice Socity 89, 3294–3298 (2010).
Huang, J., Valenzano, L. & Sant, G. Framework and Channel Modifications in Mayenite (12CaO∙7Al2O3) Nanocages By Cationic Doping. Chemestry of Material 27, 4731–4741 (2015).
Palacios, L., Bruque, S. & Aranda, M. A. G. Structure of gallium-doped mayenite and its reduction behaviour physica status solidi (b) 245, 666–672, https://doi.org/10.1002/pssb.200743425 (2008).
Mariana, I., Bertoni, T. O. M. & Julia, E. Medvedeva, Yongqiang Wang, Arthur J. Freeman, Kenneth R. Poeppelmeier. Enhanced electronic conductivity in Si-substituted calcium aluminate. Journal of applied physics 102, (113704-113701–113704-113707 (2007).
Galuskin, E. V. et al. A reinvestigation of mayenite from the type locality, the Ettringer Bellerberg volcano near Mayen, Eifel district, Germany. Mineralogical Magazine 76, 707–716 (2012).
Ren, i-G. et al. A silicon nanowire–reduced graphene oxide composite as a high-performance lithium ion battery anode material. Nanoscale 6, 3353 (2014).
Kim, S. W., Shimoyama, T. & Hosono, H. Solvated Electrons in High-Temperature Melts and Glasses of the Room-Temperature Stable Electride [Ca24Al28O64] 4+ 4e−. Science 333, 71–74 (2011).
Pan, R. K., Feng, S. & Tao, H. Z. XPS and NMR analysis on 12CaO·7Al2O3. IOP Conf. Series: Materials Science and Engineering 167 012017, 10.1088/1757-899×/167/1/012017 (2017).
Khan, K. et al. Facile synthesis of cationic doped [Ca24Al28O64]4+.(4e−) composite via rapid citrate sol-gel method. Dalton Transactions. https://doi.org/10.1039/C7DT04543C (2018).
Mortazavi, S. Z., Parvin, P., Reyhani, A., Mirershadi, S. & Sadighi-Bonabi, R. Generation of various carbon nanostructures in water using IR/UV laser ablation. Journal of Physics D: Applied Physics 46, 165303 (165309pp), https://doi.org/10.1088/0022-3727/46/16/165303 (2013).
Han, N. et al. Improved heat dissipation in gallium nitride light-emitting diodes with embedded graphene oxide pattern. Nature communications 4, 1–8, https://doi.org/10.1038/ncomms2448, www.nature.com/naturecommunications (2013).
Chanda, D. et al. The effect of surface modification by reduced graphene oxide on the electrocatalytic activity of nickel towards the hydrogen evolution reaction. Physical Chemistry Chemical Physics 17, 26864–26874 (2015).
Sagar, R. U. R., Stadler, F. J., Namvari, M. & Navale, S. T. Synthesis of scalable and tunable slightly oxidized graphene via Chemical Vapor Deposition. Journal of Colloid and Interface Science 490, 844–849, https://doi.org/10.1016/j.jcis.2016.11.073 (2016).
Acknowledgements
Financial supports from Natural Science Foundation of China (NSFC) (Grant Nos.: 61275043, 61307048, and 61171006).
Author information
Authors and Affiliations
Contributions
Research idea and paper writing was done was by Prof. Karim Khan, and paper was polished by Prof. Karim Khan, Dr. Ayesha khan tareen, Dr. Usman Khan, Dr. Adeela Nairan, Dr. Sayed Elshahat, Dr. Naseer Muhammad, Dr. Muhammad Saeed, Dr. Ashish Yadav, Dr. Luigi Bibbò, and finally Prof. Zhengbiao Ouyang gave a final touch to paper in finalizing it.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, K., Tareen, A.k., Khan, U. et al. Single step synthesis of highly conductive room-temperature stable cation-substituted mayenite electride target and thin film. Sci Rep 9, 4967 (2019). https://doi.org/10.1038/s41598-019-41512-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41512-7
This article is cited by
-
Recent Progress, Challenges, and Prospects in Two-Dimensional Photo-Catalyst Materials and Environmental Remediation
Nano-Micro Letters (2020)
-
Fe-doped mayenite electride composite with 2D reduced Graphene Oxide: As a non-platinum based, highly durable electrocatalyst for Oxygen Reduction Reaction
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.