Abstract
Inherited Retinal Dystrophies are clinically and genetically heterogeneous disorders affecting the photoreceptors. Although NGS has shown to be helpful for the molecular diagnosis of these conditions, some cases remain unsolved. Among these, several individuals harboured monoallelic variants in a recessive gene, suggesting that a comprehensive screening could improve the overall diagnosis. In order to assess the contribution of non-coding variations in a cohort of 29 patients, 25 of them with monoallelic mutations, we performed targeted NGS. The design comprised the entire genomic sequence of three genes (USH2A, ABCA4 and CEP290), the coding exons of 76 genes and two disease-associated intronic regions in OFD1 and PRPF31. As a result, likely causative mutations (8 novel) were identified in 17 probands (diagnostic rate: 58.62%), including two copy-number variations in USH2A (one deletion of exons 22–55 and one duplication of exons 46–47). Possibly damaging deep-intronic mutations were identified in one family, and another with a monoallelic variant harboured causal mutations in a different locus. In conclusion, due to the high prevalence of carriers of IRD mutations and the results obtained here, sequencing entire genes do not seem to be the approach of choice for detecting the second hit in IRD patients with monoallelic variants.
Similar content being viewed by others
Introduction
Inherited Retinal Dystrophies (IRDs) are a group of rare disorders characterized by the progressive loss of photoreceptors in the retina, with a prevalence of 1 in 3,000 individuals worldwide1. Depending on the first photoreceptor cell affected, IRDs are subdivided in rod-cone and cone-rod degenerations. The most common form of IRDs is Retinitis Pigmentosa (RP), a rod-cone disease defined by a primary death of rods, which results in night blindness and constriction of the visual field. Later in life, loss of cones leads to a decreased visual acuity2. RP can be inherited as an autosomal dominant (adRP), autosomal recessive (arRP) or X-linked (xlRP) trait, but in a large percentage of cases the mode of inheritance is unknown due to absence of family history (simplex RP, sRP). In other pathologies like cone-rod dystrophies (COD), cones degenerate first, whereas in Leber congenital amaurosis (LCA) both types of photoreceptors are damaged simultaneously3. Maculopathies like Stargardt disease (STGD) are defined by loss of central vision and accumulation of yellow flecks deposits around the macula4,5. There are also syndromic pathologies related to IRDs such as Usher syndrome (USH), in which RP is accompanied by congenital hearing impairment2. Several of these conditions share some features, which leads to overlapping phenotypes. Moreover, IRDs are characterized by huge phenotypic variability, in which clinical features, age of onset and disease progression can vary from patient to patient, even in the same family (inter- and intra-familial variability)6,7. Furthermore, IRDs are one of the most genetically heterogeneous disorders. To date, more than 300 genes have been associated (RetNet; https://sph.uth.edu/retnet/, accessed July 2018). In addition, mutations in a single gene can be associated with a broad phenotypic spectrum and a specific phenotype can be caused by mutations in multiple genes8.
Next-generation sequencing (NGS) resulted in an improvement of the diagnostic rate of this group of heterogeneous disorders9,10,11. However, even though these strategies show high efficacy in a large proportion of cases, around 40–50% of cases remain unsolved9,12. Deep-intronic variants, large rearrangements that escape genetic detection or currently unknown IRDs genes may explain these cases13. In order to increase the diagnostic rate of this group of disorders, other genomic regions not routinely analyzed must be considered.
Aberrant splicing is a well-known disease-causing mechanism. In fact, it is estimated that a significant percentage of mutations related to monogenic pathologies have an effect on splicing14,15. Indeed, deep-intronic mutations in ABCA416,17, CEP29018, USH2A19, CHM20,21, PRPF3122 or OFD123 have been described as disease-causing in IRDs. Furthermore, mutations in genes coding for spliceosome components have been found in patients with RP24,25, which enlightens the importance of alternative splicing in this condition. Structural variants, including CNVs, have also been described as a relevant cause of disease26. Specifically in IRDs, duplications and deletions have been linked to the development of syndromic and non-syndromic cases20,27,28,29. These mutations are easier to detect by whole-genome or whole gene sequencing, as coding and non-coding elements analysis allows the identification of the accurate size and both breakpoints of CNVs.
Here we applied a targeted gene panel covering the entire genomic region of three genes (ABCA4, USH2A and CEP290) and the coding exons of 76 additional genes for the molecular analysis of 29 IRDs patients with simplex or suspected autosomal recessive inheritance. For 25 of them, other methods have previously succeeded in identifying a heterozygous variant in one of these genes. Our study aimed to find a second variant in the same gene to explain the patients’ phenotype by compound heterozygous inheritance. The diagnostic rate was 58.62%% (17/29), of which 14 cases (~77.7%) were solved by the identification of USH2A mutations.
Results
Clinical features
All analyzed families (n = 29) were of Spanish origin. Index patients received a well-defined clinical diagnosis, which included either RP, USH type II (USHII), STGD, LCA or COD. Available clinical findings of the index patients of the likely genetically diagnosed families are reported in Table 1. The presumed underlying mode of inheritance was either autosomal recessive or simplex in all families. In 10 cases, DNA samples from additional family members were used for segregation analysis of the candidate variants.
NGS data quality
The panel design covered 95.3% (1,346,725 bp) of 1,412,505 target bases. The uncovered bases represented 4.7% of the total number of bases, most of them lying in non-coding regions (repeating elements: Long terminal repeats, LTRs and Long interspersed nuclear elements, LINEs). The specific uncovered bases of the genes ABCA4, CEP290 and USH2A are provided in Supplementary Table S1. Only one exonic region in exon 15 (ORF15) of RPGR remained uncovered (98–145 bp, depending on the patients). The overall mean coverage was 809X with 100% of captured bases covered, except for individual II:6 of family R, whose coverage dropped to 96.6% due to a large homozygous deletion (Fig. 1). NGS performed on an Illumina MiSeq or NextSeq systems achieved on average 9,828,453 reads per run of which 8,213,528 were mapped on target (82.73%).
Detection of CNVs in USH2A using our NGS approach. (a) IGV snapshot showing the homozygous deletion of exons 22–55 detected in family R-II:6 (Chr1:g.215,949,321_216,272,841del, hg19). The capture of whole genomic sequence of USH2A allowed us to determinate the CNVs breakpoints. (b) Schematic representation of the mutated gDNA sequence and Sanger sequencing of the breakpoint area (orange arrows) confirming the USH2A deletion (c.4628-2287_10939 + 3867del; NM_206933). (c) IGV snapshot showing the heterozygous duplication of exons 46–47 (Chr1:g.216,005,789_216,019,066dup, hg19) detected in family P-II:9 versus a control sample. The heterozygous duplication can be inspected visually using IGV paying special attention to (i) a sharp increase in the coverage and (ii) changes in the allele ratios of all the SNPs within the duplicated interval from ~50:50 to ~67:33 unmasking the presence of a total of three copies. (d) Schematic representation of the mutated gDNA sequence and Sanger sequencing of the breakpoint area (orange arrows) confirming the USH2A tandem duplication of exons 46 and 47 (c.9055 + 100_9371 + 5544dup; NM_206933).
Validation of the panel
Twenty-five out of the 29 cases included in this study carried heterozygous mutations previously detected by other techniques (Table 2), and so, they were used as positive controls for our approach. The application of the data analysis pipeline allowed the accurate re-detection of all the known variants, indicating a mutation detection rate of 100%.
Identification and assessment of candidate variants
In order to identify likely disease-causing variants for each sample, we conducted a stepwise mutation detection protocol as previously described9,30 with some modifications: heterozygous mutations in genes with one previous detected variant were prioritized and new variants, including CNVs, were looked for in non-coding regions (deep-intronic and splicing mutations) and coding regions (synonymous variants). Intronic variants were analyzed with in silico tools for their potential effect on splicing. If no mutations were found, variants in other genes were assessed as we described in the Methods section.
Sequencing of the gene panel led to the identification of a mean of 2,349 potential variants per patient. After filtering out common polymorphisms with MAF > 0.015 in any of the variant databases queried, including 1,000 Genomes, ExAC and EVS, an average of 314 rare variants per sample remained, of which a range from 1 to 4 were prioritized as described above to be co-segregated by Sanger sequencing.
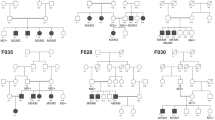
As a result, 31 pathogenic mutations were identified as likely causative in 17 probands (six familial cases and 11 simplex cases) (Supplementary Fig. S1) achieving a diagnostic rate of 58.62% (Table 1). The most frequently mutated gene in this study was USH2A (Table 3), and the most prevalent mutation was p.Cys759Phe. We found this mutation in nine patients, always in a compound heterozygous state with a deleterious allele, and only in non-syndromic RP patients (Fig. 2). All novel and known sequence variants of the genetically diagnosed patients have been submitted to the Leiden Open Variation Database, LOVD (https://databases.lovd.nl/).
USH2A mutations and genotype-phenotype correlations. (a) Schematic representation of usherin structure showing the mutated residues located within different protein domains. Isoform “a” is an N-terminal fragment of isoform “b”. Mutations in orange font are implicated in USHII, mutations in fuchsia font are associated with both non-syndromic RP and USHII and mutations in black font are associated with non-syndromic RP. SP: signal peptide; LamGL: LamG-like jellyroll fold; Lam NT: Laminin N-terminal; EGFLam: Laminin-type EGF-like (LE); FN3: fibronectin type-III; LamG: Laminin G; TM: Transmembrane domain; PDB: PDZ-binding domain. (b) Phenotype-genotype correlations of usherin mutations. Variants responsible of non-syndromic RP are represented in blue. The variant p.Cys759Phe has been detected in combination with other deleterious alleles in nine patients with non-syndromic RP. Mutations shown in fuchsia color have been detected in individuals with both non-syndromic RP and USH depending on the nature of the second variant. Variants responsible of USHII are shown in orange.
Among the candidate variants, two of them were CNVs in USH2A, comprising one homozygous deletion of exons 22–55 (c.4628–2287_10939 + 3867del) and one heterozygous duplication of exons 46 and 47 (c.9055 + 100_9371 + 5544dup) (Fig. 1). Furthermore, we also detected 18 missense, four frameshift, three nonsense, three intronic-splicing and one synonymous variant located in the exonic canonical splice site (Table 1). Eight of the 31 variants were novel and absent in public databases (ClinVar, LOVD, Pubmed, dbSNP, ExAC, GnomAD, EVS, 1000G and CSVS). Specifically, we detected three homozygous (probands of families B, D and R) and 28 compound heterozygous mutations in autosomal recessive IRDs genes. However, segregation analysis could only be performed in 10 out of 17 families, due to the difficulty in recruiting additional family members in simplex cases. In the remaining patients, candidate variants were presumed to be disease-causing since they correlated with their specific phenotype and they met the established pathogenicity criteria (see Methods section). In this study, we emphasize the importance of intronic and synonymous variants and their effect on splicing processes, which let us diagnosed two cases.
Besides the 31 disease causing mutations, we detected one heterozygous mutation in an autosomal dominant IRDs-associated gene (FSCN2, c.1345 + 6_1345 + 10 dup) in one proband affected of simplex RP (Family C-II:1). Nevertheless, segregation analysis could not be performed in this family and the in silico predictions failed to validate this mutation as a splice-altering variant (Table 1). Interestingly, this patient was also a carrier of the ABCA4 allele (p.Val2050Leu) reported as variant with conflicting interpretations of pathogenicity in the Clinvar database. Therefore, additional studies are needed to ascertain the genetic cause of the disease in this family.
Deep-intronic variants assessment
One of the main objectives of this study was to gain knowledge of the contribution of deep-intronic variants in our population. After analyzing the genomic data, only those deep-intronic variants that fulfilled the selection criteria to be considered causative mutations were selected to be segregated, when possible. A total of 5 variants were found to pass the standards: m33 (USH2A c.6326–17446_6326-17439dup) in family B-II:1, m36 (ABCA4 c.66 + 2044G > A) in family X-II:1, m37 (CEP290 c.3104-238 T > G) in family Z-II:1 and m39 (USH2A c.6806-810 A > G) and m40 (USH2A c.6050-8058G > C) in family S-II:1 (Supplementary Fig. S1). Variants m33, m36 and m37 do not segregate with the disease, as they were in cis with the previously found mutation in these patients. Regarding the other family (Family S), although segregation analysis could not be conducted, both variants (m39: USH2A c.6806-810 A > G and m40: USH2A c.6050-8058G > C) are predicted to induce the activation of a cryptic donor/acceptor site respectively. Moreover, a very low frequency in the queried databases was retrieved for these changes (0 and 13 heterozygous carriers in GnomAD, respectively).
Detection of the second mutation in patients with monoallelic variants
Among the families with a monoallelic variant in one of the genes included in the panel, 15 harboured a single previously detected variant in USH2A, eight in ABCA4 and two in CEP290 (Table 2). Remarkably, 13 out of 15 of the families with a previous USH2A variant were genetically solved by the identification of a second hit in the same gene. Interestingly, family S harbours, besides a missense mutation (c.5363 A > G, p.Asp1788Gly) in USH2A, two deep-intronic variants that, although additional studies are needed, might be causative.
However, the diagnostic yield dropped in families with a known variant in ABCA4 and CEP290, since the second potentially causative mutations were not detected in these genes. Of note, one of the cases (Family B) with a known ABCA4 mutation (c.466 A > G; p.Ile156Val) was solved by the identification of a homozygous likely disease-causing mutation in another gene: CNGB3 (c.1148del; p.Thr383Ilefs*13) (Table 1).
Clinical heterogeneity of USH2A mutations
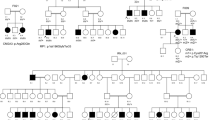
In our study, we found the USH2A p.Cys759Phe allele in compound heterozygosis in 9 patients with non-syndromic RP (Fig. 2). Three patients who received a USHII diagnosis harboured a combination of one nonsense and one frameshift mutation (family M), a homozygous CNV (family R) and one CNV and a missense mutation (family P) in USH2A. Remarkably, not all the affected members of the family P fulfilled the diagnostic criteria of USH, since two affected siblings (II:1 and II:7) exhibited a less severe phenotype consisting of non-syndromic RP (Supplementary Fig. S1). Another family with a significant intrafamilial variability was family H. In this family, only two of the three affected siblings (II:3 and II:4) suffered from RP and hearing loss (Table 1). However, the hearing loss was more likely to have a different genetic cause since the father (individual I:1) of the index patient (II:3) was affected of progressive and bilateral non-syndromic hearing loss. Additionally, here we describe two USH2A mutations, p.Glu767Serfs*21 and p.Arg303Ser, found in patients with syndromic and non-syndromic RP.
Discussion
In this study, we conducted a NGS targeted sequencing approach comprising all exons of 76 retinal disease genes, three entire genes (USH2A, ABCA4 and CEP290), and two deep-intronic regions located in OFD1 and PRPF31, to identify the genetic cause of 29 Spanish patients of IRDs, most of them carrying a monoallelic variant in USH2A, ABCA4 and CEP290.
The molecular diagnosis was achieved in 58.62% of IRDs patients (17/29). This diagnostic yield is in line with previous works30 that similarly analyzed population-specific IRDs genes, and it is somewhat higher than other studies involving more genes31,32. It demonstrates that a consistent and adapted design of the panel guarantees a good diagnostic yield while reducing sequencing costs, time and analytical effort.
In our cohort, we detected 31 likely disease-causing mutations. The majority of them were missense variants (58.1%), followed by splicing (12.9%), and frameshift variants (12.9%). Nonsense and CNVs represented 9.6% and 6.5%, respectively. The increasing number of reported deep-intronic mutations in IRD genes prompted us to include three entire genes in our panel design: ABCA4, USH2A and CEP290. These genes accumulate a high number of pathogenic deep-intronic variants reported in the literature18,19,33,34. In this group of patients, five deep-intronic variants that met the pathogenicity criteria were detected in four families (Supplementary Fig. S1). However, in three of them (families B, X and Z) the segregation analysis discarded their role in the disease aetiology. The index patient of family S (II:1) was clinically diagnosed of non-syndromic RP and harboured a rare missense variant (c.5363 A > G; p.Asp1788Gly) in USH2A previously detected by targeted NGS (Table 2). Regarding the missense mutation, only two heterozygous individuals have been identified in GnomAD, and had no entry neither in Clinvar nor LOVD. Sequencing the USH2A entire gene allowed the identification of two deep-intronic variants (c.6806-810 A > G and c.6050-8058G > C). Although segregation analysis could not be performed, no additional variants that could explain the phenotype of this family were identified in other loci. Therefore, additional studies are needed to ascertain the clinical significance of these variants.
Screening intronic sequences also enables the proper detection of CNVs since it allows the determination of structural variants breakpoints at the nucleotide level, as well as the presence of inversions35,36. Recent studies have shown that CNVs in genes such as EYS37,38 or USH2A28,39, are indeed a significant event on the appearance of IRDs27,40,41. This is in agreement with our results showing the identification and accurate detection of their breakpoints of two novel, likely pathogenic, CNVs in USH2A. Therefore, it is highly recommended that the data analysis pipeline includes a suitable algorithm for the detection of these complex alleles.
Remarkably, of the 25 patients with previously detected monoallelic variants, 13 carried a second mutated allele in the same gene (USH2A). The majority of the unsolved patients carried a previously identified variant in ABCA4. This fact can be explained by the polymorphic nature of certain genes, and specifically of certain disease-causing reported variants. In the past, when the available genetic testing techniques were based on sequencing or genotyping a few exons per sample, detecting a sequence variant was a challenging task and it was interpreted as a causal mutation as long as it correlated with family segregation analysis and it was absent in 100 control individuals. To date, the proliferation of exome and genome sequencing projects and their use in the clinical setting have allowed unmasking some of the variants previously described as pathogenic and now considered as benign changes or at least variants of unknown significance (VUS) in certain populations42,43. In this regard, two variants in ABCA4, p.Ile156Val and p.Val2050Leu, have been traditionally considered disease-causing mutations44,45. However, an extensive revision of the literature46,47,48, the relatively high MAF according to 1000G (MAF = 0.019 in IBS and 0.029 in PUR respectively) and the fact that both mutations have been reported in cis with protein-truncating variants in STGD patients46,49, suggest that the clinical significance of these missense changes must be interpreted with caution especially in the context of genetic and reproductive counselling. Another explanation for the lack of success in detecting a second mutant allele in ABCA4 may be that, even if these changes were certainly pathogenic, they may not be the cause of disease in our patients. This is especially relevant in those cases where genotype does not correlate with the phenotype, for example, for STGD associated mutations in RP patients. In this regard, the frequency of IRD carriers in the general population is known to be relatively high50, as demonstrated by the increased prevalence of IRDs in consanguineous communities51,52. Of note, we have detected two patients with the ABCA4 variants p.Ile156Val and p.Val2050Leu, respectively, and additional mutations in other loci.
The identification of p.Ile156Val in family B can be considered a chance finding. The affected member of family B (II:1) harboured the recurrent single base pair deletion in CNGB3, c.1148del; p.Thr383Ilefs*13. This mutation is the most common variant underlying achromatopsia (ACH) worldwide53,54, accounting for over 70% of all CNGB3 changes and about 40% of all ACH associated alleles55. Additionally, this variant has also been found in patients with juvenile macular degeneration56, macular malfunction56 and, recently, cone dystrophy (COD)57. Ophthalmologic examination of patient II:1 of family B confirmed the clinical diagnosis of COD. Although the pathogenicity of CNGB3 c.1148del (p.Thr383Ilefs*13) seems convincing due to the large amount of supporting studies, two homozygous individuals have been detected in healthy control databases, one in ExAC and another one in EVS (entry 8:87,656,008AG/A), but the presence of pathogenic variants in healthy individuals has already been widely documented43,58.
Likewise, the contribution to the phenotype of the ABCA4 p.Val2050Leu variant in the index patient of family C (II:1) is not entirely clear. This patient carries also a heterozygous variant in gene FSCN2. Nevertheless the pathogenicity of the FSCN2 mutation could not be ascertained due to the lack of family members for segregation studies and the poor results of the in silico predictors (Table 1). Moreover, the association of this gene with IRDs is controversial59,60. Therefore, additional studies will be required to diagnose this simplex patient.
Interestingly, two families (F and Q) which were previously assessed with a customized panel are now genetically explained with a second mutation in USH2A (Table 2). The second hits consisted of both intronic variants (c.2167 + 5G > A; r.(spl?) and (c.12067-2 A > G; r.spl, respectively. Both variants should have been detected by the previous panel approach, but likely, they were bioinformatically filtered out due to the presence of duplicates.
In our cohort, the USH2A variant p.Cys759Phe was the most commonly mutated allele. Accordingly with other studies performed worldwide, this mutation is one of the most prevalent USH2A variants associated, in almost all of the cases, with non-syndromic RP61,62. Additionally, p.Cys759Phe variant has been frequently detected in compound-heterozygous state accompanied by a deleterious allele, while homozygous cases are rare and have been the subject of controversy42. In order to assess the hypothesis that the p.Cys759Phe variant is not pathogenic per se but it would be acting in cis with another non-coding pathogenic USH2A variant nearby, we analyzed in detail the deep intronic regions of this gene in solved cases harbouring this mutation. However, we were unable to identify any shared variant that met the criteria to be classified as pathogenic19. Therefore, if additional genetic load is acting together with the p.Cys759Phe, it is possibly that it is located in other regulatory regions.
Among the other USH2A mutations, two of them (p.Glu767Serfs*21 and p.Arg303Ser) have been detected in both syndromic and non-syndromic RP patients (Families M, N, O and P). The expression of the phenotype varies depending on the nature of the second mutation. Therefore, the greater the impact on the protein function, the greater the likelihood of developing the most severe condition, in this case, USH. Remarkably, the affected members of family P were compound heterozygous for one CNV (duplication of exons 46–47) and one missense (p.Arg303Ser), previously reported to be causative of USHII61,63. The index patient (family P-II:9) presented, besides typical arRP, bilateral sensorineural hearing loss. These findings were consistent with a diagnosis of USHII. However, the other two affected siblings (II:1 and II:7) were diagnosed of non-syndromic RP. The fact that a specific combination of mutations may be associated with a wide spectrum of symptoms in the same family, can be explained by the modulating effects of other genes and/or environmental factors on phenotypic expression64.
The main aim of this study was to evaluate the contribution of deep-intronic variants in a cohort with a previously detected heterozygous mutation in ABCA4, USH2A and CEP290. In this regard, only two predicted pathogenic mutations might be considered to be disease-causing in one patient (Family S, Supplementary Fig. S1). Prediction reports of intronic variants must be interpreted with caution in a clinical context and functional studies are mandatory. Moreover, although a study involving a larger number of samples would help to clarify the role of deep-intronic variants in the aetiopathogenesis of IRDs, the results presented here seem to indicate that deep-intronic variants have a small contribution in this group of patients.
In summary, the possibility of sequencing a number of entire genes represented an intermediate strategy between targeted sequencing and whole-genome sequencing. However, due to the high prevalence of carriers of mutations in IRD genes in the general population, the large amount of data generated with this panel, and the results obtained in this study, sequencing entire genes do not seem to be the approach of choice for detecting the second hit in IRD patients with monoallelic variants.
Methods
Subjects and clinical evaluation
A total of 29 unrelated Spanish families with different IRDs were involved in this study, including all available family members for segregation analysis. This cohort was composed of 25 index patients with one previous known mutation in ABCA4, CEP290 or USH2A genes, and 4 IRDs patients that had not been studied before but with clinical suspicion of harbouring mutations in the genes included in the panel (Table 2). Prior to the study, written informed consents were obtained from all participants or their legal guardians. Study protocols followed the tenets of the Declaration of Helsinki and they were approved by the Institutional Review Boards of the University Hospital Virgen del Rocío (Seville, Spain).
Clinical diagnosis of retinal dystrophy was based on fundus examination, visual acuity, computerized testing of central and peripheral visual fields and electroretinography (ERG) findings. Furthermore, certain non-ocular features associated with retinal degenerations were evaluated in syndromic cases. Peripheral blood was collected from all subjects to extract genomic DNA using standard protocols. Previous analyses of the 25 subjects with known mutations were made by Asper Biotech Genotyping microarrays, Sanger sequencing of exon 13 of USH2A or by applying an earlier version of our custom panel9,30 (Table 2).
Custom panel development
Our IRDs custom panel was designed using the SeqCap EZ application of the NimbleDesign software (Roche, NimbleGen, Madison, WI, USA). The intended covered sequences comprised three whole genes (ABCA4, CEP290 and USH2A) known to have deep-intronic mutations associated with IRDs, as well as the coding exons and their adjacent 25 bp of 76 IRD genes (Supplementary Table S2). The genes were selected as previously described9; only those genes with pathogenic mutations in Spanish population were included. Besides, two known point mutations in deep-intronic regions of OFD1 (c.935 + 706 A > G) and PRPF31 (c.1374 + 654 C > G) were covered as well. A total of 1,239 regions were targeted, with a final panel size of 1,412,505 bp.
Library preparation and sequencing
DNA library was performed according to the manufacturer’s protocol (NimbleGen SeqCap EZ Library SR version 5.1, Roche). Briefly, 1 μg of genomic DNA was sheared using Covaris S220 (Covaris, Woburn, MA, USA) to obtain an average fragment size of 180–220 bp. A multiplex DNA library pool, generated by mixing identical amount of DNA from several samples, was captured. Quantification of libraries was made using Agilent 2100 Bionalyzer (Agilent Technologies, CA, USA), qPCR and fluorimetric techniques. Sequencing was performed on the Illumina’s MiSeq or NextSeq instruments (Illumina, San Diego, CA, USA) using a MiSeq v2 (300 cycles) and NextSeq Mid-output v2 (300 cycles) reagent kits.
Bioinformatic analysis
Data analysis was performed using our validated pipeline30 with some modifications. Burrows-Wheeler Aligner (BWA, version 0.7.12) was used to map sample reads against the hg19 human reference genome. BEDtools package (version 2.17.0) was used to analyze the percentage of reads on-target and the mean coverage in each sample. Duplicate reads were filtered out by employing PICARD’s MarkDuplicates command (version 1.95). Variant calling and filtering were carried out using GATK software (version 3.3.0) and reads with coverage <20X and strand bias (FS > 60.0) were discarded. SNVs and indels variants were then annotated using wANNOVAR65, and only those with MAF < 0.015 in 1000G, Exome Variant Server (EVS), Exome Aggregation Consortium (ExAC), genome Aggregation Database (GnomAD) and dbSNP remained for further analysis. The frequency of all candidate variants was also checked in the Collaborative Spanish Variant Server (http://csvs.babelomics.org/) including a local population database that contains population frequency information from the whole exomes of 267 unrelated individuals, representative of the healthy Spanish population (Medical Genome Project, MGP)66.
Copy-Number Variations (CNVs) were identified employing the coverage command of BEDtools. In this method, the number of reads for each chromosomal interval of the bed file was normalized using the average number of reads generated per sample. These data were then compared with the corresponding data of the other samples in the same sequencing run. A ratio around 1 implied normal dosage; deletions and duplications ratios were set on <0.6 and >1.40 respectively. All CNVs were checked in Database of Genomic Variants (DGV) and DECIPHER67.
Variants prioritization and pathogenicity assesment
Prioritization was made with a step-by-step in-house pipeline. All variants of each sample were filtered by a Minor Allele Frequency (MAF) consistent with their disease (for IRD, MAF < 0.015). In patients with one known pathogenic variant in the coding sequence of ABCA4, CEP290 or USH2A, variants in these genes were prioritized. Heterozygous exonic, splicing and intronic variants were selected for further analysis, as well as variants with low coverage (<20X). For intronic and synonymous variants, three online tools were used to assess splicing changes: NNSPLICE (http://www.fruitfly.org/seq_tools/splice.html) and two algorithms included in Human Splicing Finder (HSF and MaxEntScan; http://www.umd.be/HSF). Specific thresholds were defined based on a known deep-intronic variants validation study for two tools19: a minimum score of 2 and a score variation >15% for MaxEnt and a minimum score of 70 and score variation >10% for HSF, was necessary to pass the quality threshold. For NNSPLICE, we use default settings (cut-off >0.4) and a score difference between wild-type and mutated sequence >10% was needed to be considered for further analysis. The pathogenicity of novel candidate variants was predicted using Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/), SIFT (http://sift.bii.a-star.edu.sg) and MutationTaster (www.mutationtaster.org/). Clinical significance of known variants was also assessed using ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and/or Leiden Open Variation Database, LOVD (https://databases.lovd.nl/). Candidate variants were segregated in all available relatives by Sanger sequencing according to the manufacturer’s protocols (3730 DNA Analyzer, Applied Biosystems, Foster City, CA, USA).
To be considered causal variants, they must (i) segregate with the disease, (ii) be described as pathogenic or likely pathogenic in databases (ClinVar, OMIM) or be a novel mutation, or (iii) have clinical manifestations consistent with the ones described for this variant. Large deletions and duplications were inspected with Integrative Genomics Viewer (IGV). If no candidate variants were found in these three entire genes, mutations in other loci were taken into account as described above. The nomenclature of variants was adjusted to the Human Genome Variation Society (http://varnomen.hgvs.org/) guidelines using Mutalyzer (https://mutalyzer.nl/).
References
Wright, A. F., Chakarova, C. F., Abd El-Aziz, M. M. & Bhattacharya, S. S. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nature reviews. Genetics 11, 273–284, https://doi.org/10.1038/nrg2717 (2010).
Hartong, D. T., Berson, E. L. & Dryja, T. P. Retinitis pigmentosa. Lancet 368, 1795–1809, https://doi.org/10.1016/S0140-6736(06)69740-7 (2006).
Hamel, C. P. Cone rod dystrophies. Orphanet journal of rare diseases 2, 7 (2007).
Stargardt, K. Über familiäre, progressive Degeneration in der Maculagegend des Auges. Albrecht von Graefes Archiv für Ophthalmologie 71, 534–550, https://doi.org/10.1007/bf01961301 (1909).
Gelisken, O. & De Laey, J. J. A clinical review of Stargardt's disease and/or fundus flavimaculatus with follow-up. International ophthalmology 8, 225–235 (1985).
Hull, S. et al. The phenotypic variability of retinal dystrophies associated with mutations in CRX, with report of a novel macular dystrophy phenotype. Invest Ophthalmol Vis Sci 55, 6934–6944, https://doi.org/10.1167/iovs.14-14715 (2014).
Yuan, Z. et al. The phenotypic variability of HK1-associated retinal dystrophy. Sci Rep 7, 7051, https://doi.org/10.1038/s41598-017-07629-3 (2017).
Broadgate, S., Yu, J., Downes, S. M. & Halford, S. Unravelling the genetics of inherited retinal dystrophies: Past, present and future. Progress in retinal and eye research 59, 53–96 (2017).
Bravo-Gil, N. et al. Improving the management of Inherited Retinal Dystrophies by targeted sequencing of a population-specific gene panel. Scientific reports 6, 23910, https://doi.org/10.1038/srep23910 (2016).
Bernardis, I. et al. Unravelling the Complexity of Inherited Retinal Dystrophies Molecular Testing: Added Value of Targeted Next-Generation Sequencing. BioMed research international 6341870, 29 (2016).
Jones, K. D. et al. Next-generation sequencing to solve complex inherited retinal dystrophy: A case series of multiple genes contributing to disease in extended families. Molecular vision 23, 470–481 (2017).
Perez-Carro, R. et al. Panel-based NGS Reveals Novel Pathogenic Mutations in Autosomal Recessive Retinitis Pigmentosa. Scientific reports 6 (2016).
Zaneveld, J. et al. Comprehensive analysis of patients with Stargardt macular dystrophy reveals new genotype-phenotype correlations and unexpected diagnostic revisions. Genetics in medicine: official journal of the American College of Medical Genetics 17, 262–270 (2015).
Vaz-Drago, R., Custodio, N. & Carmo-Fonseca, M. Deep intronic mutations and human disease. Human genetics 12, 017–1809 (2017).
Anna, A. & Monika, G. Splicing mutations in human genetic disorders: examples, detection, and confirmation. 59, 253–268, https://doi.org/10.1007/s13353-018-0444-7 (2018).
Albert, S. et al. Identification and Rescue of Splice Defects Caused by Two Neighboring Deep-Intronic ABCA4 Mutations Underlying Stargardt Disease. Am J Hum Genet 102, 517–527, https://doi.org/10.1016/j.ajhg.2018.02.008 (2018).
Zernant, J. et al. Extremely hypomorphic and severe deep intronic variants in the ABCA4 locus result in varying Stargardt disease phenotypes. Cold Spring Harbor molecular case studies, https://doi.org/10.1101/mcs.a002733 (2018).
den Hollander, A. I. et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. American journal of human genetics 79, 556–561, https://doi.org/10.1086/507318 (2006).
Liquori, A. et al. Whole USH2A Gene Sequencing Identifies Several New Deep Intronic Mutations. Human mutation 37, 184–193 (2016).
Carss, K. J. et al. Comprehensive Rare Variant Analysis via Whole-Genome Sequencing to Determine the Molecular Pathology of Inherited Retinal Disease. American journal of human genetics 100, 75–90 (2017).
Garanto, A., van der Velde-Visser, S. D., Cremers, F. P. M. & Collin, R. W. J. Antisense Oligonucleotide-Based Splice Correction of a Deep-Intronic Mutation in CHM Underlying Choroideremia. Advances in experimental medicine and biology 1074, 83–89, https://doi.org/10.1007/978-3-319-75402-4_11 (2018).
Rio Frio, T. et al. A single-base substitution within an intronic repetitive element causes dominant retinitis pigmentosa with reduced penetrance. Human mutation 30, 1340–1347 (2009).
Webb, T. R. et al. Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23). Human molecular genetics 21, 3647–3654, https://doi.org/10.1093/hmg/dds194 (2012).
Ezquerra-Inchausti, M. et al. High prevalence of mutations affecting the splicing process in a Spanish cohort with autosomal dominant retinitis pigmentosa. Scientific reports 7 (2017).
Ruzickova, S. & Stanek, D. Mutations in spliceosomal proteins and retina degeneration. RNA biology 14, 544–552, https://doi.org/10.1080/15476286.2016.1191735 (2017).
Stankiewicz, P. & Lupski, J. R. Structural variation in the human genome and its role in disease. Annu Rev Med 61, 437–455 (2010).
Bujakowska, K. M. et al. Copy-number variation is an important contributor to the genetic causality of inherited retinal degenerations. Genetics in medicine: official journal of the American College of Medical Genetics 19, 643–651 (2017).
Huang, X. F. et al. Genome-Wide Detection of Copy Number Variations in Unsolved Inherited Retinal Disease. Investigative ophthalmology & visual science 58, 424–429 (2017).
Ellingford, J. M. et al. Assessment of the incorporation of CNV surveillance into gene panel next-generation sequencing testing for inherited retinal diseases. J Med Genet 55, 114–121, https://doi.org/10.1136/jmedgenet-2017-104791 (2018).
Bravo-Gil, N. et al. Unravelling the genetic basis of simplex Retinitis Pigmentosa cases. Scientific reports 7, 41937, https://doi.org/10.1038/srep41937 (2017).
Khan, K. N. et al. Advanced diagnostic genetic testing in inherited retinal disease: experience from a single tertiary referral centre in the UK National Health Service. Clinical genetics 91, 38–45 (2017).
Ge, Z. et al. NGS-based Molecular diagnosis of 105 eyeGENE((R)) probands with Retinitis Pigmentosa. Scientific reports 5 (2015).
Schulz, H. L. et al. Mutation Spectrum of the ABCA4 Gene in 335 Stargardt Disease Patients From a Multicenter German Cohort-Impact of Selected Deep Intronic Variants and Common SNPs. Investigative ophthalmology & visual science 58, 394–403 (2017).
Bax, N. M. et al. Heterozygous deep-intronic variants and deletions in ABCA4 in persons with retinal dystrophies and one exonic ABCA4 variant. Human mutation 36, 43–47 (2015).
Shirts, B. H. et al. Deep sequencing with intronic capture enables identification of an APC exon 10 inversion in a patient with polyposis. Genetics in medicine: official journal of the American College of Medical Genetics 16, 783–786 (2014).
Suzuki, T. et al. Precise detection of chromosomal translocation or inversion breakpoints by whole-genome sequencing. Journal of human genetics 59, 649–654 (2014).
Pieras, J. I. et al. Copy-number variations in EYS: a significant event in the appearance of arRP. Investigative ophthalmology & visual science 52, 5625–5631, https://doi.org/10.1167/iovs.11-7292 (2011).
Gonzalez-del Pozo, M. et al. Mutation screening of multiple genes in Spanish patients with autosomal recessive retinitis pigmentosa by targeted resequencing. PloS one 6, e27894, https://doi.org/10.1371/journal.pone.0027894 (2011).
Steele-Stallard, H. B. et al. Screening for duplications, deletions and a common intronic mutation detects 35% of second mutations in patients with USH2A monoallelic mutations on Sanger sequencing. Orphanet journal of rare diseases 8, 1750–1172 (2013).
Nishiguchi, K. M. et al. Whole genome sequencing in patients with retinitis pigmentosa reveals pathogenic DNA structural changes and NEK2 as a new disease gene. Proceedings of the National Academy of Sciences of the United States of America 110, 16139–16144 (2013).
Eisenberger, T. et al. Increasing the yield in targeted next-generation sequencing by implicating CNV analysis, non-coding exons and the overall variant load: the example of retinal dystrophies. PloS one 8 (2013).
Gonzalez-Del Pozo, M. et al. Re-evaluation casts doubt on the pathogenicity of homozygous USH2A p.C759F. American journal of medical genetics. Part A, https://doi.org/10.1002/ajmg.a.37003 (2015).
Xue, Y. et al. Deleterious- and disease-allele prevalence in healthy individuals: insights from current predictions, mutation databases, and population-scale resequencing. American journal of human genetics 91, 1022–1032 (2012).
Papaioannou, M. et al. An analysis of ABCR mutations in British patients with recessive retinal dystrophies. Investigative ophthalmology & visual science 41, 16–19 (2000).
Allikmets, R. et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nature genetics 15, 236–246, https://doi.org/10.1038/ng0397-236 (1997).
Passerini, I. et al. Novel mutations in of the ABCR gene in Italian patients with Stargardt disease. Eye 24, 158–164 (2010).
Cornelis, S. S. et al. In Silico Functional Meta-Analysis of 5,962 ABCA4 Variants in 3,928 Retinal Dystrophy Cases. Human mutation 38, 400–408 (2017).
Sciezynska, A. et al. Next-generation sequencing of ABCA4: High frequency of complex alleles and novel mutations in patients with retinal dystrophies from Central Europe. Experimental eye research 145, 93–99 (2016).
Weisschuh, N. et al. Mutation Detection in Patients with Retinal Dystrophies Using Targeted Next Generation Sequencing. PloS one 11, e0145951, https://doi.org/10.1371/journal.pone.0145951 (2016).
Nishiguchi, K. M. & Rivolta, C. Genes associated with retinitis pigmentosa and allied diseases are frequently mutated in the general population. PloS one 7, e41902, https://doi.org/10.1371/journal.pone.0041902 (2012).
Bittles, A. Consanguinity and its relevance to clinical genetics. Clinical genetics 60, 89–98 (2001).
Sharon, D. & Banin, E. Nonsyndromic retinitis pigmentosa is highly prevalent in the Jerusalem region with a high frequency of founder mutations. Molecular vision 21, 783–792 (2015).
Sundin, O. H. et al. Genetic basis of total colourblindness among the Pingelapese islanders. Nature genetics 25, 289–293 (2000).
Wiszniewski, W., Lewis, R. A. & Lupski, J. R. Achromatopsia: the CNGB3 p.T383fsX mutation results from a founder effect and is responsible for the visual phenotype in the original report of uniparental disomy 14. Human genetics 121, 433–439 (2007).
Kohl, S. et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. European journal of human genetics: EJHG 13, 302–308 (2005).
Nishiguchi, K. M., Sandberg, M. A., Gorji, N., Berson, E. L. & Dryja, T. P. Cone cGMP-gated channel mutations and clinical findings in patients with achromatopsia, macular degeneration, and other hereditary cone diseases. Human mutation 25, 248–258, https://doi.org/10.1002/humu.20142 (2005).
Gupta, S. et al. Whole exome sequencing unveils a frameshift mutation in CNGB3 for cone dystrophy: A case report of an Indian family. Medicine 96, 0000000000007490 (2017).
Kobayashi, Y. et al. Pathogenic variant burden in the ExAC database: an empirical approach to evaluating population data for clinical variant interpretation. Genome medicine 9, 017–0403 (2017).
Gamundi, M. J. et al. Sequence variations in the retinal fascin FSCN2 gene in a Spanish population with autosomal dominant retinitis pigmentosa or macular degeneration. Molecular vision 11, 922–928 (2005).
Zhang, Q., Li, S., Xiao, X., Jia, X. & Guo, X. The 208delG mutation in FSCN2 does not associate with retinal degeneration in Chinese individuals. Investigative ophthalmology & visual science 48, 530–533 (2007).
Aller, E. et al. Genetic analysis of 2299delG and C759F mutations (USH2A) in patients with visual and/or auditory impairments. European journal of human genetics: EJHG 12, 407–410, https://doi.org/10.1038/sj.ejhg.5201138 (2004).
Rivolta, C., Sweklo, E. A., Berson, E. L. & Dryja, T. P. Missense mutation in the USH2A gene: association with recessive retinitis pigmentosa without hearing loss. Am J Hum Genet 66, 1975–1978, https://doi.org/10.1086/302926 (2000).
Baux, D. et al. Enrichment of LOVD-USHbases with 152 USH2A genotypes defines an extensive mutational spectrum and highlights missense hotspots. Human mutation 35, 1179–1186 (2014).
Ebermann, I. et al. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. The Journal of clinical investigation 120, 1812–1823 (2010).
Chang, X. & Wang, K. wANNOVAR: annotating genetic variants for personal genomes via the web. Journal of medical genetics 49, 433–436 (2012).
Dopazo, J. et al. 267 Spanish Exomes Reveal Population-Specific Differences in Disease-Related Genetic Variation. Molecular biology and evolution 33, 1205–1218 (2016).
Firth, H. V. et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. American journal of human genetics 84, 524–533 (2009).
Lewis, R. A. et al. Genotype/Phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. American journal of human genetics 64, 422–434, https://doi.org/10.1086/302251 (1999).
Allikmets, R. et al. Organization of the ABCR gene: analysis of promoter and splice junction sequences. Gene 215, 111–122 (1998).
Jaijo, T. et al. Microarray-based mutation analysis of 183 Spanish families with Usher syndrome. Investigative ophthalmology & visual science 51, 1311–1317, https://doi.org/10.1167/iovs.09-4085 (2010).
Jaijo, T. et al. Functional analysis of splicing mutations in MYO7A and USH2A genes. Clinical genetics 79, 282–288 (2011).
McGee, T. L., Seyedahmadi, B. J., Sweeney, M. O., Dryja, T. P. & Berson, E. L. Novel mutations in the long isoform of the USH2A gene in patients with Usher syndrome type II or non-syndromic retinitis pigmentosa. Journal of medical genetics 47, 499–506, https://doi.org/10.1136/jmg.2009.075143 (2010).
Aller, E. et al. Identification of 14 novel mutations in the long isoform of USH2A in Spanish patients with Usher syndrome type II. Journal of medical genetics 43, e55, https://doi.org/10.1136/jmg.2006.041764 (2006).
Dreyer, B. et al. A common ancestral origin of the frequent and widespread 2299delG USH2A mutation. American journal of human genetics 69, 228–234 (2001).
Baux, D. et al. Molecular and in silico analyses of the full-length isoform of usherin identify new pathogenic alleles in Usher type II patients (2007).
Mendez-Vidal, C. et al. Whole-exome sequencing identifies novel compound heterozygous mutations in USH2A in Spanish patients with autosomal recessive retinitis pigmentosa. Molecular vision 19, 2187–2195 (2013).
Kaiserman, N., Obolensky, A., Banin, E. & Sharon, D. Novel USH2A mutations in Israeli patients with retinitis pigmentosa and Usher syndrome type 2. Archives of ophthalmology 125, 219–224, https://doi.org/10.1001/archopht.125.2.219 (2007).
Acknowledgements
This work was supported by Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Economy and Competitiveness, Spain and co-funded by European Union (ERDF/ESF, “Investing in your future”) [PI15-01648], CIBERER ACCI [ER16P1AC702/2017] and Regional Ministry of Economy, Innovation, Science and Employment of the Autonomous Government of Andalusia [CTS-1664]. The CIBERER is an initiative of the ISCIII, Spanish Ministry of Economy and Competitiveness. MM-S is supported by fellowship from Regional Ministry of Economy, Innovation, Science and Employment of the Autonomous Government of Andalusia (CTS-1664).
Author information
Authors and Affiliations
Contributions
G.A. and S.B. conceived and designed this work. E.R.-R. performed the ophthalmic evaluations. M.G.-P., M.M.-S., N.B.-G. and C.M.-V. conducted the experiments and M.G.-P., M.M.-S., N.B.-G., C.M.-V. and A.C. analyzed the results. M.G.-P. and M.M.-S. wrote the manuscript. G.A. and S.B. revised the paper critically for important intellectual content. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-del Pozo, M., Martín-Sánchez, M., Bravo-Gil, N. et al. Searching the second hit in patients with inherited retinal dystrophies and monoallelic variants in ABCA4, USH2A and CEP290 by whole-gene targeted sequencing. Sci Rep 8, 13312 (2018). https://doi.org/10.1038/s41598-018-31511-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31511-5
This article is cited by
-
A comprehensive WGS-based pipeline for the identification of new candidate genes in inherited retinal dystrophies
npj Genomic Medicine (2022)
-
USH2A gene variants cause Keratoconus and Usher syndrome phenotypes in Pakistani families
BMC Ophthalmology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.