Abstract
Baseline plasma samples of 490 randomly selected antiretroviral therapy (ART) naïve patients from seven hospitals participating in the first nationwide Ethiopian HIV-1 cohort were analysed for surveillance drug resistance mutations (sDRM) by population based Sanger sequencing (PBSS). Also next generation sequencing (NGS) was used in a subset of 109 baseline samples of patients. Treatment outcome after 6– and 12–months was assessed by on-treatment (OT) and intention-to-treat (ITT) analyses. Transmitted drug resistance (TDR) was detected in 3.9% (18/461) of successfully sequenced samples by PBSS. However, NGS detected sDRM more often (24%; 26/109) than PBSS (6%; 7/109) (p = 0.0001) and major integrase strand transfer inhibitors (INSTI) DRMs were also found in minor viral variants from five patients. Patients with sDRM had more frequent treatment failure in both OT and ITT analyses. The high rate of TDR by NGS and the identification of preexisting INSTI DRMs in minor wild-type HIV-1 subtype C viral variants infected Ethiopian patients underscores the importance of TDR surveillance in low– and middle–income countries and shows added value of high-throughput NGS in such studies.
Similar content being viewed by others
Introduction
Ethiopia is heavily affected by the human immunodeficiency virus type 1 (HIV-1) epidemic with an estimated number of 700,000 infected persons, although the adult prevalence has decreased to 1.1% in 20161,2. Antiretroviral therapy (ART) has been widely accessible since 20053. The first-line consists of fixed-dose combinations (FDC) of two nucleoside/nucleotide reverse transcriptase inhibitors (NRTI; zidovudine (ZDV) or tenofovir (TDF) plus lamivudine (3TC) or emtricitabine (FTC)), and a non-nucleoside RTI (NNRTI; efavirenz (EFV) or nevirapine (NVP))4. Prevention of mother-to-child transmission (PMTCT) was launched in 2003 using single-dose nevirapine (sdNVP). In 2014, 360,000 patients were estimated to receive ART5. However, more than a quarter of Ethiopian patients are lost-to-follow-up (LTFU), in some regions up to 40%4,6. Also, the absence of monitoring of viral load and the limited availability of second-line ART, can be expected to contribute further to a high rate of therapy failure with drug resistance, as seen in other low and middle income countries (LMIC)7,8.
A recent report from WHO revealed a global increase in pretreatment drug resistance (PDR)9. In Ethiopia, there are no data so far of transmitted drug resistance (TDR) or PDR at the national level, except very limited information from some parts of the country. A study from Addis Ababa using a sensitive allele-specific polymerase chain reaction reported a 6.5% TDR prevalence10 and two studies from Northwest Ethiopia reported a 3.3%11 and a 5.6% TDR prevalence, respectively12. Due to this very limited information about the situation in Ethiopia with regard to HIV drug resistance, we assessed TDR by population-based Sanger sequencing (PBSS) among ART naïve patients, included in a large nationwide cohort study, and compared the results with next-generation sequencing (NGS). Thus, none of our patients were known to have been treated with ART before inclusion in the study. In addition, due to the recent introduction of integrase strand transfer inhibitors (INSTI) in certain African countries, we also analysed the NGS results for INSTI associated drug resistance mutations (DRM).
Results
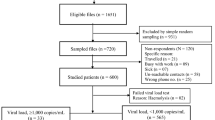
Among the 874 ART naïve HIV-1 infected patients enrolled in the study, 676 and 459 had VL data at month six and 12, respectively. Ninety (13.3%) and 61 (13.3%) had VL ≥150 copies/ml (detection limit of the assay) and 57 (8.4%) and 34 (7.4%) had VL ≥1000 copies/ml (WHO definition of virologic failure), respectively.
As described in the methodology section, baseline samples of randomly selected 490 patients (females: 58%; median age: 33 years) were tested for PDR by PBSS and assessed mutations associated with NRTI−, NNRTI−, and PI− drug classes. In addition, baseline samples of 109 virologic treatment failure (n = 71) and virologic suppressor (n = 38) patients were analyzed by NGS (females: 55%; median age: 30 years), where PDR associated with NRTI-, NNRTI-, PI- and INSTI- drug classes were considered (Table 1).
Outcomes of ART and sequencing
Of the 490 patients, 408 (83.3%) were still on treatment at month six and the remaining were either LTFU (n = 33) or dead (n = 49) (Fig. 1). Plasma HIV-1 RNA (VL) was not tested in 20 subjects and among those with a VL data, 316 (81.4%) had undetectable viremia, and 72 (18.6%) detectable viremia. At month 12, 383 (78.2%) out of 490 subjects were still on treatment. VL was not tested in 114 subjects and among those with an available VL, 228 (84.8%) had undetectable viremia, and 41 (15.2%) had detectable viremia. Eleven patients had died and 14 were LTFU, respectively, between month six and 12.
Study profile of patients. PBSS: population-based Sanger sequencing; NGS: next generation sequencing. NRTI: nucleoside analogue reverse transcriptase inhibitors; NNRTI: non-nucleoside RTI; PI: protease inhibitors; INSTI: integrase inhibitors. DRM: drug resistance mutations (surveillance drug resistance mutations were described for NRTI, NNRTI and PI).
For PBSS, a pol-sequence was obtained in 461 out of 490 (94%) samples at baseline, 47 out of 51 (93%) at month six and 30 out of 33 (91%) at month 12 (Fig. 1). For NGS, a result was obtained for all 109 samples with contigs ranging from one to ten. Of these, the best contig was selected. Three samples gave a fragmented contig, which were rectified manually. Another sample showed a large deletion in the RNaseH and integrase region. The sequences clustered with HIV-1C, except one CRF02_AG by the three subtyping tools listed in the methodology section.
Baseline sDRM detected by PBSS and impact on treatment outcome
At baseline, 18 (3.9%) of the 461 patients with sequence data had sDRM (NRTI: n = 9; NNRTI: n = 7; PI: n = 2) (Figure 1; Table 2). None of the patients had dual drug class sDRM. Three patients had two mutations of the same drug class. There was no correlation between the presence of sDRM and study site, sex, and CD4 + T-cell count (data not shown), but the odds of having sDRM decreased significantly as participant’s age increased (OR: 0.93; 95% CI: 0.87–0.99) and increased with higher baseline viral load (OR: 2.67; 95% CI: 1.25–5.71).
Fourteen out of the 18 patients with sDRM were still on ART at month 12, but six of them had no VL results. Patients with the RTI-sDRM had higher odds of virologic failure (defined as VL >150 copies/ml) after month six and 12 than those without RTI-sDRM (OR: 3.6; 95% CI: 1.2–11.1 and OR: 9.00; 95% CI: 1.9–43.3, the latter adjusted for tuberculosis co-infection). Also when the WHO treatment failure definition (VL >1000 copies/ml) was used, those with RTI-sDRM had higher odds of failure both at month six and 12 (OR: 6.5; 95% CI: 2.1–20.3 and OR: 7.4; 95% CI: 1.5–35.0, respectively). In the ITT analysis, patients with RTI-sDRM had significantly higher treatment failure rates at the six months, for both the 150 and 1000 copies/ml cut-off, than those without the mutations (OR: 2.9; 95% CI: 1.0–7.9 and OR: 3.8; 95% CI: 1.4–10.5, respectively), but not at month 12 (p = 0.053 and p = 0.099, respectively).
Acquired DRM detected by PBSS
At month six, 47 sequences were obtained from 51 patients who had a VL >1000 copies/ml and attempted for sequencing. Of those, 37 (79%) had one to seven major NRTI and/or NNRTI DRMs, and no major PI DRM (NRTIs + NNRTI: 25 (68%); only NRTI: seven (19%); only NNRTI: five (14%)) (see Supplementary Table S1). At month 12, 25 (83%) of 30 sequences from 33 failing patients attempted for sequence analyses had one to eight major NRTI and/or NNRTI DRMs and no major PI DRM (NRTIs and NNRTI DRMs: 16 (64%); only NRTIs: 6 (24%); NNRTIs DRMs: three (12%)).
Among those patients with sDRM at baseline who reached month six and had VL data, six out of eight patients with NRTI sDRM and two of two with PI sDRM, but only one of the five patients with NNRTI sDRM (K103N) had undetectable viremia (Table 2).
Baseline DRM detected by NGS
All NGS attempts were successful and baseline sequences were thus obtained from 109 patients. Patients with virological treatment failure (n = 71) had lower CD4+ T-cells at baseline than the virologic suppressors (n = 38) (112 cells/µl vs 150cells/µl; p = 0.02). No significant differences were found for other biological and demographic parameters in relation to the virologic treatment outcome.
Altogether NGS detected DRM at baseline in 28 patients (NRTI; n = 8; NRTI + NNRTI: n = 1; NRTI + INSTI: n = 1; NRTI + PI: n = 1; NNRTI: n = 10; PI: n = 3; INSTI DRM: n = 2; three drug classes: n = 2). NGS detected RTI or PI sDRM (>1% frequency) significantly more often (23.9%; 26/109) than PBSS (6.4%; 7/109) (p < 0.0001). In addition, INSTI DRM was found in five subjects.
The NGS DRMs (range one to three) were found in 23 (32.4%) of the 71 patients who at month six and/or 12 had a virological failure (>1000 copies/ml) (NRTI: n = 12; NNRTI: n = 9; PI: n = 5; INSTI: n = 4) (Table 3). Five (13.2%) out of 38 patients with undetectable viremia at month six and/or 12 had one DRM each at baseline (NRTI: n = 1; NNRTI: n = 2; PI: n = 1; INSTI: n = 1). There was no significant difference between patients with RTI and/or PI sDRM and those without the sDRM with regard to study sites, age, WHO clinical stage, CD4 cells or VL (data not shown). However, females had higher proportion of sDRM than males (19/60 vs 7/49, p < 0.05).
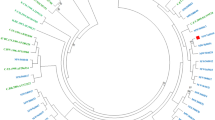
The INSTI-DRMs detected by NGS were E138K (n = 2; 1.3% and 2.1%, respectively), Q148R (n = 1; 1.6%), Q148H (n = 1; 1.5%), and T66I (n = 1; 22.3%) (Table 3). These patients were from all study sites, except Jimma and the Army unit (details of study sites depicted in the methodology section). No clustering was found among the viral strains with INSTI DRM (Fig. 2).
Impact of baseline DRM detected by NGS and correlation with PBSS
From baseline samples of failing patients, NGS detected any RTI sDRM (at >1% frequency of the population) significantly more often (28.2%; 20/71) than PBSS (8.54%; 6/71) (p = 0.004; Fisher exact test) (Table 3). PBSS failed to detect six out of 14 (42.9%) sDRM from four patients despite that the NGS assay detected sDRM corresponding to greater than 20% of the viral population. These six sDRM were derived from four different patients. Patients who failed ART with >150 copies/ml at month six and/or 12 had more frequently one or more NRTI, NNRTI and/or PI sDRM by NGS at baseline as compared to the virologic suppressors (OR: 6.4; 95% CI: 1.6–26.4 adjusted for NRTI regimens and CD4 cell counts) (Table 3). This was also holds true when only patients with sDRM NRTI and/or NNRTI were considered (20/71 versus 3/38) (p < 0.05).
Next we checked whether sDRM detected by NGS appeared at virological treatment failure (>1000 copies/ml) at month six and/or 12, as determined by PBSS (Table 4). Among 16 patients who failed at month six, only six out of 25 NGS NRTI or NNRTI sDRM at baseline appeared at month six. All these sDRM were detected at a high proportion at baseline (T215S: 99.3%, L210W: 30.3%, Y181C: 38.3%, Y181C: 97.4%, G190S: 99.6%, Y181C: 82.9%). However, other six sDRM at high baseline proportions were not detected at month six or 12 (K101E: 45.0%, D67G: 45.6%, L74I: 40.6%, G190E: 98.1%; K219N: 23.4%, Y188C: 51.9%).
Discussion
The present study is the first countrywide representative survey of transmitted drug resistance (TDR), based on the first large national ART cohort study in Ethiopia13,14. Analysing 461 pol sequences by PBSS, we found a low frequency, 3.9%, of treatment-naïve patients with sDRM. In a selected sub-set of 109 patients, additional DRMs were found by NGS, including major INSTI DRMs in five patients. Patients with TDR failed therapy more frequently both in OT and ITT analysis, suggesting a clinical impact of these mutations.
By PBSS, NRTI and NNRTI sDRM were found as expected, but also non-polymorphic accessory PI sDRM in two patients, despite the infrequent use of PI in Ethiopia in 2009–2011. An inclusion criterion in the ACM cohort was self-reported no earlier use of ART. If correctly self-reported, the prevalence of TDR was 3.9% and no regional difference within Ethiopia was observed. However, it shall be emphasized that the patients were recruited in 2009–2011 and that the present situation of pretreatment resistance (PDR) may have been changed. TDR in LMIC has increased, primarily NNRTI TDR, over time in sub-Saharan Africa (SSA)9. In addition, it should be noted that our patients had low CD4 cell counts at start of ART and were most likely not newly infected. Therefore, the TDR rate might be underestimated in our study since some drug resistant variants frequently disappear from the major viral population after a period of no ART. The increase has been steepest in east Africa up to a 7.4% prevalence eight to nine years after rollout of ART. An update until 2016, but now including all PDR, confirms this trend and the predictions of the prevalence of NNRTI PDR for 2016 were 11% (95% CI 7.5–15.9) and 15.5% (95% CI 7.7–28.8) in Southern- and Eastern-Africa, respectively9. Data from Ethiopia was however not included in these reports. Smaller and regional studies using PBSS have reported low frequencies, 3.3% in 200311 and 0% in 200515, which increased in later studies, 5.6% in 200812, and 7.2% in 201016. Our nationwide data from 2009–2011 in a larger number of patients did not however suggest an increasing trend of TDR in Ethiopia up to then.
A higher number of sDRM was identified by NGS, which is in line with our earlier report of a high detection rate (6.5%) of NNRTI TDR in Addis Ababa, 2009–2010, using a sensitive allele-specific PCR10. Thus, additional DRMs were detected in 17 patients selected for the NGS assay. Of these, mutated viral populations representing more than 20% were found in four patients represented, which should have been possible to be able to detect with our PBSS assay. Although the selection of these patients were biased, the discrepancy between PBSS and NGS suggests that NGS facilitates detection of HIV-1 sDRMs in LMICs and reveals a higher prevalence of PDR to the same or lower cost if high-throughput approaches are used17.
In a study conducted on small number of patients (n = 45) from Gondar, Ethiopia recruited in 2008, using PBSS, no major INSTI DRM was found18. Interestingly, in our study major INSTIs mutations (T66I, E138K, Q148R, and Q148H) were found in five patients albeit at a low abundance. At the time when the study started (2009–2011), to our knowledge, no patient in Ethiopia had been treated with an INSTI and still these drugs are not an integrated part of the Ethiopian ART regimes. It cannot be excluded that INSTIs DRMs have been introduced in Ethiopia through patients who have been treated outside the country. However, our phylogenetic analysis showed no clustering of the strains with INSTIs DRM and the patients came from five different study sites all over Ethiopia. It seems therefore unlikely these strains have been transmitted from INSTI treated subjects. Also, we found no evidence of cross-contamination of INSTI-resistant strains in our laboratory, which is strictly separated from the clinical diagnostic laboratory. A possibility is that wild-type HIV-1C strains in Ethiopia may harbor low abundance of INSTI DRMs. All of the identified DRMs alone or in combination associated with resistance to raltegravir and/or elvitegravir.
Recently, dolutegravir (DTG) has been planned to be used in some African countries, as fixed dose combination given once daily. Importantly, the INSTI DRM E138K contributes to reduced susceptibility to DTG in combination with other INSTI DRM. Also, Q148R and Q148H are associated with low-level or intermediate resistance to DTG, which should be administered twice daily if these DRM are present. A similar pattern is found for cabotegravir and bictegravir19,20. Our finding warrants therefore expanded analysis of minor quasispecies with regard to INSTI DRMs in different African patient populations in order to identify how often such low abundance DRM can be find.
The impact of preexisting INSTI DRMs on clinical treatment response has been discussed earlier21,22. E.g. the E157Q mutation has been reported in 1.7% and 5.6% of viral sequences from ART-naïve patients, depending on subtype23 and been implied to affect treatment response24. On the other hand, low abundance INSTI DRMs were not shown to have impact on treatment outcome21,22. However, these latter studies used allele-specific PCR detecting a significantly lower proportion of mutated virus than our NGS method, which has 1% cut-off. Therefore, a potential clinical impact of our findings still remains to be evaluated.
Patients with baseline pol-sDRM failed ART more frequently at month six. Also additional sDRM were identified pre-ART with the NGS assay. A dose-effect association between the level of low-abundance NNRTI-resistant mutants and a 5% threshold of mutant frequency has been suggested to be clinically relevant25. In our study, the number of patients with NGS sDRM was too small to allow identification of a threshold. However, among 16 patients who failed at month six, only sex out of 25 NGS NRTI or NNRTI sDRM at baseline were detected at month six by PBSS. All these six sDRM had been detected at a high proportion at baseline. The lack of detection of the minor sDRM in the follow-up samples could possibly indicate they had a limited or no impact on the emergence of drug resistance at the follow-up time points. However, further study is recommended to assess their impact as a secondary mutation for the emergence of DRM.
In conclusion, we have analysed TDR in the largest nationwide Ethiopian cohort so far and found that in 2009-2011 the rate was still low, 3.9%, using PBSS, but TDR before treatment was associated with a poorer treatment outcome. Also, our NGS results showed that the rate of 3.9% is an underestimation although we could not confirm that the low abundance DRM had a clinical impact. Interestingly, we identified preexisting INSTI DRM in wild-type HIV-1C from treatment naïve patients. Our data shows the importance of surveillance for TDR and PDR in LMIC and suggests an added value of using high-throughput NGS in such studies.
Material and Methods
Study Population
Through October 2009 to December 2011, a total of 874 ART naïve patients were recruited to the Advanced Clinical Monitoring (ACM) of ART in Ethiopia cohort, and started ART, as per the national guideline4. The subjects were from seven universities13,14, distributed geographically all over the country: Tikur Anbessa Specialized Hospital in Addis Ababa- Central region; Gondar– Northwest; Jimma– West; Mekelle– North; Harrar– East; Hawassa– South; the Army unit providing service to mobile military staff, which is located in Addis Ababa (Fig. 3). Our study was conducted on 490 subjects (age ≥ 14 years), randomly selected after stratifying by study sites (70 from each site), who were followed until the end of 2013 (Table 1). The following FDCs were given: TDF + 3TC + EFV (n = 222), TDF + 3TC + NVP (n = 39), ZDV + 3TC + EFV (n = 60), ZDV + 3TC + NVP (n = 144), stavudine (d4T) + 3TC + EFV (n = 15), d4T + 3TC + EFV (n = 9), and abacavir (ABC) + 3TC + EFV (n = 1).
Clinical and routine laboratory tests were performed at the study sites. Ten ml whole blood was collected and processed for each patient at baseline, month six and 12. Plasma samples were centrally stored at the Ethiopian Health and Nutrition Research Institute (EHNRI) at −80 °C after transport on dry ice. Quantification of VL was performed by NucliSENS easyQ® HIV-1 Nucleic Acid Sequence-Based-Amplification (NASBA) (BioMérieux Diagnostics) with a detection limit of 150 HIV-1 RNA copies/ml. CD4 T-cell count was determined at the hospital laboratories by BD FACSCalibur machines (Becton Dickinson, San Jose, USA). Data was entered into a site database and later uploaded to the central database at EHNRI, from which the following data were extracted: sex, age, WHO clinical stage, ART regimen, CD4 cell count and VL.
Population-based Sanger sequencing (PBSS)
PBSS was attempted on 490 baseline samples as well as on 51 and 33 samples with VL ≥1000 copies/ml at month six and 12, respectively. HIV RNA was extracted from 140 µl plasma using the QIAamp® RNA extraction mini-kit (Qiagen, Hilden, Germany). cDNA synthesis was done using RevertAid H-minus reagents (Life technologies, Paisley, UK). The first-round PCR was done using JA203F-C (forward) and JA206R-C (reverse) primer pair, followed by the second-round PCR, using JA204F-C (forward) and JA205R-C (reverse) primer pair26. The amplified fragments were purified (QIAquick PCR Purification Kit, Qiagen, Hilden, Germany) and sequenced with JA204F-C and JA205R-C PCR-primers plus PR2R (5′-GGATTTTCAGGCCCAATTTTTG-3′) and RT07 (5′-AAGCCAGGAATGGATGGCCCA-3′). This method has been used extensively at our laboratory. Positive PCR reactions are obtained in 100% of plasma samples containing the equivalent of 500 HIV-1 RNA copies per PCR reaction. In practice the assay gives positive results in the vast majority of plasma samples containing >500 copies/ml with a sensitivity to detect 20% mutated variants in the viral population. A comparison between the original assay26 and our slightly assay modified with primers specifically designed for HIV-1C has shown equal results.
Sequences were aligned, edited and analysed using the BioEdit software version 7.2.6.1 (http://www.mbio.ncsu.edu/bioedit/bioedit.html). Primary DRM were identified by the calibrated population resistance tool (http://cpr.stanford.edu/cpr.cgi) at Stanford HIVDR Database. Acquired DRM were identified by the Stanford HIVdb Program (hivdb.stanford.edu). DRMs associated with NRTI-, NNRTI-, and PI- drug classes were considered in this assay.
Next generation sequencing (NGS)
NGS was performed on 109 baseline samples of all patients, who had viremia at month six and/or 12 (n = 71), and from randomly selected patients with undetectable viremia (n = 38), as described27. In brief, fragment I (HXB2: 790 – 5096) covering Gag-pol was amplified, gel purified, and fragmented on the Coveris S200 followed by library preparation using NEBNext UltraTM DNA library Prep Kit. Forty-eight libraries were then pooled at equimolar (10 nM each) and run on Illumina HiSeq. 2500. The FASTQ file was demultiplexed and the consensus sequence was created for each sample followed by realignment again with the consensus sequence as input. The variant calling was performed at amino acid (AA) level. Only AA covering 5000× per position was considered quality passed. Based on the error calculation generated by PCR and NGS, any mutation >1% was considered. WHO list of DRM for surveillance of TDR was used to interpret sDRM for NRTIs, NNRTIs, PIs, and the Stanford drug resistance summaries for INSTIs (hivdb.stanford.edu).
HIV-1 subtyping and phylogenetic analysis
Subtyping was done by Recombinant Identification Program (http://www.hiv.lanl.gov/content/sequence/RIP/RIP.html), REGA HIV Subtyping Tool v3, (http://dbpartners.stanford.edu:8080/RegaSubtyping/stanford-hiv/typingtool) and COMET HIV-1 (http://comet.retrovirology.lu). Maximum likelihood phylogenetic analysis was performed using Molecular Evolutionary Generics Analysis version 7.0 (MEGA 7) software.
Treatment outcome measures
The outcomes at month six and 12 were analysed by both on-treatment (OT) and intention-to-treat (ITT) approaches. In the OT analysis, two VL cut offs were used for the definition of virological treatment failure; >150 copies/ml and >1000 copies/ml, respectively. For ITT, treatment failure was defined as either failure to attain undetectable viremia (either <150 copies/ml or <1000 copies/ml), LTFU or death.
Statistical analysis
Descriptive statistics (mean, median, standard deviation, percentiles for numerical variables, frequencies and percentages for categorical variables) were used to summarize sociodemographic, clinical, immunological, and virological parameters. Prevalence and types of DRM at baseline were investigated for their possible relationship with sociodemographic and clinical characteristics using t-test (for continuous variables), and Chi-square or Fisher’s exact test (for categorical variables). The impact of pretreatment sDRM (RTI, PI) detected by PBSS and NGS assays on virologic treatment outcome at month six and 12 was assessed by using a multivariable model testing for different confounding factors including gender, age, WHO clinical stage, functional status, TB, CD4 cell count, baseline VL, and NRTI regimens. P-value < 0.05 was considered statistically significant. Data analysis was performed using STATA software 14 (Stata Corp. College Station, Texas, USA).
Ethical approval and informed consent
Scientific and ethical approvals were obtained from the National Research Ethics Review Committee in Ethiopia (3.10|528|06) and the Institutional Review Board (IRB) of EHNRI (Reference No. E.H.N.R.I 6.13/163). Written informed consent was obtained from all patients. All the methods were performed in accordance with approved institutional guidelines.
The sequences generated by Sanger sequencing in this study are deposited in Gene Bank [accession numbers: MG009597-MG010057].
References
HAPCO. Country progress report on the HIV response. Federal HIV/AIDS Prevention and Control Office (FHAPCO), March 2014 (2014).
ARC/HAPCO. HIV/AIDS Estimates and Projections in Ethiopia, 2011–2016. AIDS Resource Center (ART). Federal HIV/AIDS Prevention and Control Office (2017).
Assefa, Y. et al. Scaling up antiretroviral treatment and improving patient retention in care: lessons from Ethiopia, 2005–2013. Global Health 10, 43, https://doi.org/10.1186/1744-8603-10-43 (2014).
HAPCO. Guidelines for management of opportunistic infections and antiretroviral treatment in adolescents and adults in Ethiopia. Federal HIV/AIDS Prevention and Control Office. Federal Ministry of Health, March 2008. Available at: http://www.who.int/hiv/pub/guidelines/ethiopia_art.pdf, accessed 15 October 2013 (2008).
WHO. ETHIOPIA Update sheet on HIV - AIDS programme 2014. WHO Country Office for ETHIOPIA (2015).
Castelli, F., Pietra, V., Diallo, I., Schumacher, R. F. & Simpore, J. Antiretroviral (ARV) Therapy in Resource Poor Countries: What do we Need in Real Life? The open AIDS journal 4, 28–32, https://doi.org/10.2174/1874613601004020028 (2010).
Hamers, R. L., Sigaloff, K. C., Kityo, C., Mugyenyi, P. & de Wit, T. F. Emerging HIV-1 drug resistance after roll-out of antiretroviral therapy in sub-Saharan Africa. Current opinion in HIV and AIDS 8, 19–26, https://doi.org/10.1097/COH.0b013e32835b7f94 (2013).
Svard, J. et al. Drug resistance testing through remote genotyping and predicted treatment options in human immunodeficiency virus type 1 infected Tanzanian subjects failing first or second line antiretroviral therapy. PloS one 12, https://doi.org/10.1371/journal.pone.0178942 (2017).
WHO. Hiv Drug Resistance Report 2017 (2017).
Ekici, H. et al. Minority drug-resistant HIV-1 variants in treatment naive East-African and Caucasian patients detected by allele-specific real-time PCR. PloS one 9, e111042, https://doi.org/10.1371/journal.pone.0111042 (2014).
Kassu, A. et al. Molecular epidemiology of HIV type 1 in treatment-naive patients in north Ethiopia. AIDS research and human retroviruses 23, 564–568, https://doi.org/10.1089/aid.2006.0270 (2007).
Mulu, A., Lange, T., Liebert, U. G. & Maier, M. Clade homogeneity and Pol gene polymorphisms in chronically HIV-1 infected antiretroviral treatment naive patients after the roll out of ART in Ethiopia. BMC infectious diseases 14, 158, https://doi.org/10.1186/1471-2334-14-158 (2014).
Kalu, A. W. et al. Monophylogenetic HIV-1C epidemic in Ethiopia is dominated by CCR5-tropic viruses-an analysis of a prospective country-wide cohort. BMC infectious diseases 17, 37, https://doi.org/10.1186/s12879-016-2163-1 (2017).
Alula, M. Teklu et al. Establishing a Multicenter Longitudinal Clinical Cohort Study in Ethiopia: Advanced Clinical Monitoring of Antiretroviral Treatment Project. Ethiop J Health Sci 27, 3–16, https://doi.org/10.4314/ejhs.v27i1.2S (2017).
Abegaz, W. E. et al. Threshold survey evaluating transmitted HIV drug resistance among public antenatal clinic clients in Addis Ababa, Ethiopia. Antiviral therapy 13(Suppl 2), 89–94 (2008).
Huruy, K., Kassu, A., Mulu, A. & Wondie, Y. Immune restoration disease and changes in CD4+ T-cell count in HIV- infected patients during highly active antiretroviral therapy at Zewditu memorial hospital, Addis Ababa, Ethiopia. AIDS research and therapy 7, 46, https://doi.org/10.1186/1742-6405-7-46 (2010).
Inzaule, S. C. et al. The evolving landscape of HIV drug resistance diagnostics for expanding testing in resource-limited settings. AIDS reviews 19 (2017).
Mulu, A., Maier, M. & Liebert, U. G. Lack of integrase inhibitors associated resistance mutations among HIV-1C isolates. J Transl Med 13, https://doi.org/10.1186/s12967-015-0734-3 (2015).
Yoshinaga, T. et al. Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrobial agents and chemotherapy 59, 397–406, https://doi.org/10.1128/AAC.03909-14 (2015).
Tsiang, M. et al. Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrobial agents and chemotherapy 60, 7086–7097, https://doi.org/10.1128/AAC.01474-16 (2016).
Charpentier, C. et al. . High frequency of integrase Q148R minority variants in HIV-infected patients naive of integrase inhibitors. Aids 24, 867–873, https://doi.org/10.1097/QAD.0b013e3283367796 (2010).
Liu, J. et al. Analysis of low-frequency mutations associated with drug resistance to raltegravir before antiretroviral treatment. Antimicrobial agents and chemotherapy 55, 1114–1119, https://doi.org/10.1128/AAC.01492-10 (2011).
Charpentier, C. & Descamps, D. Resistance to HIV Integrase Inhibitors: About R263K and E157Q Mutations. Viruses 10, https://doi.org/10.3390/v10010041 (2018).
Neogi, U. et al. Ex-vivo antiretroviral potency of newer integrase strand transfer inhibitors cabotegravir and bictegravir in HIV type 1 non-B subtypes. Aids 32, 469–476, https://doi.org/10.1097/QAD.0000000000001726 (2018).
Avila-Rios, S. et al. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 WHO survey. The lancet. HIV 3, e579–e591, https://doi.org/10.1016/S2352-3018(16)30119-9 (2016).
Lindstrom, A. & Albert, J. A simple and sensitive ‘in-house’ method for determining genotypic drug resistance in HIV-1. Journal of virological methods 107, 45–51 (2003).
Aralaguppe, S. G. et al. Multiplexed next-generation sequencing and de novo assembly to obtain near full-length HIV-1 genome from plasma virus. Journal of virological methods 236, 98–104, https://doi.org/10.1016/j.jviromet.2016.07.010 (2016).
Acknowledgements
Samples and data were collected by the Advanced Clinical Monitoring of Antiretroviral Treatment in Ethiopia (ACM), funded by CDC Cooperative Agreement 5U2GPSOOO85 8. Part of this work has been supported by the Swedish Research Council, Swedish International Developing Agency, the Swedish Institute, the EDCTP and the EuResist consortium. We acknowledge Addis Ababa University (AAU) College of Health Sciences (especially Drs Ahmed Reja and Dawit Wondimagegn), and the ACM steering committee (especially Dr. Achamyeleh Alebachew). A supplementary table is available.
Author information
Authors and Affiliations
Contributions
N.F.T. designed the study, performed laboratory experiments, analysed sequences and data, and drafted the manuscript. A.W.K. designed the study, performed laboratory experiments, and reviewed the manuscript. S.G., D.F. and B.T. designed the study, and reviewed the manuscript. S.A. supervised the laboratory experiments, and reviewed the manuscript. G.M. supervised the statistics and reviewed the manuscript. U.N. reviewed the manuscript. A.S. designed the study, interpreted clinical data, supervised all aspects of the study, developed and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Telele, N.F., Kalu, A.W., Gebre-Selassie, S. et al. Pretreatment drug resistance in a large countrywide Ethiopian HIV-1C cohort: a comparison of Sanger and high-throughput sequencing. Sci Rep 8, 7556 (2018). https://doi.org/10.1038/s41598-018-25888-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25888-6
This article is cited by
-
Molecular tools are crucial for malaria elimination
Molecular Biology Reports (2024)
-
Increased acquired protease inhibitor drug resistance mutations in minor HIV-1 quasispecies from infected patients suspected of failing on national second-line therapy in South Africa
BMC Infectious Diseases (2021)
-
Pretreatment resistance mutations and treatment outcomes in adults living with HIV-1: a cohort study in urban Malawi
AIDS Research and Therapy (2020)
-
Increased HIV-1 pretreatment drug resistance with consistent clade homogeneity among ART-naive HIV-1 infected individuals in Ethiopia
Retrovirology (2020)
-
A viral genome wide association study and genotypic resistance testing in patients failing first line antiretroviral therapy in the first large countrywide Ethiopian HIV cohort
BMC Infectious Diseases (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.