Abstract
To curb HIV infection rate in Tanzania, antiretroviral therapy (ART) has been scaled up since 2006, and in 2019, the country shifted to regimen including dolutegravir as a default first line. We assessed the success of ART and the contribution of HIV drug resistance (HIVDR) to unsuppressed viral loads. Between February and May 2023 a cross-sectional survey with random sampling was conducted in the six clinics in an urban cohort in Dar es Salaam. Patients with unsuppresed viral loads (local criteria viral load (VL) ≥ 1000 copies/mL) were tested for HIVDR mutations using the WHO adapted protocol for plasma samples. Mutations were interpreted using the Stanford HIVDR database. In total 600 individuals participated in this survey, the majority were female (76.83%), mean age (\(\pm\) standard deviation) was 44.0 (\(\pm\) 11.6) years. The median duration on ART (interquartile range) was 6.5 (3.9–10.2) years. Approximately 99% were receiving tenofovir + lamivudine + dolutegravir as a fixed dose combination. VL testing was successful in 99.67% (598/600) of survey patients and only 33 had VL ≥ 1000 copies/mL, resulting in a viral suppression level of 94.48% (565/598, 95% CI 92.34–96.17%). For 23 samples, protease and reverse transcriptase (RT) genotyping were successful, with 13 sequences containing RT inhibitor surveillance drug resistance mutations (SDRMs) (56.5%). No SDRM against protease inhibitors were detected. Thirty samples were successfully genotyped for integrase with 3 sequences (10.08%) containing integrase strand transfer inhibitor (INSTI) SDRMs. In samples successfully genotyped in the three genetic regions, 68.18% (16/22) had a genotypic susceptibility score (GSS) ≥ 2.5 for the concurrent regimen, implying factors beyond drug resistance caused the unsuppressed viral load. For five patients, GSS indicated that HIVDR may have caused the unsuppressed viral load. All three patients with INSTI resistance mutations were highly resistant to dolutegravir and accumulated nucleoside and non-nucleoside RT inhibitor HIVDR mutations. Although in this cohort the last 95 UNAIDS target was almost achieved, HIVDR mutations, including INSTIs resistance mutations were detected in HIV-positive individuals taking ART for at least one year. We recommend the design and implementation of high-impact interventions to prevent the increase of HIVDR, failure of dolutegravir and address the non-resistance factors in the study area.
Similar content being viewed by others
Introduction
As of 2021, 38.4 million people were infected with the human immunodeficiency virus (HIV) globally1, 1.7 million of them living in Tanzania. The 2021 national HIV survey ranked Dar es Salaam region among the top three with the highest HIV prevalence. Mbeya region had the highest prevalence (14%), followed by Iringa region (13%) and Dar es Salaam region (11%)2. To curb the HIV epidemic, UNAIDS put forward the 95–95–95 targets for 2025: 95% of all people living with HIV are diagnosed, 95% of diagnosed are treated, with 95% of them achieving viral load suppression3. Worldwide, 75% of all people living with HIV have currently access to antiretroviral therapy (ART) and more than 1.3 million in Tanzania (76% of diagnosed) are receiving ART4.
In 2006, Tanzania scaled-up the use of ART for treatment of HIV-1 infection5. Criteria to start and change therapy are currently following the country-wide policy to test and treat with a default first line therapy and move to a default second line therapy when viral load rises above 1000 copies/ml for two consecutive tests, amounting to therapy failure in the Tanzanian context2. The country adopted a triple drug first-line ART regimen, consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) combined with either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor. The initial default first-line ART regimen included nevirapine based regimen (tenofovir, lamivudine and nevirapine)6. Later, nevirapine was replaced by efavirenz, and in 2019, the country shifted to using an integrase inhibitor (dolutegravir)7. This shift was for all patients, whether they were starting treatment or whether they were already on a first line regimen. Shifting to integrase inhibitors was influenced by the reported high-level drug resistance to, and poor virologic outcomes associated with the use of NNRTIs8.
Dolutegravir (DTG), an integrase strand transfer inhibitor (INSTI), has proven highly effective in suppressing HIV replication when used in combination ART9. DTG-based regimen, specifically the combination of tenofovir, lamivudine and DTG (TLD) is now the preferred first-line regimen for adults living with HIV in Tanzania7. This regimen has demonstrated high effectiveness in suppressing viral load, is cost-effective, well-tolerated by patients10,11,12 and has high genetic barrier to resistance13. TLD is described to be an important milestone for treated patients in suppressing the viral replication11,12. However, the transition to DTG is now facing challenges of emerging HIV resistance to INSTIs14,15 and circulating HIV integrase genotypes linked to drug resistance16. Resistance to DTG has emerged in various settings13. Common dolutegravir resistance mutations, such as R263K, G118R, H51Y, and N155H, diminish drug effectiveness by reducing its binding affinity to the integrase enzyme17.
The national HIV survey conducted in 2021 revealed that approximately 17% (221,000) of those on ART did not achieve VL suppression defined as having two consecutive viral loads ≥ 1000 copies/mL4. This unsuppressed viral load can be attributed to not correctly taking the therapy (e.g., due to adherence or counselling problems), or to HIV drug resistance (HIVDR) which could be pre-existing or newly emerging drug resistance mutations14,18,19.
To protect the current and future activity of regimens with INSTI inhibitors, the scale of the problem needs to be assessed. This calls for regular monitoring and surveillance of drug resistance20, in addition to the viral load testing for routine therapy follow up, in order to provide timely data on the emerging and timing of dolutegravir resistance21. This monitoring and surveillance may not only help to inform treatment algorithms for patients whose dolutegravir-based ART may fail, but it can also provide crucial information to inform future HIV treatment guidelines22 and prevent possible emergence of dolutegravir resistance. Furthermore, monitoring of emerging HIVDR could help protect the long-term efficacy of available treatment regimens23.
In a conceptual systems map, Kiekens et al.24 indicated three main loops contributing to HIVDR in sub-Saharan Africa. One of these is that overreliance on new drugs with a high genetic barrier to resistance contributes to an increase in HIVDR. To avoid this to come true, a systems approach is needed and quantitative data need to be collected. HIVDR surveillance data and its association with other factors will contribute to assess the problem of HIVDR at a systems level and uncover potential leverage points that could be addressed with intervention25. In this study we used the Dar es Salaam urban cohort of patients from the Ilala district to determine the virologic suppression rate and the prevalence of HIVDR as well as the circulating resistance patterns. We also determined the factors associated with unsuppressed viral loads. With this information, a baseline for intervention studies at the study site will be available.
Materials and methods
Study design and patient
A cross-sectional survey was conducted between February and May 2023 in the Dar es Salaam Urban Cohort Study (DUCS) platform of the Ilala district in Dar es Salaam26. DUCS area (Ukonga and Gongolamboto administrative wards) has a total of six HIV care and treatment clinics (CTCs); three of which are public owned facilities, two are military owned while one is privately owned (faith-based). All adults (18 years and above) living with HIV and resident in the DUCS platform area and receiving antiretroviral therapy in CTCs found in the DUCS area were eligible to participate in the study. Patients who had received ART for less than one year were excluded from the study.
Sample size and sampling technique
The sample size (n) was computed using a cross sectional formulae for a finite patient; n = N(z2) p(1-p)/d2(N-1) + (z2) p(1-p)27 based on the assumption of an estimated prevalence (p) of 0.68%15 for detecting any HIVDR in adult Tanzanian patient taking ART. Using type I error of 5% (z = 1.96) and a margin of error (d) of ± 0.55%, a minimum sample size of 565 patients was required. With an oversampling of 20%, to allow for non-responders, drop outs and failures in testing, 720 from the 1651 eligible patients were selected using a simple random sampling (using SPSS version 29, Chicago Inc., USA)28. Furthermore, a proportional sampling method was used to obtain a representative sample from all six HIV CTCs found in the DUCS platform area.
Patient recruitment procedures
Phone contacts were extracted from patient files, with up to three call attempts. Patients visiting the clinic were informed about the study, provided informed consent, completed a questionnaire on electronic tablets, and had venous blood drawn. Regardless of consent status, patients received reimbursement for transport costs and a one-kilogram sugar incentive. This process ensured an organized and incentive-driven approach to patient recruitment and study participation.
Data collection process
The study questionnaire was designed following a comprehensive literature review12,24,25,29,30. It consisted of questions about patient social demographic information such as; age, sex, employment status, place of residence and marital status, questions designed to reflect on factors associated with HIVDR such as HIV status disclosure and health insurance. Patient clinical information such as regimen used, treatment line and duration on ART were collected from the record files of the patients (Table 1).
Blood sample handling, processing and storage
Blood sample collection, transportation and storage were treated according to the Temeke Regional Referral Hospital- Specialized Laboratory protocol31. Eight ml of venous blood was drawn using two EDTA tubes each with 4 ml. Whole blood was separated into plasma within 6 h of collection, before separation the whole blood was kept at 2–8 °C. Centrifugation was performed for 10 min at 1000–2000×g, then supernatant (plasma) was kept at − 20 °C with a maximum of 3 times of freeze–thaw cycles.
Viral load testing and HIV drug resistance analysis
Viral load testing was performed using Roche cobas® 6800/8800 systems32 (software version 1.3, publication 4.2) with a lower limit of detection of 15 copies/mL. Given that we followed the current Tanzania national HIV treatment guidelines2, only samples with viral load count ≥ 1000 copies/mL were subjected to analysis of HIVDR mutations. We used Management and Development for Health (MDH) WHO adapted protocol (Unpublished) to perform HIVDR testing using the 3500xL genetic analyser (Applied Biosystems), a capillary sequencer using Sanger-style sequencing. Analysis of mutations were performed using Stanford University HIVDR database version 9.4. Moreover, calibrated patient resistance tool (CPR version 8.1) was used to determine proportion of mutation suggestive of transmitted HIVDR resistance33. Before importing the generated sequences (fasta format) to Stanford University HIVDR database, the raw sequence (abi format) was assembled, aligned and edited using a RECall (v2.32)- web based sequence analysis then saved as fasta file34. The sequenced codons were 6–99, 1–251, and 1–288, for protease, reverse transcriptase and integrase proteins, respectively. We used Stanford HIVDR33 and COMET35 to assign the HIV sub-types, and Rega HIV sub-typing tool 3.46 database36 was consulted to resolve any disagreements between Stanford and COMET. The VL testing and the genotyping data were linked to the questionnaire information in the DUCS database for analysis.
Statistical analysis
Descriptive statistics of the socio-demographic characteristics were summarized using frequencies (n) and percentages (%). In accordance with the Tanzania national HIV treatment guideline7, which was adapted from the WHO37 guidelines, patients were considered to have a suppressed viral load when the count was < 1000 copies/mL, while ≥ 1000 copies/mL was considered unsuppressed viral load. In addition, in some analysis, viral load counts were stratified into three categories: < 50 copies/mL, 50–999 copies/mL, and ≥ 1000 copies/mL. The viral suppression was calculated by dividing the number of patients with viral load count below 1,000 copies/mL over successfully viral load tested patients. In this study we estimated the prevalence of HIVDR as the proportions of patients’ samples which harboured surveillance drug resistance mutations to those patients’ with successfully genotyped sequences.
We determined the 95% CI for viral suppression and proportion of patients with any major HIVDR mutations using the binomial approximation38. We used mean and standard deviations or median and interquartile range (IQR) to estimate the measure of central tendencies for symmetrical and asymmetrical continuous data, respectively. Factors associated with unsuppressed viral loads (1000 copies/mL and above) were determine using binary logistic regression model. Crude and adjusted odds ratios (cOR and aOR) were the effect measures for univariate and multivariate for regression analysis, respectively. All variables in univariate analysis were used in the multivariate analysis. We used the confidence interval of 95% (95%CI), and the p-value \(<\) 0.05 was considered statistically significant.
Statistics were done using SPSS software (SPSS version 29, Chicago Inc., USA) and Excel spreadsheet 2022 (Microsoft Corporation, Redmond, WA). The Genotypic Susceptibility Score (GSS) for the combination antiretroviral therapy (regimen) was determined by adding the score of each drug used in the regimen, using the Stanford HIVDB scoring system39. The interpretations of these tests were categorized as 'susceptible', 'possible resistance', and 'resistance', which were assigned scores of 1, 0.5, and 0, respectively40. Possible resistance included potential low-level resistance, low-level resistance, and intermediate resistance. Patient was considered susceptible to the regimen if the GSS was \(\ge\) 2.
Ethics declarations
The study was approved by Muhimbili University of Health and Allied Sciences (MUHAS)-Ethical Review Board (Ref No. MUHAS/REC/2020/243) and the National Committee on Medical Research Ethics (Ref No. NIMR/HQ/R.8c/Vol.1/1870). Permissions to access the CTCs were requested from the President's Office—Regional Administration and Local Government and CTC managers, Dar es Salaam Regional Medical Officer, Ilala District Medical Officer and CTCs managers. This study complied with the Declaration of Helsinki for medical research involving human subjects.
Results
Overview of recruitment and genotyped samples
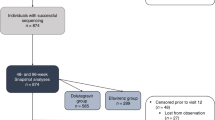
Between February and May 2023, 7083 treated patients were screened from the HIV care and treatment clinics in the DUCS platform for eligibility criteria and 1651 patients were found eligible for inclusion (Fig. 1). With regard to the exclusion criteria, the most common reasons for exclusion were (i) not living in the study area (59.45%); (ii) being on ART for less than one year (12.55%); and, younger than 18 years old (3.48%). None who attended their appointment refused to give informed consent. Viral loads were determined in 600 patients, the success rate was 598 samples (99.67%), with 5.52% (n = 33) having unsuppressed viral load. Genetic sequencing was successful in the protease and reverse transcriptase regions for 23 patients’ samples (69.70%) and in the integrase region for 30 patients’ samples (90.90%).
Characteristics of the surveyed patients
For the survey patients, we collected socio-demographic and other characteristics potentially associated with unsuppressed viral load, summarized in Table 1. The majority was female, the mean age was 44 years, and the education level was mainly primary education (Table 1). About half of patients were attending the military-operated CTCs, most patients spent less than 30 min waiting during clinic visits, and the median time since first HIV diagnosis was 6.8 years. Approximately, 99% of survey patients were taking TLD regimen.
According to the local criteria of therapy success (VL < 1000 copies/mL) 94.48% (565/598, 95% CI 92.34–96.17%) were successfully treated, which comes close to 95%, the goal of UNAIDS for 2025. Those with a viral load count < 50 copies/mL accounted for 83.95% (502/598), those with 50–999 copies/mL made up 10.54% (63/598), while those with ≥ 1000 copies/mL constituted 5.52% (33/598).
Both univariate and multivariate analysis found an association between unsuppressed viral loads and time since first HIV-positive test, patients diagnosed since less than 2 years had lower odds of having unsuppressed viral load (aOR 0.13, 95% CI 0.02–0.69, p = 0.017) as compared to those with at least 10 years since diagnosis. However, patients with self-reported other underlying chronic disease conditions also had lower odds of unsuppressed viral load (aOR 0.18, 95% CI 0.05–0.73, p = 0.016) as compared to their counterparts (Table 1).
Characteristics and treatment history of patients with viral load \(\ge\) 1000 copies/mL
The mean age (± standard deviation) of participants was 42.97 (± 13.16) years, while the mean duration on ART was 6.79 (± 3.67) years (Table 2). Moreover, mean (± standard deviation) time since the start of dolutegravir-based regimen was 32.6 (± 12.95) months. Out of 33 patients, 32 (96.97%) had been initiated on a DTG-based regimen either as first therapy or switched from another first line, all of which because of a national policy change. Many of these patients also had previous treatment changes, and two patients were switched away from DTG. The reason for previous changes is not known, however, given that all were switched from a first line suggests the previous changes might also have been policy changes or might have been related to side effects.
Of 33 patients with high viral load (≥ 1000 copies/mL) despite taking ART, 30 (90.91%) were using a DTG-based regimen, mostly TDF + 3TC + DTG, two patients were using ABC + 3TC + LPV/r, and one was taking ABC + 3TC + ATV/r. Of those patients, one had never used a DTG-based regimen but had a history of using TDF and 3TC drugs. Patient MC-243 was already receiving a second line PI-containing regimen, when switching to DTG, suggesting this patient had failed TDF + 3TC + EFV, and might have already accumulated TDF and 3TC resistance. This makes the DTG-containing regimen TLD particularly vulnerable when used after failure of a first line therapy in Tanzania, where first line failure may not have been diagnosed at the time of the switch. The risk of using functional monotherapy is then too high. One patient, CR-063, had not yet been on a dolutegravir-based regimen. Two patients, CR-119 and MC-400, failed their DTG-containing regimen and were already switched to a protease inhibitor-containing therapy when sampled (Table 2).
HIV drug resistance (HIVDR)-associated mutations
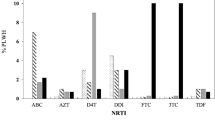
Among NRTI resistance mutations, M184V was the most common 9/23 while for NNRTIs, A98G, G190A, and K103N was the most common 5/23. Furthermore, for INSTIs, E138K was the common 4/30 genotyped mutation (Fig. 2). There were no HIVDR against PIs. The most common HIV-1 subtype was A: 45.16% (14/31), followed by C 35.0% (11/31) then D 9.58% (3/31), and recombinant/ double infection A,D 9.68% (3/31).
Surveillance drug resistance mutations (SDRMs) and HIV-1 subtypes
Out of 33 samples eligible for genotyping, 93.94% (31/33) samples were successfully genotyped in at least one genomic region, while 66.67% (22/33) were successfully genotyped in all genomic regions tested, protease, reverse transcriptase and integrase (Fig. 1 and Table 3). For one sample, protease and reverse transcriptase were successfully genotyped but integrase failed, and for eight samples integrase was successfully genotyped but protease and reverse transcriptase failed. The reason for this high protease and reverse transcriptase (PRRT) failure rate is not clear, viral load was very high in most of them. In almost half of the genotyped patients (13 of 31), surveillance drug resistance mutations (SDRMs) were detected (Table 3). Five SDRMs (T66I, G118R, E138K, G140A, Q148K) associated with INSTI resistance were detected, of which E138K was the most common 4/30.
Prevalence of surveillance drug resistance mutations (SDRMs)
In total 23 sequences were analyzed for protease and reverse transcriptase (PRRT) mutations (Table 4). Of which 13 sequences contained RT SDRMs (56.5%). Thirty sequences were analyzed for integrase where 3 sequences (10.08%) contained INSTI SDRMs. Worrying is that for those samples where full genotypic information was available (n = 22), all three with INSTI SDRMs also had NRTI and NNRTI SDRMs, adding up to almost 14%.
Drug susceptibility assessment
The drug susceptibility assessment based on the patterns of mutations per patient is illustrated in Fig. 3. The detection of high-level resistance against NRTs and NNRTIs was consistent with patients' treatment history. Furthermore, patients with INSTI mutations and high level INSTI resistance (MC-243, MC-367 and CR-048) had a history of using DTG-based regimen (Table 2).
To assess whether the estimated susceptibility was relevant for the concurrent treatment regimen, a GSS to the concurrant regimen was calculated for the 22 for whom the full PRRT and INSTI genotype was available (Table 5). This revealed that 68.18% (16/22) had a total GSS of \(\ge\) 2.5 towards the concurrent regimen, suggesting that for the majority of patients other factors than their drug resistance profile was causing unsuppressed viral load. For one patient, the total GSS was 2.0, but with an active DTG. Careful management and consideration of alternative therapeutic options may be necessary for this patient to maintain effective treatment and prevent further resistance development especially to DTG. Three had a total GSS less than 1, and two had a total GSS of 1.5. All three patients with INSTI resistance mutations were highly resistant to DTG, the other patients are at risk for developing DTG resistance (Table 5). This highlights the importance of monitoring and potentially adjusting treatment to avoid virologic failure and further increase of drug resistance in these individuals. Given that time since HIV diagnosis and whether or not there were other chronic diseases was associated with unsuppressed viral load (Table 1), we added this information to the table for illustration purposes.
Discussion
This study aimed at determining the prevalence of viral suppression and HIV drug resistance (HIVDR) among a random sample of 600 HIV treatment-experienced adults (18 years and above) living in Ukonga and Gongolamboto, Dar es Salaam, three years after the introduction of dolutegravir (DTG)-based antiretroviral therapy (ART). The survey constitutes a baseline at the study site, in order to inform and evaluate upcoming intervention studies. In this survey the prevalence of viral suppression (defined by viral load (VL) < 1000 copies/ml) was 94.48%, this is a substantial milestone on the last Joints United Nations AIDS Programme on HIV/AIDS3 (UNAIDS target where 95% of people on ART should have suppressed their viral loads by 2025).
The high virological suppression rate in the studied area is probably linked to the policy change of replacing non-nucleoside reverse transcriptase (NNRTI)-based regimen by DTG-based regimen (tenofovir + lamivudine + DTG or TLD) in first line. This policy change resulted in 99% of the surveyed patients taking the TLD regimen. The treatment success rate in our survey is consistent with the recent national representative survey carried out in 2021, in which viral suppression among adults was 96.16%15. The national survey was conducted 18 months after DTG introduction, while patients in our survey were on average already three years on a DTG-containing regimen. Prior to DTG, viral suppression rate was 87.7% among patients with age between 15 and 64 years, as documented in the Tanzania national survey of 2016–201741, the last survey before introduction of DTG-based regimen in 20197. This shows that the introduction of DTG-based regimen successfully managed to bring treatment success to the majority of treated patients. Previously, Kiekens et al.24 described that on a broader scale, the accessibility of highly potent ART combined with second-generation integrase inhibitors, which possess a robust genetic resistance barrier, presents a novel and encouraging avenue for treatment24. The dependence on new ART options should be addressed, especially considering that there are currently limited new ART medications in development. The focus should be on optimizing the use of existing ART regimens and exploring alternative strategies to manage HIV and prevent HIVDR, rather than anticipating the introduction of new drugs in the near future. As Kiekens et al. posit, overreliance on new drugs is one of the drivers of HIVDR.
While treatment success was high, the proportion of those harbouring any SDRMs among those with unsuppressed viral load (VL ≥ 1000 copies/mL) was also high (41.39%). Treatment success viral load thresholds are much higher in Tanzania2 compared to Western countries44,45 (which aim for VL < 50 instead of 1000 copies/mL). In addition, Tanzanian guidelines allow HIVDR testing2 only for samples with unsuppressed viral load. This study adhered to Tanzanian guidelines to ensure that the study followed local protocols, as the current survey is intended to be repeated in the context of local interventions consistent with the healthcare practices and infrastructure in the region. The high proportion of SDRM is therefore not surprising. Resistance was most commonly observed against NNRTIs (52.1%) and NRTIs (34.88%) similar as in other studies in Tanzanian15,16,42,43. The analysis of PRRT mutations in 23 sequences revealed SDRMs in 56.5% of the studied patients, while no protease SDRMs were detected. This is consistent with the history of treatment guidelines in Tanzania. In the period 20065–2018, the first treatment line included 2NRTIs plus one NNRTI. PIs were, and still are, mainly used for patients who failed the first line regimen7. Comparatively, the examination of integrase sequences from 30 samples revealed that 10% contained INSTI SDRMs. The detected INSTI mutations, T66I, G118R, E138K, G140A, and Q148K are associated with reduced susceptibility to DTG. E138K and G140A also affect elvitegravir and cabotegravir, in combination with Q148A, G140A reduces the susceptibility to raltegravir and elvitegravir by more than 100-fold while susceptibility to dolutegravir and bictegravir could be reduced by up to fivefold. T66I mainly affects the susceptibility of EVG by tenfold, with a minimal effect to other INSTIs33.
The proportion with INSTI mutations is higher than the previously documented prevalence of 5.8% by Kamori et al15. This indicates a potential increase in the occurrence of INSTI resistance mutations, which could have significant implications for the effectiveness of integrase-targeting antiretroviral therapies. However, it is important to consider the scope and context of both studies when interpreting and comparing these findings. We found that HIV-1 subtype was A: 45.16% (14/31), followed by C 35.0% (11/31) then D 9.58% (3/31). Our results agree with the findings from the neighbouring country Kenya which reported subtype A (70.3%) as the dominant subtypes46,47. The Tanzanian study revealed subtype C (60.87%), followed by subtype A (41.30) were the commonly prevailing subtypes among adolescents and young adults48. A national representative survey found that C was the predominant circulating HIV-1 subtype in adults, 45.3% followed by A, 35.7%15. Globally, subtype C accounts for 48% of worldwide HIV infections, primarily clustered in eastern and southern Africa, while subtype A (12%) and subtype D (2%) constitute subsequent proportions49.
Our data substantiate the ongoing concern regarding the development of drug resistance mutations in reverse transcriptase, which can compromise the efficacy of antiretroviral therapies given that nucleoside reverse transcriptase inhibitors (NRTIs) are the backbone of any regimen, including8,42,43,50. The most worrying is that in our study, 13.63% of successfully sequenced patients’ samples for PRRT and integrase had triple class resistance; NRTI and NNRTI and INSTI. Given that patients had been switched to DTG because of the policy change and not because of treatment failure, the high levels of NRTI and NNRTI resistance would indicate that these patients may not have been followed up closely enough to timely switch therapy. This may have contributed to the surprisingly high rate of unsuppressed viral load under the DTG-regimen, some with but most without DTG resistance, after only three years of DTG-containing therapy. This was anticipated in our previous research when considering the drivers of HIVDR at systems level24.
Unique in our study is that we had treatment information, this helped to assess the relevance of the resistance profile for the therapy that the patients were receiving, we examined their GSS to the concurrent regimen. Surprisingly, 68.18% of patients with unsuppressed viral load exhibited GSS values of ≥ 2.5 for their concurrent regimen, implying that factors other than their drug resistance profiles might be contributing to the unsuppressed viral load. This underscores the complex interplay of multiple factors beyond resistance mutations, including issues related to adherence, healthcare access, and psychosocial aspects24. This multifaceted perspective on treatment failure emphasizes the need for a holistic approach to understanding and managing HIV treatment outcomes25. Those with a low GSS but an active DTG face the risk of developing DTG resistance. This underscores the significance of continuous monitoring and timely treatment adjustments to prevent virologic failure and subsequent resistance escalation13. In addition, knowledge about drug resistance development and treatment adherence24,25 are also important. The patients with DTG resistance also exhibited resistance to reverse transcriptase (RT) inhibitors. We may speculate here that DTG resistance mainly developed in patients that had already failed their first line regimen with reverse transcriptase inhibitors, a failure that might have gone undiagnosed. These patients might have accumulated DTG resistance because they had in fact been receiving functional monotherapy. In countries that switch all first line patients to TLD, this DTG-containing regimen may be at risk when first line failure went undiagnosed.
Despite its effectiveness, the emergence of dolutegravir resistance poses a significant concern in HIV treatment15,16. In high-income countries with well-established healthcare systems and access to a diverse range of antiretroviral drugs, the prevalence of dolutegravir resistance remains relatively low even years after its introduction (below 3%)51. This can be attributed to regular treatment monitoring, early detection of resistance, and the availability of alternative treatment options, which collectively contribute to a more controlled resistance landscape. However, in low and middle-income countries, where healthcare resources and treatment options may be limited, the prevalence of dolutegravir resistance becomes more concerning ranging from 1 to 20%52. Factors such as delayed diagnosis, restricted access to resistance testing, and suboptimal adherence to treatment can facilitate the development and dissemination of resistance mutations in these settings17. This shows how development of HIVDR is context dependent, and battling HIVDR has to be done at a systems level.
In the regression analysis, we found that patients diagnosed since less than 2 years had lower odds (0.13) of having an unsuppressed viral load as compared to those with at least 10 years since diagnosis. This could be attributed to various factors, such as closer monitoring, more accessible healthcare services, and timely interventions during the early stages of diagnosis for patients that were diagnosed under the test and treat strategy7. This finding aligns with expectations in resource-constrained settings, where recently diagnosed individuals might receive more focused attention and support (unpublished data). Surprisingly, patients self-reporting other underlaying chronic disease conditions also had lower odds (0.18) of unsuppressed viral load as compared to their counterparts. This finding suggests that patients with coexisting chronic diseases might be receiving more comprehensive medical care and support, which inadvertently contributes to better HIV treatment outcomes. This observation underscores again that HIVDR has to be addressed at systems level.
While interpreting the results of our study, it is crucial to acknowledge certain limitations that might affect the generalizability of the findings. Firstly, our research was limited to the urban patient, potentially excluding valuable insights from rural or suburban areas, where healthcare access and disease patterns may differ. Secondly, we excluded patients who were not living in the study area, while attending clinics in the study area. This points to a subset of patients (60%) who attend clinics away from the area they live in, also for our study area, we are aware of patients attending clinics in other areas, mostly to avoid stigma. The absence of these patients in our survey may affect the results. It is not clear whether this may underestimate (those experiencing or fearing more stigma are absent in our survey) or overestimate (attending clinics away from where you live may reduce the consequences of stigma) treatment success in our survey.
Our study focused on patients who were on treatment for at least one year (13% had less than 1 year of ART in our study area), which might overlook potential differences in disease progression and treatment response among those with shorter treatment durations. Additionally, by only considering participants aged 18 years and above, we may have missed important aspects of the condition's impact on younger age groups, for whom treatment approaches and outcomes could vary significantly. Given the low success rate of PRRT genotyping, caution should be exercised when interpreting the genotyping results and drawing conclusions based solely on the genotyped data. Our interpretation, mostly considered the samples successfully genotyped for PPRT and integrase. Another limitation was that our dataset included information on the previous treatment regimen only for patients with unsuppressed viral loads. In addition, as per Tanzanian guidelines, we were not able to assess the presence of HIVDR mutations in patients with a detectable viral load below 1000 copies/mL. As a result, we were unable to ascertain the confounding effect of potential pre-existing HIVDR mutations in patients who transitioned to DTG-containing regimen from a different first line regimen. Whether and how much this could have influenced the observation that patients diagnosed since less than 2 years had lower odds of unsuppressed viral load can therefore not be estimated.
Conclusions
This study represents a foundational baseline investigation conducted in a designated intervention site, providing comprehensive insights into viral suppression, HIVDR and assessing GSS as a multifaceted metric to evaluate treatment effectiveness. In our study site, approximately 95% of patients achieved viral suppression under therapy, successfully reaching the last of the 95–95–95 UNAIDS targets. This achievement was attributed to the replacement of NNRTIs with DTG in the first-line treatment, which played a significant role. However, it is essential to note that HIVDR mutations, including INSTIs resistance mutations, were detected in this survey. Given that the overall viral suppression rate is high, the prevalence of dolutegravir resistance remains low but concerning, reaching already 10% of genotyped patient, all with triple class resistance. Without appropriate interventions, HIVDR could potentially threaten the effectiveness of the currently available treatment regimen with INSTIs. Therefore, it is crucial to maintain high patient levels of viral suppression and continually survey for HIVDR to integrase inhibitors. The fact that in most of those with unsupressed viral load, the estimated activity of the treatment was still sufficient to suppress viral replication, shows how important other factors are for treatment success, and that a systems approach is needed.
We strongly recommend the design and implementation of high-impact interventions to prevent any further increase in HIVDR in both the study area and Tanzania as a whole. This action is vital to safeguard the potency and effectiveness of this new class of INSTIs. To ensure successful interventions, a transdisciplinary human-centered approach should be adopted, examining the problem at a systems level. A systems approach offers a holistic vision on the complex issue of HIVDR, recognizing the interconnected nature of medical, social, and systemic factors that contribute to treatment effectiveness. This comprehensive strategy will help address the challenges associated with HIVDR and secure the long-term success of treatment efforts.
Data availability
Data used for analysis to produce this manuscript are available for secondary analysis from the corresponding author or the DUCS Platform database management upon request.
Abbreviations
- ART:
-
Antiretroviral therapy
- CTC:
-
Care and treatment clinic
- DUCS:
-
Dar es Salaam urban cohort study
- DTG:
-
Dolutegravir
- HIVDR:
-
HIV drug resistance
- NRTIs:
-
Nucleoside reverse transcriptase inhibitors
- NNRTIs:
-
Non-nucleoside reverse transcriptase inhibitors
- PIs:
-
Protein inhibitors
- INSTIs:
-
Integrase strand transfer inhibitors
- ARVs:
-
Antiretrovirals
- TLD:
-
Tenofovir plus lamivudine plus dolutegravir
- PRRT:
-
Protease and reverse transcriptase
References
UNAIDS. Fact sheet - Latest global and regional statistics on the status of the AIDS epidemic. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (2022).
The United Republic of Tanzania. National Guidelines for Management of HIV and AIDS (Issue April). https://differentiatedservicedelivery.org/wp-content/uploads/national_guidelines_for_the_management_of_hiv_and_aids_2019.pdf (2019).
UNAIDS. (2014). Understanding Fast-Track: Accelerating Action to End the AIDS Epidemic by 2030. https://www.unaids.org/en/resources/documents/2014/JC2686_WAD2014report.
HIV and AIDS Estimates Country factsheets United Republic of Tanzania 2021. (n.d.). Retrieved January 11, 2023, from file:///Users/georgebwire/Downloads/Country-factsheets-United-Republic-of-Tanzania-2021Change.pdf
Isingo, R. et al. Trends in the uptake of voluntary counselling and testing for HIV in rural Tanzania in the context of the scale up of antiretroviral therapy. Trop. Med. Int. Health 17(8), 1. https://doi.org/10.1111/j.1365-3156.2011.02877.x (2012).
Sangeda, R. Z. et al. Development of HIV Drug Resistance in a Cohort of Adults on First-Line Antiretroviral Therapy in Tanzania during the Stavudine Era. Microbiol. Res. 12(4), 847–861. https://doi.org/10.3390/microbiolres12040062 (2021).
National AIDS Control Programme. The United Republic of Tanzania. (2019). HIV Treatment Guideline. https://differentiatedservicedelivery.org/wp-content/uploads/national_guidelines_for_the_management_of_hiv_and_aids_2019.pdf.
World Health Organization. (n.d.). HIV Drug Resistance Report 2021. In WHO Press (Issue July 2017). Retrieved February 28, 2023, from https://www.who.int/publications/i/item/9789240038608.
Optimization, O., & The, E. Dolutegravir (DTG) and the fixed dose combination (FDC) of tenofovir/lamivudine/dolutegravir (TLD). 2, 1–10 (2018).
Nabitaka, V. M. et al. High acceptability and viral suppression of patients on Dolutegravir-based first-line regimens in pilot sites in Uganda: A mixedmethods prospective cohort study. PLoS ONE https://doi.org/10.1371/journal.pone.0232419 (2020).
Mutagonda, R. F., Mlyuka, H. J., Maganda, B. A., & Kamuhabwa, A. A. R. Adherence, effectiveness and safety of dolutegravir based antiretroviral regimens among hiv infected children and adolescents in Tanzania. J. Int. Assoc. Prov. AIDS Care 21. https://doi.org/10.1177/23259582221109613 (2022).
Kilapilo, M. S., Sangeda, R. Z., Bwire, G. M., Sambayi, G. L., Mosha, I. H., & Killewo, J. Adherence to Antiretroviral Therapy and Associated Factors Among People Living With HIV Following the Introduction of Dolutegravir Based Regimens in Dar es Salaam, Tanzania. J. Int. Assoc. Prov. AIDS Care 21. https://doi.org/10.1177/23259582221084543 (2022).
Kouamou, V., Inzaule, S. & Manasa, J. Dolutegravir drug-resistance monitoring in Africa. Lancet HIV 8(11), e664–e666. https://doi.org/10.1016/S2352-3018(21)00268-X (2021).
Henerico, S. et al. Primary resistance against integrase strand transfer inhibitors in integrase strand transfer inhibitor-naive patients failing first- and second-line ART in Tanzania. J. Antimicrob. Chemother. 77(11), 3138–3143. https://doi.org/10.1093/jac/dkac295 (2022).
Kamori, D. et al. Emerging integrase strand transfer inhibitor drug resistance mutations among children and adults on ART in Tanzania: findings from a national representative HIV drug resistance survey. J. Antimicrob. Chemother. https://doi.org/10.1093/jac/dkad010 (2023).
Masoud, S. et al. Circulating HIV-1 integrase genotypes in tanzania: implication on the introduction of integrase inhibitors-based antiretroviral therapy regimen. AIDS Res. Hum. Retroviruses 36(6), 539–543. https://doi.org/10.1089/aid.2020.0021 (2020).
Rhee, S. et al. A systematic review of the genetic mechanisms of dolutegravir resistance. J. Antimicrob. Chemother. 74, 3135–3149. https://doi.org/10.1093/jac/dkz256 (2019).
Boender, T. S. et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J. Antimicrob. Chemother. 71(10), 2918–2927. https://doi.org/10.1093/jac/dkw218 (2016).
Ngarina, M., Kilewo, C., Karlsson, K., Aboud, S., Karlsson, A., Marrone, G., Leyna, G., Ekström, A. M., & Biberfeld, G. Virologic and immunologic failure, drug resistance and mortality during the first 24 months postpartum among HIV-infected women initiated on antiretroviral therapy for life in the Mitra plus Study, Dar es Salaam, Tanzania. BMC Infect. Dis. 15(1). https://doi.org/10.1186/s12879-015-0914-z (2015).
HIV Drug Resistance Strategy. https://apps.who.int/iris/bitstream/handle/10665/343175/9789240030565-eng.pdf (2021).
Schramm, B., Temfack, E., Descamps, D., Nicholas, S., Peytavin, G., Bitilinyu-Bangoh, J. E., Storto, A., Lê, M. P., Abdi, B., Ousley, J., Kalua, T., Calvez, V., Jahn, A., Marcelin, A.-G., & Szumilin, E. Viral suppression and HIV-1 drug resistance 1 year after pragmatic transitioning to dolutegravir first-line therapy in Malawi: a prospective cohort study. www.thelancet.com/hiv (2022).
Da Silva, J., Pals, S., Chang, J., Hackett, S., Godfrey, C., & Raizes, E. Monitoring emerging human immunodeficiency virus drug resistance in Sub-Saharan Africa in the Era of Dolutegravir. J. Infect. Dis. 225(3), 364–366. https://doi.org/10.1093/infdis/jiab382 (2022).
Juma, J. M. et al. Monitoring prevention or emergence of HIV drug resistance: Results of a population-based foundational survey of early warning indicators in mainland Tanzania. BMC Infect. Dis. 14(1), 1–8. https://doi.org/10.1186/1471-2334-14-196 (2014).
Kiekens, A. et al. Exploring the mechanisms behind HIV drug resistance in sub-Saharan Africa: Conceptual mapping of a complex adaptive system based on multi-disciplinary expert insights. BMC Public Health 22(1), 1–15. https://doi.org/10.1186/s12889-022-12738-4 (2022).
Kiekens, A. et al. Factors associated with HIV drug resistance in Dar es Salaam, Tanzania: Analysis of a complex adaptive system. Pathogens 10(12), 1. https://doi.org/10.3390/pathogens10121535 (2021).
Leyna, G. H. et al. Profile: The Dar Es Salaam health and demographic surveillance system (Dar es Salaam HDSS). Int. J. Epidemiol. 46(3), 801–808. https://doi.org/10.1093/ije/dyw324 (2017).
Arya, R., Antonisamy, B. & Kumar, S. Sample size estimation in prevalence studies. Indian J. Pediatr. 79(11), 1482–1488. https://doi.org/10.1007/s12098-012-0763-3 (2012).
Arifin, W. N. Random sampling and allocation using SPSS. Educ. Med. J. 4(1), 1. https://doi.org/10.5959/eimj.v4i1.4 (2012).
Kiekens, A., Bwire, G. M., Decouttere, C., Jordan, M. R., Mangara, A., Mosha, I. H., Rinke De Wit, T., Sangeda, R. Z., Swalehe, O., Vandaele, N., Killewo, J., & Vandamme, A. M. HIV and SARS-CoV-2: The interplay of two wicked problems. BMJ Global Health 7(8). https://doi.org/10.1136/bmjgh-2022-009105 (2022).
Sangeda, R. Z. et al. Predictors of non adherence to antiretroviral therapy at an urban HIV care and treatment center in Tanzania. Drug Healthc. Patient Saf. 10, 79–88. https://doi.org/10.2147/DHPS.S143178 (2018).
Kenya Accreditation Service. Temeke Regional Referral Hospital—Specialized Laboratory. https://kenas.go.ke/cabs/entry/temeke-regional-referral-hospital-specialized-laboratory/ (2023).
Tschäpe, J. et al. Multisite Performance Evaluation of the cobas 5800 System and Comparison to the cobas 6800/8800 Systems for Quantitative Measurement of HBV, HCV, and HIV-1 Viral Load. Microbiol. Spectr. 10(6), 1. https://doi.org/10.1128/spectrum.03125-22 (2022).
Stanford University HIV Drug Resistance Database. (n.d.). Retrieved June 9, 2023, from https://hivdb.stanford.edu/.
Woods, C. K. et al. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J. Clin. Microbiol. 50(6), 1936–1942. https://doi.org/10.1128/JCM.06689-11 (2012).
Luxembourg Institute of Health Context-based Modelling for Expeditious Typing (COMET). (n.d.). Retrieved June 9, 2023, from https://comet.lih.lu/.
Rega HIV Sub-typing Tool. (n.d.). Retrieved June 9, 2023, from https://www.genomedetective.com/app/typingtool/hiv.
WHO. (2023). The role of HIV viral suppression in improving individual health and reducing transmission. https://www.who.int/publications/i/item/9789240055179.
Sample Size Calculator. (n.d.). Retrieved June 9, 2023, from https://sample-size.net/confidence-interval-proportion/.
Charpentier, C. et al. High virological suppression regardless of the genotypic susceptibility score after switching to a dolutegravir-based regimen: Week 48 results in an observational cohort. J. Antimicrob. Chemother. 73(6), 1665–1671. https://doi.org/10.1093/jac/dky062 (2018).
Jordan, M. R. et al. High levels of HIV drug resistance among adults failing second-line antiretroviral therapy in Namibia. Medicine (United States) 99(37), E21661. https://doi.org/10.1097/MD.0000000000021661 (2020).
National Bereau of Statistics. (n.d.). Tanzania HIV Impact Survey 2016–2017. Retrieved March 4, 2023, from https://www.tacaids.go.tz/documents/research.
Dow, D. E., Shayo, A. M., Cunningham, C. K. & Mmbaga, B. T. HIV-1 drug resistance and virologic outcomes among tanzanian youth living with HIV. Pediatr. Infect. Dis. J. 38(6), 617–619. https://doi.org/10.1097/INF.0000000000002288 (2019).
Mziray, S. R., Kumburu, H. H., Assey, H. B., Sonda, T. B., Mahande, M. J., Msuya, S. E., & Kiwelu, I. E. Patterns of acquired HIV-1 drug resistance mutations and predictors of virological failure in Moshi, Northern Tanzania. PLoS One 15(9 September). https://doi.org/10.1371/journal.pone.0232649 (2020).
Centers for Disease Control and Prevention. HIV in the United States by Region: Viral Suppression. https://www.cdc.gov/hiv/statistics/overview/viral-suppression.html (2023).
Lesko, C. R., Chander, G., Moore, R. D. & Lau, B. Variation in estimated viral suppression associated with the definition of viral suppression used. AIDS (London, England) 34(10), 1519–1526. https://doi.org/10.1097/QAD.0000000000002579 (2020).
Neilson, J. R. et al. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi. Kenya. J. Virol. 73(5), 4393–4403. https://doi.org/10.1128/jvi.73.5.4393-4403.1999 (1999).
Adhiambo, M. et al. Human immunodeficiency virus (Hiv) type 1 genetic diversity in hiv positive individuals on antiretroviral therapy in a cross sectional study conducted in Teso Western Kenya. Pan Afr. Med. J. 38, 1–12. https://doi.org/10.11604/pamj.2021.38.335.26357 (2021).
Rugemalila, J. et al. HIV virologic response, patterns of drug resistance mutations and correlates among adolescents and young adults: A cross-sectional study in Tanzania. PLoS One 18(1), 1. https://doi.org/10.1371/journal.pone.0281528 (2023).
Lihana, R. W., Ssemwanga, D., Abimiku, A. & Ndembi, N. Update on HIV-1 diversity in Africa: A decade in review. AIDS Rev. 14(2), 83–100 (2012).
Gupta-Wright, A., Corbett, E. L., Fielding, K., Grint, D. J., International, D., J van Oosterhout, M. J., Alufandika, M., Heaney, J., Byott, M., Nastouli, E., Gupta-Wright, A., Fielding, K., van Oosterhout, J. J., Alufandika, M., Grint, D. J., Chimbayo, E., Heaney, J., Byott, M., Nastouli, E., & Gupta, R. K. Virological failure, HIV-1 drug resistance, and early mortality in adults admitted to hospital in Malawi: an observational cohort study. In Articles Lancet HIV (Vol. 7). https://mafft.cbrc (2020).
de Salazar, A. et al. Transmitted drug resistance to integrase-based first-line human immunodeficiency virus antiretroviral regimens in Mediterranean Europe. Clin. Infect. Dis. 76(9), 1628–1635. https://doi.org/10.1093/cid/ciac972 (2023).
Inzaule, S. C. et al. Primary resistance to integrase strand transfer inhibitors in patients infected with diverse HIV-1 subtypes in sub-Saharan Africa. J. Antimicrob. Chemother. 73(5), 1167–1172. https://doi.org/10.1093/jac/dky005 (2018).
Acknowledgements
Authors express their gratitude to the DUCS research team and health care workers from the studied HIV care and treatment clinics for their participation in field data collection, and also Management and Development for Health (MDH) Laboratory scientists who performed the laboratory tests. We thank the local authorities and health facilities for granting permission to access to record files of the patients, use their premises to conduct the interviews, and the phlebotomy rooms for blood sample taking. We acknowledge Anneleen Kiekens for setting the research baseline (generating systems map of the study area) and providing invaluable guidance to GMB in the initial stage. The author (GMB) extends gratitude for the mentorship in HIV Implementation Science provided by Christopher R. Sudfeld and Muhammad Bakari.
Funding
This research was supported by VLIR-UOS: grant numbers, TZ2019SIN263 (South Initiatives), TZ2020JOI032A101 (JOINT), TZ2022TEA530A101 (TEAM), and Global Minds Scholarship. The funder did not take part in the study design, data collection and analysis, and manuscript review.
Author information
Authors and Affiliations
Contributions
G.M.B. participated in conceptualization, designing the study, field and laboratory data collection and analysis, interpretation and drafting the manuscript. B.G.A., I.H.M., M.S.K., A.M. and M.S.K. participated in field data collection. P.K. participated in data collection and cleaning. J.V. participated in data analysis and manuscript revision. M.R.J. and J.P.S. participated in manuscript revision. D.S., D.T., A.S. and W.M. participated in laboratory data collection. C.D., O.S., J.K. and N.V. participated in conceptualization and manuscript review. R.Z.S. participated in conceptualization, study design, and manuscript revision. A.M.V. participated in conceptualization, study design, data analysis and manuscript revision. All authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bwire, G.M., Aiko, B.G., Mosha, I.H. et al. High viral suppression and detection of dolutegravir-resistance associated mutations in treatment-experienced Tanzanian adults living with HIV-1 in Dar es Salaam. Sci Rep 13, 20493 (2023). https://doi.org/10.1038/s41598-023-47795-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47795-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.