Abstract
Huntington disease is a fatal neurodegenerative disorder caused by a CAG repeat expansion in the gene encoding the huntingtin protein. Expression of the mutant protein disrupts various intracellular pathways and impairs overall cell function. In particular striatal neurons seem to be most vulnerable to mutant huntingtin-related changes. A well-known and commonly used model to study molecular aspects of Huntington disease are the striatum-derived STHdh cell lines generated from wild type and huntingtin knock-in mouse embryos. However, obvious morphological differences between wild type and mutant cell lines exist, which have rarely been described and might not have always been considered when designing experiments or interpreting results. Here, we demonstrate that STHdh cell lines display differences in cell size, proliferation rate and chromosomal content. While the chromosomal divergence is considered to be a result of the cells’ tumour characteristics, differences in size and proliferation, however, were confirmed in a second non-immortalized Huntington disease cell model. Importantly, our results further suggest that the reported phenotypes can confound other study outcomes and lead to false conclusions. Thus, careful experimental design and data analysis are advised when using these cell models.
Similar content being viewed by others
Introduction
Huntington disease (HD) is an inherited, fatal, neurodegenerative disorder. It results from a CAG repeat expansion in the gene HTT, coding for the huntingtin protein. The mutation is translated into an elongated polyglutamine repeat in huntingtin, which leads to the disruption of various cellular signalling pathways and results in impaired cell function and ultimately cell death, particularly of striatal neurons1,2. To study cellular and molecular mechanisms contributing to the HD pathogenesis, numerous cell and animal models have been generated. The STHdh cell lines were generated from an HD knock- in mouse model3, which carries the endogenous Hdh gene (mouse Huntington disease gene homolog) with a chimeric exon 14 and is characterized by a mild behavioural phenotype and neuropathological features5. These cell lines derive from striatal primordia3 and express wild-type and mutant huntingtin at endogenous levels6. The precise genetic context and the striatal origin of the cells make the STHdh cell lines a widely used model in HD research. By comparing immortalized striatal precursor cells from wild type mice (STHdh Q7/Q7 cells) to precursor cells derived from heterozygous and homozygous Hdh Q111 knock-in mice (STHdh Q7/Q111 and STHdh Q111/Q111 cells), differences in a variety of HD-related cellular pathways have been discovered or confirmed, for instance an involvement of huntingtin in calcium handling deficits and mitochondrial dysfunction7,8,9,10,11 or effects on various signalling cascades12,13,14. Despite the to date unquestioned usefulness and importance of this model, obvious but rarely reported differences in size11, shape15 and proliferation rate might demand caution when using the STHdh cell lines. The origin of these differences, their importance for HD, as well as the consequences for the interpretation of study outcomes remains largely unaddressed.
In this study, we show that the STHdh cell lines exhibit divergent characteristics, which interfere with commonly used assays and hamper the direct comparison of both cell lines. We further show that these features are partially shared by mouse embryonic fibroblast (MEFHdh) cell lines generated from the same animal model and their wild type littermates, which implies a common, HD-related mechanism beyond immortalization artefacts. Overall, these findings argue for a thorough characterization of every cell line used and the inclusion of such confounding factors in the experimental design.
Results
Reduced cell size is a characteristic of STHdh Q111/Q111 and MEFHdh Q111/Q111 cells
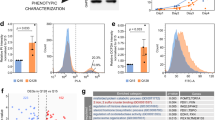
We performed a morphometric analysis of homozygous STHdh Q111/Q111 (STQ111) and wild type STHdh Q7/Q7 (STQ7) cells by light microscopy and flow cytometry analysis. Measurement of the surface area of cells attached to the culture dish revealed a significantly smaller cell surface area in the mutant STHdh cells (Fig. 1a and b; P < 0.001). The smaller cell size of STHdh Q111/Q111 was also found in detached cells, both when measuring the surface area from microscopic images (Supplementary Fig. S1) and on a larger scale by flow cytometry analysis (Fig. 1c and d). Here, the relative mean forward-scatter area (FSC-A), which is positively related to cell size, was 32% lower in STHdh Q111/Q111 than in STHdh Q7/Q7 cells (Fig. 1d; P = 0.013). Similar differences were also observed after differentiation into neuron-like cells (Supplementary Fig. S2).
Cell size difference in Q111 knock-in cells. (a) Representative pictures of STHdh Q7/Q7 (STQ7) and STHdh Q111/Q111 (STQ111) cells, and (b) ImageJ-based surface area quantification of STHdh cells attached to the culture dish surface n = 3 experiments, unpaired t-tests; ***P < 0.001. (c) Representative histograms of STHdh cells and (d) quantification of the cell size of live cells in suspension, based on the relative mean forward scatter area (FSC-A); n = 4 experiments, unpaired t-tests; *P < 0.05. (e–h) Results of size determination for MEFHdh Q7/Q7 (MEFQ7) and MEFHdh Q111/Q111 (MEFQ111) cells, respectively; *P < 0.05, **P < 0.01.
To assess whether this cell size phenotype is cell line-specific or whether it might be considered a general feature of HD, we performed the same set of experiments in a fibroblast cell line established from the same mouse model (MEFHdh cells). Like in the STHdh cells, the mutant MEFHdh Q111/Q111 (MEFQ111) cells had a smaller cell surface area compared to the wild type MEFHdh Q7/Q7 (MEFQ7) cells’, when the cells were attached to the culture dish (Fig. 1f; P = 0.03). Although the difference did not reach statistical significance when manually analysing cell surface area in detached cells (Supplementary Fig. S1; P = 0.13), it was detected again via flow cytometry analysis (Fig. 1g and h; P = 0.002). The relative mean FSC-A of MEFHdh Q111/Q111 cells was 31% lower compared to MEFHdh Q7/Q7 cells, comparable to the values retrieved for STHdh cells (Fig. 1h). Flow cytometry analysis further revealed a higher heterogeneity of the MEFHdh cell population compared to STHdh cells, as represented by a broader distribution of cell sizes and two distinct peaks in the FSC-A plot (Fig. 1g), possibly due to the biological origin of these cell lines16.
STHdh but not MEFHdh cells show considerable chromosome abnormalities
As changes in DNA content can lead to alterations in cell size17,18 and are a common feature of cell line stabilization19 and cell passaging20,21, we performed a karyotype analysis to clarify whether the cell size differences observed in both cell lines are explained by changes in ploidy.
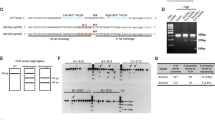
Karyotyping revealed a variety of chromosomal abnormalities in STHdh cells. Even more importantly, the chromosomal changes differed between STHdh Q111/Q111 and STHdh Q7/Q7 cells in qualitative and quantitative terms (Fig. 2a and b). In detail, STHdh Q7/Q7 cells showed a hyperpentaploid, female, murine karyotype with chromosome numbers between 104 and 115. Different numerical anomalies as well as a variable number of additional, structurally abnormal chromosomes (three to eight marker chromosomes) were detected. About 40% of the cells showed at least one, but up to four additional copies of chromosome 3, 8, 9, 10, 14, 16 and 17. Interestingly, nearly 100% of the analysed cells showed two to six additional copies of chromosome 15, 18 and 19. Loss of at least one, but up to four copies was found for chromosome 4, 6, 7, 11, 12 and 13 in 40% of the cells. In contrast, STHdh Q111/Q111 cells showed a hypo- to hypertetraploid, female, murine karyotype (77–82 chromosomes) with a high number (seven to nine) of marker chromosomes. Loss of one to four copies was found for chromosome 1, 4, 6, 7, 12, 14 and 18 - similar to STHdh Q7/Q7 cells. Nearly all cells had one to three additional copies of chromosome 15 and 19. The total number of chromosomes was significantly lower in STHdh Q111/Q111 cells compared to STHdh Q7/Q7 cells (Fig. 2c; P < 0.001).
STHdh cells display marked and divergent chromosome abnormalities. (a) Representative karyograms from STHdh Q7/Q7 (STQ7) and (b) STHdh Q111/Q111 (STQ111) cells with (c) quantification of the chromosome numbers; n = 5 experiments, unpaired t-tests; ***P < 0.001. (d–f) Result of karyogram analysis for MEFHdh Q7/Q7 (MEFQ7) and MEFHdh Q111/Q111 (MEFQ111) cells, respectively; n = 8 experiments.
In contrast, MEFHdh cells did not show marked chromosomal abnormalities (Fig. 2d and e). In detail, MEFHdh Q7/Q7 cells showed a mainly diploid, murine, male karyotype with only some tetraploid cells (Fig. 2d). Apart from a small number of single cell anomalies, no chromosomal losses were detected. A few cells showed additional copies of chromosome 16 and 17. MEFHdh Q111/Q111 cells showed a mainly diploid, female, murine karyotype and only a few tetraploid cells (Fig. 2e). Nearly all cells showed a numerically normal karyotype. About 50% of the cells were found to have an additional chromosome 17. The total number of chromosomes did not differ between MEFHdh Q111/Q111 and MEFHdh Q7/Q7 cells (Fig. 2f).
STHdh Q111/Q111 and MEFHdh Q111/Q111 cells show a higher proliferation rate
We further examined the proliferation rate of STHdh and MEFHdh cells, as both mutant cell lines appeared to proliferate at different rates during regular passaging.
Quantification of the increase in cell number after 3 days of cultivation revealed an elevated proliferation rate of STHdh Q111/Q111 compared to STHdh Q7/Q7 cells (Fig. 3a, P = 0.02). A trend towards increased proliferation rate was detected in MEFHdh Q111/Q111 compared to MEFHdh Q7/Q7 cells after 7 days of cultivation (Fig. 3b; P = 0.073), although both MEFHdh cell lines did not proliferate as much as STHdh cells.
Both mutant cell lines exhibit increased proliferation rates. (a) Manually determined cell count of STHdh cells after 3 days; n = 5 experiments, unpaired t-tests; *P < 0.05 and (b) manually determined cell count of MEFHdh cells after 7 days; n = 5 experiments; unpaired t-tests. (c) Representative overlays of signal intensity of STHdh Q7/Q7 and STHdh Q111/Q111 DAPI-stained cells and (d) representative overlay of signal intensity of MEFHdh Q7/Q7 and MEFHdh Q111/Q111 DAPI-stained cells with exemplary indication of cell cycle; n = 3.
In order to clarify whether the enhanced proliferation had been the result of increased cell division or reduced cell death, we performed a cell cycle analysis and measured the amount of viable and apoptotic cells.
First, the proportion of cells in the different phases of the cell cycle was analysed by measuring the DNA content via DAPI staining intensity in detached, fixed cells. This assay confirmed the difference in ploidy between STHdh Q7/Q7 and STHdh Q111/Q111 cells, as there was a noticeable right shift in the curve obtained for STHdh Q7/Q7 cells, corresponding to an overall increased DNA content (Fig. 3c). This shift, however, made the automated analysis by the analysis software unreliable, and was therefore not quantified. MEFHdh cells, on the other hand and in line with their similar karyograms, exhibited similar distribution patterns of cell populations with different DNA content (Fig. 3d). In this case, the analysis showed a significant decrease in cells in the G0/G1 phase (MEFHdh Q7/Q7 65.73 ± 2; MEFHdh Q111/Q111 39.17 ± 1; P = 0.0003), alongside a tendency to an increase in cells in the S (MEFHdh Q7/Q7 4.1 ± 2; MEFHdh Q111/Q111 8.2 ± 0.2; P = 0.07) and G2/M phase (MEFHdh Q7/Q7 22.9 ± 4; MEFHdh Q111/Q111 38.1 ± 0.2; P = 0.03). The observed differences in cell cycle progression were in line with the observation that MEFHdh cells containing the huntingtin knock-in mutation proliferate more than wild type cells.
Second, we analysed the amount of viable and apoptotic cells by flow cytometry analysis (Fig. 4). We found STHdh Q111/Q111 cells to have a higher proportion of viable cells (Fig. 4b, P = 0.047), and in turn a lower proportion of apoptotic cells compared to STHdh Q7/Q7 cells, although the latter did not reach statistical significance. Similar results were obtained for MEFHdh cells, showing a significantly higher proportion of viable cells (Fig. 4e; P = 0.026) and, in this case, a significantly lower number of apoptotic cells (Fig. 4f; P = 0.017) in MEFHdh Q111/Q111 cells compared to their wild type control.
Cell viability is not reduced in STHdh and MEFHdh mutant cell lines. Results from cell size- and cell number-independent flow cytometry analysis: (a) Representative scatterplots of flow cytometry analysis of STHdh cells and (b and c) quantification from flow cytometry analysis of Annexin V/7′AAD staining; n = 4 experiments. VC: viable cells, EAC: early apoptotic cells, LAC: late apoptotic cells, NC: necrotic cells. Quantification of apoptotic cells combines results for EAC and LAC; unpaired t-tests; *P < 0.05. (d–f) Results of MEFHdh cells, respectively; unpaired t-tests; *P < 0.05.
The cell size and proliferation phenotypes in STHdh Q111/Q111 cells might impede the interpretation of standard cell viability assays
When investigating cell viability in our study, we used flow cytometry, a method that should theoretically be independent of cell size and cell proliferation. However, common cell viability tests depend considerably on these parameters. Thus, we reassessed cell viability and cell death using the standard cell viability assays, PrestoBlue® and LDH assay, respectively (Fig. 5).
The cell size and proliferation phenotypes in STHdh Q111/Q111 cells impede the interpretation of standard cell viability assays. Results from the cell size- and cell number-dependent tests for STHdh cells: (a) PrestoBlue®, n = 3 experiments and (b) LDH assay, n = 3 experiments. Unpaired t-tests; *P < 0.05. (c and d) Results from size- and cell number-dependent tests for MEFHdh cells, respectively; unpaired t-tests; *P < 0.05.
Analysis of the data revealed contradicting results when compared to the outcomes from flow cytometry. The PrestoBlue® assay consistently showed lower signals in STHdh Q111/Q111 cells (Fig. 5a; P = 0.031) and the LDH assay revealed increased LDH release in STHdh Q111/Q111 compared to STHdh Q7/Q7 cells (Fig. 5b; P = 0.022), suggesting that mutant cells are characterized by reduced viability and increased cell death, in contrast to the first findings. Differentiation of STHdh cells led to a similar readout as flow cytometry (Supplementary Fig. S3).
The results obtained for MEFHdh cells differed from the results obtained for STHdh cells. MEFHdh Q111/Q111 cells had similar signals as MEFHdh Q7/Q7 cells in the PrestoBlue® assay (Fig. 5c; P = 0.656), but showed reduced LDH release (Fig. 5d; P = 0.034). These findings were comparable to the results obtained by flow cytometry.
Chromosomal abnormalities in STHdh Q111/Q111 cells might impede the interpretation of western blot analyses
Since we observed that STHdh Q111/Q111 cells differ markedly from the control STHdh Q7/Q7 cell line in terms of chromosomal constitution, we investigated possible consequences of these alterations on the protein levels of commonly used loading controls for western blot analysis. The four proteins, β-actin (Actb, chromosome 5), GAPDH (Gapdh, chromosome 6), α-tubulin (Tuba1a, chromosome 15) and vinculin (Vcl, chromosome 14) are located on different chromosomes.
Western blot analysis of RIPA cell lysates revealed strong trends toward decreased levels of α-tubulin and vinculin in STHdh Q111/Q111 cells compared to STHdh Q7/Q7 cells (Fig. 6b and c; P = 0.06, P = 0.03), in accordance with the reduced number of chromosomes 15 and 14 in STHdh Q111/Q111 cells. In contrast, these differences were not found in MEFHdh cells (Fig. 6e and f), where no differences in the number of chromosomes 15 and 14 were detected. Furthermore, the levels of β-actin were comparable in STHdh Q111/Q111 and STHdh Q7/Q7 cells (Fig. 6b and c) as well as in MEFHdh Q111/Q111 and MEFHdh Q7/Q7 cells (Fig. 6e and f), in accordance with the similar numbers of chromosome 5 in mutant and control cell lines. Interestingly, despite equal numbers of chromosome 6, levels of GAPDH were elevated in STHdh Q111/Q111 and tendentially in MEFHdh Q111/Q111 cell lines, compared to their wild type counterparts (Fig. 6b,c; P = 0.06, e and f; P = 0.02).
Chromosomal abnormalities impede the interpretation of western blot results in STHdh cells. (a) SYPRO Ruby staining, (b) western blots and (c) corresponding quantification of marker proteins in STHdh cells. Unpaired t-tests; *P < 0.05. (d) SYPRO Ruby staining, (e) western blots and (f) corresponding quantification of marker proteins in MEFHdh cells. Unpaired t-tests; *P < 0.05.
Discussion
STHdh cells represent a widely used cell culture model for studying cellular and molecular aspects of HD. Differences in cell morphology, growth and differentiation have previously been mentioned by other groups15,22, but to date, these differences have not been assessed quantitatively. Our study demonstrates clear differences in cell size, proliferation and ploidy between mutant and wild type STHdh cells, and suggests a strong influence of these phenotypes on other readouts.
In the first description of the STHdh cell lines, it was stated that STHdh Q111/Q111 cells are of similar size as STHdh Q7/Q7 cells, while cell proliferation was even decreased in the mutant cells and accompanied by an increase in DNA content3. Later studies, however, either do mention a reduced cell size of STHdh Q111/Q111 cells11,15, or the results are at least suggestive of such a phenotype (although not specifically discussed in these papers)23,24,25. This might indicate that the phenotypes observed in our study had developed over time, possibly due to the tumour character of the cell lines. On the other hand, a reduced cell size was also found in our MEFHdh Q111/Q111 cell line compared to the respective control, despite the absence of large scale chromosomal changes. In addition, cell size differences in striatal neurons have been reported for the R6/2 model26,27 and the YAC128 model28, two transgenic mouse models of HD, and it has been suspected for HD patients29. It remains uncertain, if the reduced cell size should be considered an artefact or could be an HD-related feature, although it might be concluded that huntingtin is at least somehow involved in cell size regulation, as it is, as well, known to interact with cytoskeletal proteins30.
The multiple numerical anomalies and structurally abnormal chromosomes we found in both STHdh cell lines are typical for stable cell lines and long-term passaging19,20,21. Importantly, these abnormalities were found in cell populations that had been passaged for a maximum of six times between their purchase and the respective karyogram analysis. As this is a normal amount of passages required to carry out experiments, the abnormalities are likely to appear in other laboratories in a similar magnitude. Thus, users should be aware that the cell lines might not show the characteristics according to the original publication.
We further found the STHdh Q111/Q111 as well as MEFHdh Q111/Q111 cells to have an increased proliferation rate. It had been reported earlier that mutant huntingtin is involved in cell division in cell models and Drosophila 31, as well as Hdh Q111/111 knock-in mice, STHdh Q111/Q111 cells and MEFHdh Q111/Q111 cells, as it alters the orientation of the mitotic spindle32. Although cell proliferation had not been measured in that study, the authors demonstrate that this leads to changes in neurogenesis in the developing cortex, highlighting the importance of this phenotype.
It is perceivable that differences in cell size, proliferation rate and chromosomal content might constitute confounding factors, and might complicate the interpretation of study outcomes due to adding several variables which cannot properly be controlled for. We demonstrated that assays based on cell size and number, such as the PrestoBlue® and LDH assay, revealed lower basal cell viability and increased cell mortality in STHdh Q111/Q111 cells. Similar results have previously been published by others using the same assays33,34,35 or comparable methods7. However, the results could not be recreated in an assay that was likely to not depend on cell size or cell number. Thus, the earlier reported baseline difference in cell viability between STHdh Q111/Q111 and STHdh Q7/Q7 cells is questionable. Interestingly, our results were even indicative of increased cell viability in both, STHdh Q111/Q111 and MEFHdh Q111/Q111. Effects on pro-survival functions in STHdh Q111/Q111 cells would need to be further investigated, as they have been reported to be reduced for other cell models of HD36,37, whereas Akt signalling, implicated in neuronal survival38, has been shown to be increased in mutant STHdh cells14. Clearly, STHdh cells do not represent the hallmarks of the advanced disease. Intranuclear inclusions, amongst others, found in in vitro and in vivo models, are not found in STHdh cells3. Therefore, the disease stage they model might not necessarily be characterized by a reduction in cell viability under normal conditions.
Confounding effects of the chromosomal abnormalities found in the STHdh cell lines were further expected for western blot analyses. Our investigations revealed important aspects to be considered when choosing a loading control for western blot analysis in STHdh cells. The protein levels of α-tubulin and vinculin were lower in STHdh Q111/Q111, but not in MEFHdh Q111/Q111, when compared to their respective controls and can be interpreted as a direct effect of the lower copy number of the chromosomes 14 and 15 in the STHdh Q111/Q111 cells. These observations correspond to previous studies, which reported on analogous proteomic changes resulting from variations of the gene copy number in cancer cells or aneuploid cell lines39,40. On the other hand, our observation of an elevated GAPDH expression in both STHdh Q111/Q111 and MEFHdh Q111/Q111 cells has already been shown in other HD models. As GAPDH is a well-known interaction partner of huntingtin, these results further render GAPDH as an inadequate loading control in HD research41,42,43.
Although our study is important, as it demonstrates features of the extensively used STHdh model that need to be considered when working with this cell model, and as it highlights the MEFHdh cells as useful controls in in vitro studies, there are some limitations that we would like to point out. First, our MEFHdh cells were generated from embryos of different sex. The MEFHdh cells were generated 12 days after a 48-hour breeding period, and sex differentiation in the mouse embryo begins as early as E1044. Therefore, although we consider the influence of sex determination on cell size, proliferation rate and chromosomal content at that point negligible, we cannot rule it out. Thus, we highly recommend the generation of sex-matched MEFHdh cell lines for further studies. Second, the two cell lines characterized here originate from the same HD animal model. As such, they share several drawbacks that need to be considered. Hdh Q111 knock-in mice, like most other animal models of HD, are designed to express mutant huntingtin with high numbers of polyglutamine repeats to provoke possibly early and strong phenotypes (reviewed by Ferrante et al.45), even though such high repeat numbers are only found in patients with the rare juvenile form of HD. In this regard, it should be noted that cell models46 and animal models47 with lower CAG-repeats have been generated to recapitulate the commonly found mutation lengths. Furthermore, both STHdh and MEFHdh cell lines are not isogenic. Q7 alleles represent the wild type mouse alleles, while Q111 alleles are human mouse chimera of exon1. For this reason, there are additional differences in the gene sequence between Q7 and Q111 alleles than the CAG repeat expansion. On the other hand, STHdh and MEFHdh cells differ fundamentally regarding immortalization and biological origin. STHdh cells are comparable to other immortalized cell lines with regard to immortalization artefacts19,20,21,48, as shown here by the altered chromosome numbers. This is a drawback, as the supposedly complementary Q7 and Q111 cell lines have apparently acquired divergent features over time. Moreover, it needs to be considered that p53, a tumour suppressor protein affected in immortalized cell lines49,50, is a transcriptional regulator of huntingtin 51 and implicated in the pathogenesis of HD52. In this regard, the MEFHdh cells used here represent a better cell model, as these were not immortalized and therefore the genetic integrity was less corrupted. However, the MEFHdh cells presented milder phenotypes regarding cell size and proliferation, which is likely to be due to their heterogeneous cell composition16. In this regard, the clonal and neuronal character of STHdh cells might lead to stronger and more robust phenotypes than embryonic fibroblasts. The clonal character, however, once more underscores the importance of an additional model, to exclude artefacts. Finally, it would always be advantageous to confirm phenotypes in cell and animal models of HD that are based on a different genetic background.
In summary, STHdh cell lines are a generally useful model to study mechanisms behind the molecular pathogenesis of HD, because they provide the proper cellular as well as genetic context of HD due to their striatal origin and the knock-in model they derive from. However, the possible bias due to differences in cell size, proliferation and chromosomal content need to be considered when planning and interpreting results. In this regard, assays in which cell size and cell number play an important role for the outcome, and cannot be controlled for, should be avoided. Differentiation of the STHdh cells into neuron-like cells might at least overcome the problem regarding cell proliferation. Nevertheless, for time-course experiments the increased proliferation rate, as it was, as well, observed in MEFHdh cells needs to be considered. A simple solution for treatment studies would be to not directly compare results from STHdh Q7/Q7 to STHdh Q111/Q111, but to rather compare treatment effects in the two cell lines independently. Finally, using a second in vitro or an in vivo model to confirm results is beneficial to determine the HD-dependency of the phenotype investigated. Our study emphasizes that it is of importance to regularly check the basic characteristics of an employed cell model and to consider putative alterations for experimental design and analysis.
Methods
Ethics Statement
Experiments for the generation of MEFHdh cells were performed at the University of Tuebingen. The protocol was approved by the local ethics committee at Regierungspraesidium Tuebingen and carried out in accordance with the German Animal Welfare Act and the guidelines of the Federation of European Laboratory Animal Science Associations, based on European Union legislation (Directive 2010/63/EU).
STHdh cells
STHdh cell lines, originally generated at the laboratory of Dr. Marcy MacDonald (Harvard Medical School, Boston)3, were purchased from Coriell Cell Repositories (Coriell Institute for Medical Research). Cell passages 4–12 were used for the experiments.
MEFHdh cells
A heterozygous breeding of Hdh Q111 knock-in mice was set up and maintained for 48 hours. After 12 days, the pregnant female was sacrificed by inhalation of CO2. The embryos were extracted by caesarean sectioning, decapitated immediately and placed individually in sterile, ice-cold, Dulbecco’s phosphate-buffered saline (DPBS) (Invitrogen). Limbs, brain and visceral organs were removed. The remaining tissue was transferred into a sterile well of a 6-well plate with fresh DPBS, which was then replaced by 2 ml of culture media (Dulbecco’s Modified Eagle Medium (DMEM) with 1% penicillin/streptomycin and 10% fetal calf serum (FCS), Gibco®, Thermo Fisher Scientific). The tissue was incubated for 1 h at 37 °C and 5% CO2. After this, the tissue was transferred into a 100 mm dish with 10 ml culture media (pre-warmed to 37 °C), and minced with a scalpel. Pieces were transferred to a T75 cell culture flask with 10 ml of fresh media and incubated at 37 °C and 5% CO2 for 3 days. Afterwards, media was changed and the cells were incubated until they reached 90% confluency. Cells were then trypsinized (1 ml 0.25% trypsin-EDTA (Invitrogen) for 5 min at 37 °C and 5% CO2) and gently resuspended using a 1 ml pipette for subcultivation. For the experiments, a wild type and a homozygous culture were picked.
Cell handling and treatment
STHdh and MEFHdh cells were maintained in DMEM supplemented with 10% FCS (GibcoTM) and 1% penicillin/streptomycin (GibcoTM) at 37 °C in 5% CO2. STHdh media was additionally complemented by adding 1% geneticin (A2912, Biochrome). Both, STHdh and MEFHdh cells were routinely tested negative for contamination by mycoplasma using the Venor®GeM Mycoplasma detection kit (Merck). Unless specifically stated differently, STHdh cells were undifferentiated. For differentiation into neuron-like cells a previously described differentiation protocol3 was used. For this, cells were incubated in differentiation cocktail for 24–48 h.
Flow cytometry
Undifferentiated STHdh and MEFHdh cells were recorded using a flow cytometry LSR II cytofluorometer (BD Bioscience). A total of 200,000 ungated events were analysed with the flow cytometry-DIVA software version 6.1.3 (BD Bioscience) and overlays were processed with FCS Express software version 4.0.230 (De Novo Software). Differentiated STHdh cells were recorded with a CyAn™ ADP flow cytometer (Beckman Coulter). A total of 20,000 ungated events were analysed with Summit V4.3.01 software (Dako Colorado, Inc.).
Cell size determination
Cells were seeded in 6-well plates and grown to 60–70% confluency. Cell size was measured for cells attached to the surface of the culture dish as well as for detached cells after trypsinization each with 3 replicates per cell line. A total of 270 cells per genotype were analysed in 3 independent experiments (30 cells/well; 3 wells/experiment). Pictures of the cells were taken using an Eclipse TS100 Inverted Routine microscope (Nikon) with a digital camera at 20x magnification and analysed with ImageJ v1.4753. For attached cells, the area of the cells was approximated by measuring the area of a polygon that was assigned to each cell. For detached cells, the area of a round shape was measured that was applied to each cell individually. The scale was determined by the length of the counting chamber grid.
Chromosome analysis
Chromosome preparations from cultured cells and GTG-banding were performed using standard techniques. For each cell line, 17 mitoses were numerically analysed and 5–8 mitoses were structurally analysed. For cytogenetic analyses, for all cell lines, cells from early passages (P4-P6) were harvested using a standard protocol and was followed by G-banding. Images of well spread metaphase chromosomes were captured using a CCD camera. Karyotyping was performed using the IKAROS software (MetaSystems, Altlussheim, Germany). Chromosome classification followed the guidelines of the International Committee on Standardized Genetics nomenclature for mice54.
Determination of proliferation rate
Three replicates of STHdh (40,000 cells per well) and MEFHdh cells (100,000 cells per well) were seeded in 6-well plates. After 3 days (STHdh) or 7 days (MEFHdh), cells were harvested by trypsinization (250 µl 0.25% trypsin-EDTA (GibcoTM) for 5 min at 37 °C and 5% CO2), washed and counted again. At least three independent experiments were performed.
Determination of DNA content
DNA content was measured using the NucleoView NC-3000 (ChemoMetec). Reagents were provided by the manufacturer and cells were treated according to the manufacturer’s instructions. In brief, cells were detached from the culture flask, washed with DPBS (GibcoTM) and lysed. The cells were stained with DAPI, at a saturating concentration (10 µg/ml), stabilized and immediately analysed with the device. Data was analysed with the NucleoView NC-3000 software, Version 2.1.25.12 (ChemoMetec).
Viability assays
Cell viability and cell death were determined using commercially available kits (PrestoBlue® cell viability reagent, InvitrogenTM; Cytotoxicity Detection Kit (LDH), Roche), following the provider’s instructions. Briefly, 10,000 cells were seeded in a 96-well plate and incubated overnight. Culture media was transferred into a new 96 well plate for the LDH assay. Cells left in the original plate received fresh media containing PrestoBlue®. The fluorescence intensity (PrestoBlue® assay) was measured after 1 h; the absorption (LDH assay) was measured according to manufacturer’s instructions, using the plate reader MWGt Synergy HT (BioTek Instruments) and the software Gen5 2.01 (BioTek).
In addition, cell viability and cell death were measured using flow cytometry. For this, cells were grown in 75 ml culture flasks and harvested by gentle trypsinization (0.25% Trypsin-EDTA; Gibco®, Thermo Fisher Scientific). Cells were centrifuged at 400 × g for 5 min and washed twice with 1× Annexin V Binding Buffer (eBioscience). Cells were labelled with Alexa Fluor® 647 Annexin V (Biolegend) and 7-Amino-Actinomycin (7′AAD) (BD Pharmingen). Data was recorded by flow cytometry to determine the number of Annexin V/7′AAD-positive cells.
Cell lysate preparation
For preparation of lysates, STHdh and MEFHdh cells were trypsinized and collected by centrifugation at 350 × g for 5 min. The pellet was washed once with cold DPBS (Gibco®, Thermo Fisher Scientific), centrifuged again and lysed in RIPA buffer (50 mM Tris pH 7.5, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate and 1% Triton X-100, containing protease inhibitors) for 25 min on ice, while vortexing briefly every 5 min. Afterwards, samples were centrifuged at 13,200 × g for 15 min at 4 °C. Supernatant was pipetted into a pre-cooled reaction tube, adding glycerol to final concentration of 10%, and stored at −80 °C until further analysis.
Western blotting, SYPRO Ruby staining and immunodetection
Protein concentrations of RIPA lysates were determined spectrophotometrically using Bradford reagent (Bio-Rad Laboratories). Western blot analysis was performed following standard procedures. Briefly, 30 µg of protein were separated electrophoretically using 10% Bolt® Bis-Tris Plus Gels (Thermo Fisher Scientific). Proteins were transferred on Amersham™ Protran™ Premium 0.2 µm nitrocellulose membranes (GE Healthcare) using a TE22 Transfer Tank (Hoefer).
After transfer, total protein was stained with SYPRO Ruby Protein Blot Stain (Thermo Fisher Scientific) according to manufacturer’s instructions and detected at 600 nm using the LI-COR ODYSSEY® FC imaging system (LI-COR Biosciences).
After SYPRO Ruby staining, membranes were blocked with 5% Slimfast in TBS at room temperature for 1 h and probed overnight at 4 °C with the following primary antibodies: mouse anti-β-actin (1:10.000; clone AC-15, A5441, Sigma Aldrich), mouse anti-GAPDH (1:1000; clone GA1R, ab125247, Abcam), mouse anti-α-tubulin (1:5000; clone DM1A, CP06, EMD Millipore) and rabbit anti-vinculin (1:1000; clone E1E9V, #13901, Cell signalling). Afterwards, membranes were incubated at room temperature for 1 h with the respective secondary IRDye antibodies goat anti-mouse 680LT and goat anti-rabbit 800CW (all 1:10,000; LI-COR Biosciences). Fluorescence signals were detected with the LI-COR ODYSSEY® FC and quantified with ODYSSEY® Server software version 4.1 (LI-COR Biosciences). Quantified signals were normalized to total protein as detected before using SYPRO Ruby Protein Stain.
Statistical Analysis and Data availability
All data are presented as individual measurements (grey shapes) with mean and standard error of the mean (SEM). Statistical analysis was performed with GraphPad Prism 6.00 for Windows (GraphPad Software, Inc). Statistical significance of two group data sets was determined using two- tailed, unpaired Student’s t-test, with Welch’s correction. The significance threshold was set to P < 0.05. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Vonsattel, J. P. et al. Neuropathological classification of Huntington’s disease. Journal of neuropathology and experimental neurology 44, 559–577 (1985).
Zuccato, C. & Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5, 311–322 (2009).
Trettel, F. et al. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet 9, 2799–2809 (2000).
Menalled, L. B. Knock-In Mouse Models of Huntington’s Disease. NeuroRX 2, 465–470 (2005).
Wheeler, V. C. et al. Early phenotypes that presage late-onset neurodegenerative disease allow testing of modifiers in Hdh CAG knock-in mice. Human Molecular Genetics 11, 633–640, https://doi.org/10.1093/hmg/11.6.633 (2002).
Wheeler, V. C. et al. Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Human Molecular Genetics 9, 503–513, https://doi.org/10.1093/hmg/9.4.503 (2000).
Gines, S. et al. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington’s disease knock-in mice. Human Molecular Genetics 12, 497–508, https://doi.org/10.1093/hmg/ddg046 (2003).
Oliveira, J. M. A. et al. Mitochondrial-Dependent Ca2+Handling in Huntington’s Disease Striatal Cells: Effect of Histone Deacetylase Inhibitors. The Journal of Neuroscience 26, 11174–11186, https://doi.org/10.1523/jneurosci.3004-06.2006 (2006).
Choo, Y. S., Johnson, G. V., MacDonald, M., Detloff, P. J. & Lesort, M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet 13, 1407–1420, https://doi.org/10.1093/hmg/ddh162 (2004).
Seong, I. S. et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet 14, 2871–2880, https://doi.org/10.1093/hmg/ddi319 (2005).
Milakovic, T. & Johnson, G. V. Mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin. J Biol Chem 280, 30773–30782, https://doi.org/10.1074/jbc.M504749200 (2005).
Xifro, X., Garcia-Martinez, J. M., Del Toro, D., Alberch, J. & Perez-Navarro, E. Calcineurin is involved in the early activation of NMDA-mediated cell death in mutant huntingtin knock-in striatal cells. J Neurochem 105, 1596–1612, https://doi.org/10.1111/j.1471-4159.2008.05252.x (2008).
Ferrante, A. et al. Expression, pharmacology and functional activity of adenosine A1 receptors in genetic models of Huntington’s disease. Neurobiol Dis 71, 193–204, https://doi.org/10.1016/j.nbd.2014.08.013 (2014).
Gines, S., Ivanova, E., Seong, I. S., Saura, C. A. & MacDonald, M. E. Enhanced Akt signaling is an early pro-survival response that reflects N-methyl-D-aspartate receptor activation in Huntington’s disease knock-in striatal cells. J Biol Chem 278, 50514–50522, https://doi.org/10.1074/jbc.M309348200 (2003).
Reis, S. A. et al. Striatal neurons expressing full-length mutant huntingtin exhibit decreased N-cadherin and altered neuritogenesis. Hum Mol Genet 20, 2344–2355, https://doi.org/10.1093/hmg/ddr127 (2011).
Singhal, P. K. et al. Mouse embryonic fibroblasts exhibit extensive developmental and phenotypic diversity. Proc Natl Acad Sci USA 113, 122–127, https://doi.org/10.1073/pnas.1522401112 (2016).
Lee, H. O., Davidson, J. M. & Duronio, R. J. Endoreplication: polyploidy with purpose. Genes & Development 23, 2461–2477, https://doi.org/10.1101/gad.1829209 (2009).
Gregory, T. R. Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biological Reviews 76, 65–101, doi:undefined (2001).
Stewart, N. & Bacchetti, S. Expression of SV40 large T antigen, but not small t antigen, is required for the induction of chromosomal aberrations in transformed human cells. Virology 180, 49–57 (1991).
Gaztelumendi, N. & Nogués, C. Chromosome Instability in mouse EmbryonicStem Cells. Scientific Reports 4, 5324, https://doi.org/10.1038/srep05324 (2014).
Wang, Y. et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis 4, e950, https://doi.org/10.1038/cddis.2013.480 (2013).
Lim, D. et al. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem 283, 5780–5789, https://doi.org/10.1074/jbc.M704704200 (2008).
Acuna, A. I. et al. A failure in energy metabolism and antioxidant uptake precede symptoms of Huntington’s disease in mice. Nature communications 4, 2917, https://doi.org/10.1038/ncomms3917 (2013).
Blazquez, C. et al. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain 134, 119–136, https://doi.org/10.1093/brain/awq278 (2011).
Lajoie, P. & Snapp, E. L. Changes in BiP availability reveal hypersensitivity to acute endoplasmic reticulum stress in cells expressing mutant huntingtin. J Cell Sci 124, 3332–3343, https://doi.org/10.1242/jcs.087510 (2011).
Chopra, V. et al. A small-molecule therapeutic lead for Huntington’s disease: Preclinical pharmacology and efficacy of C2-8 in the R6/2 transgenic mouse. Proceedings of the National Academy of Sciences 104, 16685–16689, https://doi.org/10.1073/pnas.0707842104 (2007).
Levine, M. S. et al. Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knockin mouse models of Huntington’s disease. J Neurosci Res 58, 515–532 (1999).
Slow, E. J. et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Human Molecular Genetics 12, 1555–1567, https://doi.org/10.1093/hmg/ddg169 (2003).
Vonsattel, J. P. G., Keller, C. & Pilar Amaya, Md Neuropathology of Huntington’s Disease. Handbook of Clinical Neurology 89, 599–618 (2008).
Munsie, L. et al. Mutant huntingtin causes defective actin remodeling during stress: defining a new role for transglutaminase 2 in neurodegenerative disease. Human Molecular Genetics 20, 1937–1951, https://doi.org/10.1093/hmg/ddr075 (2011).
Godin, J. D. et al. Huntingtin Is Required for Mitotic Spindle Orientation and Mammalian Neurogenesis. Neuron 67, 392–406, https://doi.org/10.1016/j.neuron.2010.06.027.
Molina-Calavita, M. et al. Mutant Huntingtin Affects Cortical Progenitor Cell Division and Development of the Mouse Neocortex. The Journal of Neuroscience 34, 10034–10040, https://doi.org/10.1523/jneurosci.0715-14.2014 (2014).
Ribeiro, M., Silva, A. C., Rodrigues, J., Naia, L. & Rego, A. C. Oxidizing effects of exogenous stressors in Huntington’s disease knock-in striatal cells–protective effect of cystamine and creatine. Toxicol Sci 136, 487–499, https://doi.org/10.1093/toxsci/kft199 (2013).
Oliveira, A. M. et al. Protective effects of 3-alkyl luteolin derivatives are mediated by Nrf2 transcriptional activity and decreased oxidative stress in Huntington’s disease mouse striatal cells. Neurochemistry International 91, 1–12 (2015).
Ruiz, C. et al. Protection by glia-conditioned medium in a cell model of Huntington disease. PLoS Currents 4, e4fbca54a2028b, https://doi.org/10.1371/4fbca54a2028b (2012).
Rigamonti, D. et al. Wild-type huntingtin protects from apoptosis upstream of caspase-3. Journal of Neuroscience 20, 3705–3713 (2000).
Li, S.-H., Cheng, A. L., Li, H. & Li, X.-J. Cellular Defects and Altered Gene Expression in PC12 Cells Stably Expressing Mutant Huntingtin. The Journal of Neuroscience 19, 5159–5172 (1999).
Dudek, H. et al. Regulation of Neuronal Survival by the Serine-Threonine Protein Kinase Akt. Science 275, 661 (1997).
Geiger, T., Cox, J. & Mann, M. Proteomic changes resulting from gene copy number variations in cancer cells. PLoS Genet 6, e1001090, https://doi.org/10.1371/journal.pgen.1001090 (2010).
Stingele, S. et al. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol Syst Biol 8, 608, https://doi.org/10.1038/msb.2012.40 (2012).
Bae, B. I. et al. Mutant huntingtin: nuclear translocation and cytotoxicity mediated by GAPDH. Proc Natl Acad Sci USA 103, 3405–3409, https://doi.org/10.1073/pnas.0511316103 (2006).
Burke, J. R. et al. Huntingtin and DRPLA proteins selectively interact with the enzyme GAPDH. Nat Med 2, 347–350 (1996).
Senatorov, V. V., Charles, V., Reddy, P. H., Tagle, D. A. & Chuang, D. M. Overexpression and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase in a transgenic mouse model of Huntington’s disease. Mol Cell Neurosci 22, 285–297 (2003).
Eggers, S. & Sinclair, A. Mammalian sex determination—insights from humans and mice. Chromosome Research 20, 215–238, https://doi.org/10.1007/s10577-012-9274-3 (2012).
Ferrante, R. J. Mouse Models of Huntington’s Disease and Methodological Considerations for Therapeutic Trials. Biochimica et biophysica acta 1792, 506–520, https://doi.org/10.1016/j.bbadis.2009.04.001 (2009).
Maiuri, T. et al. Huntingtin is a scaffolding protein in the ATM oxidative DNA damage response complex. Human Molecular Genetics 26, 395–406, https://doi.org/10.1093/hmg/ddw395 (2017).
von Horsten, S. et al. Transgenic rat model of Huntington’s disease. Hum Mol Genet 12, 617–624 (2003).
Mittelman, D. & Wilson, J. H. The fractured genome of HeLa cells. Genome Biology 14, 111, https://doi.org/10.1186/gb-2013-14-4-111 (2013).
Segawa, K., Minowa, A., Sugasawa, K., Takano, T. & Hanaoka, F. Abrogation of p53-mediated transactivation by SV40 large T antigen. Oncogene 8, 543–548 (1993).
Ali, S. H. & DeCaprio, J. A. Cellular transformation by SV40 large T antigen: interaction with host proteins. Seminars in Cancer Biology 11, 15–22, https://doi.org/10.1006/scbi.2000.0342 (2001).
Feng, Z. et al. p53 tumor suppressor protein regulates the levels of huntingtin gene expression. Oncogene 25, 1 (2006).
Bae, B. I. et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron 47, 29–41, https://doi.org/10.1016/j.neuron.2005.06.005 (2005).
Abràmoff, M. D. M., Paulo, J. & Ram, Sunanda, J. Image Processing with ImageJ. Biophotonics International (2004).
Eppig, J. T. Rules for Nomenclature of Mouse Chromosome Aberrations, http://www.informatics.jax.org/mgihome/nomen/anomalies.shtml (2015).
Acknowledgements
J.J.W. was funded by the Baden-Wuerttemberg Foundation (research grant number P-BWS-SPII/3-08). The work was supported by the European Union 7th Framework Program (FP7/2012), Project “SWITCH-HD”, under grant agreement No. 324495 to H.P.N. L.E.C. was a postdoctoral fellow of the SWITCH-HD project. We are grateful to Alissa Mittnik and Tanja Wlodkowski for making the first cell size analyses in STHdh cells, Midea Ortiz Rios for supportive cell culture work and Eva Haydt for harvesting the different cultures for karyotyping as well as Jeannette Schoene for technical assistance.
Author information
Authors and Affiliations
Contributions
L.E.C., C.W., J.J.W., O.R. and H.P.N. developed the conceptual framework for the study. E.S., C.W., A.-C.K. and L.E.C. performed cell size and proliferation analyses. U.A.M.-H. performed karyotyping. J.J.W. performed the analysis of protein markers. E.S., C.W., A.-C.K., N.R. and L.E.C. determined cell viability. L.E.C., C.W., J.J.W., E.S., U.A.M.-H., and H.P.N. interpreted the data. L.E.C., C.W., J.J.W. and E.S. prepared the Figures. L.E.C., E.S., C.W., J.J.W. and H.P.N. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singer, E., Walter, C., Weber, J.J. et al. Reduced cell size, chromosomal aberration and altered proliferation rates are characteristics and confounding factors in the STHdh cell model of Huntington disease. Sci Rep 7, 16880 (2017). https://doi.org/10.1038/s41598-017-17275-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17275-4
This article is cited by
-
Elevated SLC7A2 expression is associated with an abnormal neuroinflammatory response and nitrosative stress in Huntington’s disease
Journal of Neuroinflammation (2024)
-
Mitochondrial Abnormalities and Synaptic Damage in Huntington’s Disease: a Focus on Defective Mitophagy and Mitochondria-Targeted Therapeutics
Molecular Neurobiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.